Macro- and Trace-Element Intake from Human Milk in Australian Infants: Inadequacy with Respect to National Recommendations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants and Study Design
2.2. Sample Collection
2.3. Analytical Methods
2.4. Infant Growth Parameters
2.5. Statistical Analysis
3. Results
3.1. Cohort Characteristics
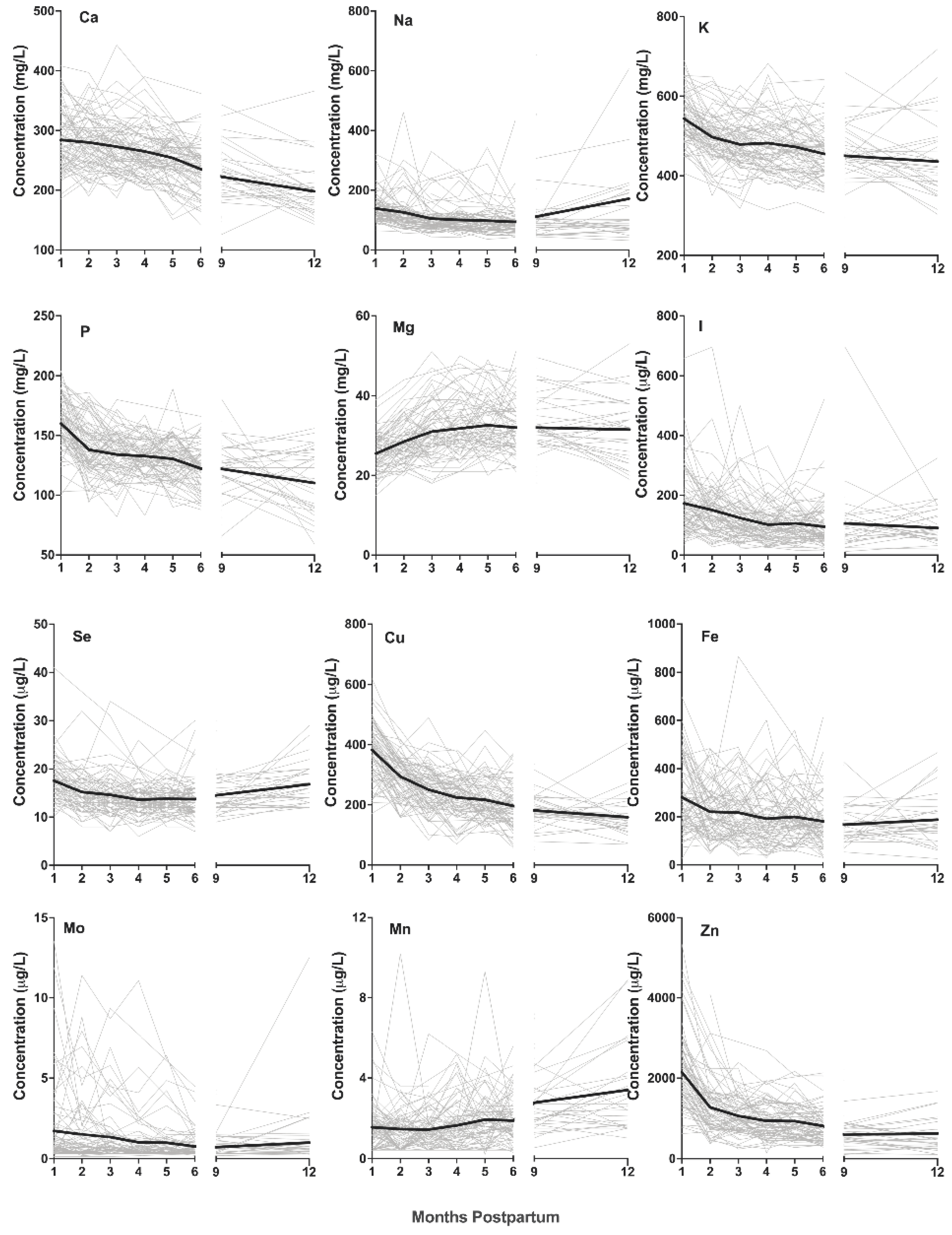
3.2. Changes in Macro- and Trace-Element Concentrations over the First 12 Months Postpartum
3.3. Changes in Macro- and Trace-Element Intake over the First Six Months Postpartum
3.4. Relationship between Intake and Infant Growth Parameters
4. Discussion
Limitations and Further Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmer, J.P. Handbook of Milk Composition; Jensen, R.G., Ed.; Academic Press: San Deigo, CA, USA, 1995. [Google Scholar]
- Andreas, N.J.; Kampmann, B.; Le-Doare, K.M. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Binns, C.; Fraser, M.L.; Lee, A.H.; Scott, J. Defining exclusive breastfeeding in Australia. J. Paediatr. Child Health 2009, 45, 174–180. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.Y.; Pundir, S.; McKenzie, E.; Keijer, J.; Kussmann, M. Maternal Circulating Vitamin Status and Colostrum Vitamin Composition in Healthy Lactating Women—A Systematic Approach. Nutrients 2018, 10, 687. [Google Scholar] [CrossRef] [Green Version]
- Allen, L.H.; Dror, D.K. Introduction to Current Knowledge on Micronutrients in Human Milk: Adequacy, Analysis, and Need for Research. Adv. Nutr. 2018, 9, 275S–277S. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee on Diet and Health. Diet and Health: Implications for Reducing Chronic Disease Risk; National Academies Press: Washington, DC, USA, 1989. [CrossRef]
- Allen, L.H.; Donohue, J.A.; Dror, D.K. Limitations of the Evidence Base Used to Set Recommended Nutrient Intakes for Infants and Lactating Women. Adv. Nutr. 2018, 9, 295S–312S. [Google Scholar] [CrossRef] [Green Version]
- National Health and Medical Research Council, Australian Government Department of Health and Aging, New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; National Health and Medical Research Council: Canberra, Australia, 2006.
- Krachler, M.; Li, F.S.; Rossipal, E.; Irgolic, K. Changes in the Concentrations of Trace Elements in Human Milk during Lactation. J. Trace Elem. Med. Biol. 1998, 12, 159–176. [Google Scholar] [CrossRef]
- Krebs, N.F.; Reidinger, C.J.; Hartley, S.; Robertson, A.D.; Hambidge, K.M. Zinc supplementation during lactation: Effects on maternal status and milk zinc concentrations. Am. J. Clin. Nutr. 1995, 61, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Overview of Nutrients in Human Milk. Adv. Nutr. 2018, 9, 278S–294S. [Google Scholar] [CrossRef] [Green Version]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Ramsay, D.T.; Doherty, D.A.; Hartmann, P. Volume and Frequency of Breastfeedings and Fat Content of Breast Milk Throughout the Day. Pediatrics 2006, 117, e387–e395. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.M.; Aiello, M.O.; Fujita, M.; Hinde, K.; Milligan, L.; Quinn, E. Field and laboratory methods in human milk research. Am. J. Hum. Biol. 2013, 25, 1–11. [Google Scholar] [CrossRef]
- Kent, J.C.; Gardner, H.; Geddes, D.T. Breastmilk Production in the First 4 Weeks after Birth of Term Infants. Nutrients 2016, 8, 756. [Google Scholar] [CrossRef] [Green Version]
- Kent, J.C.; Hepworth, A.R.; Sherriff, J.L.; Cox, D.B.; Mitoulas, L.R.; Hartmann, P.E. Longitudinal changes in breastfeeding patterns from 1 to 6 months of lactation. Breastfeed Med. 2013, 8, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Bilston-John, S.H.; Narayanan, A.; Lai, C.T.; Rea, A.; Joseph, J.; Geddes, D.T. Daily and within-feed variation of macro- and trace-element concentrations in human milk and implications for sampling. Food Chem. 2021, 363, 130179. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Child Growth Standards Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age. Methods and Development; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Daniels, L.; Gibson, R.S.; Diana, A.; Haszard, J.J.; Rahmannia, S.; Luftimas, D.E.; Hampel, D.; Shahab-Ferdows, S.; Reid, M.; Melo, L.; et al. Micronutrient intakes of lactating mothers and their association with breast milk concentrations and micronutrient adequacy of exclusively breastfed Indonesian infants. Am. J. Clin. Nutr. 2019, 110, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Solomons, N.W.; Scott, M.E.; Koski, K.G. Minerals and Trace Elements in Human Breast Milk Are Associated with Guatemalan Infant Anthropometric Outcomes within the First 6 Months. J. Nutr. 2016, 146, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Osendarp, S.J.; Santosham, M.; Black, R.E.; Wahed, M.; van Raaij, J.M.; Fuchs, G.J. Effect of zinc supplementation between 1 and 6 mo of life on growth and morbidity of Bangladeshi infants in urban slums. Am. J. Clin. Nutr. 2002, 76, 1401–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umeta, M.; West, C.E.; Haidar, J.; Deurenberg, P.; Hautvast, J.G. Zinc supplementation and stunted infants in Ethiopia: A randomised controlled trial. Lancet 2000, 355, 2021–2026. [Google Scholar] [CrossRef]
- Vuori, E.; Mäkinen, S.M.; Kara, R.; Kuitunen, P. The effects of the dietary intakes of copper, iron, manganese, and zinc on the trace element content of human milk. Am. J. Clin. Nutr. 1980, 33, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; De Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J.; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Salgueiro, M.J.; Zubillaga, M.B.; Lysionek, A.E.; Caro, R.A.; Weill, R.; Boccio, J.R. The role of zinc in the growth and development of children. Nutrients 2002, 18, 510–519. [Google Scholar] [CrossRef]
- Pathak, P.; Kapil, U. Role of trace elements zinc, copper and magnesium during pregnancy and its outcome. Indian J. Pediatr. 2004, 71, 1003–1005. [Google Scholar] [CrossRef]
- Trabzuni, D.M.; Ibrahim, H.S.; Ewaidah, E.H. An assessment of iodine status of Saudi lactating mothers and its relation to iodine intake in Riyadh City. Ecol. Food Nutr. 1998, 37, 297–307. [Google Scholar] [CrossRef]
- Rossipal, E.; Krachler, M.; Li, F.; Micetic-Turk, D. Investigation of the transport of trace elements across barriers in humans: Studies of placental and mammary transfer. Acta Paediatr. 2000, 89, 1190–1195. [Google Scholar] [CrossRef]
- Kumpulainen, J.; Salmenperä, L.; Siimes, M.A.; Koivistoinen, P.; Perheentupa, J. Selenium status of exclusively breast-fed infants as influenced by maternal organic or inorganic selenium supplementation. Am. J. Clin. Nutr. 1985, 42, 829–835. [Google Scholar] [CrossRef]
- Trafikowska, U.; Sobkowiak, E.; Butler, J.; Whanger, P.; Zachara, B. Organic and Inorganic Selenium Supplementation to Lactating Mothers Increase the Blood and Milk Se Concentrations and Se Intake by Breast-fed Infants. J. Trace Elem. Med. Biol. 1998, 12, 77–85. [Google Scholar] [CrossRef]
- Vormann, J. Magnesium: Nutrition and Homoeostasis. AIMS Public Health 2016, 3, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Lönnerdal, B. Milk and Nutrient Intake of Breast-Fed Infants from 1 to 6 Months. J. Pediatr. Gastroenterol. Nutr. 1983, 2, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Karra, M.V.; Kirksey, A.; Galal, O.; Bassily, N.S.; Harrison, G.G.; Jerome, N.W. Zinc, calcium, and magnesium concentrations in milk from American and Egyptian women throughout the first 6 months of lactation. Am. J. Clin. Nutr. 1988, 47, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Vanstone, C.A.; Weiler, H.A. Normative Data for Bone Mass in Healthy Term Infants from Birth to 1 Year of Age. J. Osteoporos. 2012, 2012, 672403. [Google Scholar] [CrossRef] [PubMed]
- Dorea, J.G. Breast milk calcium and phosphorus concentrations. Nutrition 2000, 16, 146–147. [Google Scholar] [CrossRef]
- Meinel, B.; Bode, J.; Koenig, W.; Richter, F.-W. Contents of Trace Elements in the Human Liver before Birth. Biol. Neonate 1979, 36, 225–232. [Google Scholar] [CrossRef]
- Kujawa, M. Trace Element Metabolism in Man and Animals. 3. Proceedings of the 3rd International Symposium; von M. Kirchgessner, H., Ed.; Institut fur Ernahrungsphysiologie: Dummerstorf, Germany, 1978. [Google Scholar]
- Casey, C.E.; Hambidge, K.M.; Neville, M.C. Studies in human lactation: Zinc, copper, manganese and chromium in human milk in the first month of lactation. Am. J. Clin. Nutr. 1985, 41, 1193–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farré, R.; Martínez-Costa, C.; Silvestre, D.; Lagarda, M.J.; Brines, J.; Clemente, G. Copper, Iron, and Zinc Contents in Human Milk During the First Three Months of Lactation: A Longitudinal Study. Biol. Trace Elem. Res. 2001, 80, 01–11. [Google Scholar] [CrossRef]
- Lönnerdal, B. Trace Element Transport in the Mammary Gland. Annu. Rev. Nutr. 2007, 27, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Domellöf, M.; Cohen, R.J.; Rivera, L.L.; Hernell, O.; Lönnerdal, B. Iron Supplementation Affects Growth and Morbidity of Breast-Fed Infants: Results of a Randomized Trial in Sweden and Honduras. J. Nutr. 2002, 132, 3249–3255. [Google Scholar] [CrossRef] [Green Version]
- Rivera, J.A.; González-Cossío, T.; Flores, M.; Romero, M.; Rivera, M.; Téllez-Rojo, M.M.; Rosado, J.L.; Brown, K.H. Multiple micronutrient supplementation increases the growth of Mexican infants. Am. J. Clin. Nutr. 2001, 74, 657–663. [Google Scholar] [CrossRef] [Green Version]
- Lönnerdal, B. Development of iron homeostasis in infants and young children. Am. J. Clin. Nutr. 2017, 106, 1575S–1580S. [Google Scholar] [CrossRef] [Green Version]
- Allen, L.H.; Hampel, D. Human Milk as the First Source of Micronutrients. Nestle Nutr. Inst. Workshop Ser. 2020, 93, 67–76. [Google Scholar]

| Characteristic | Mean ± SEM | Range |
|---|---|---|
| Maternal age at infant birth (n = 81) | 33.2 ± 0.52 | 25.1–46.4 |
| Parity (n = 83) | 2.1 ± 0.1 | 1–4 |
| Maternal ethnicity (n = 83) | ||
| Caucasian | 74 | |
| Other | 9 | |
| 24-hour infant breast milk intake (mL, n = 59) | 776.3 ± 24.0 | 348–1344 |
| Maternal supplementation (n = 50) Calcium Magnesium Iron Zinc | n = 10 n = 18 n = 38 n = 8 | |
| HM collection+ (n = 83) | ||
| Left breast/right breast | 29/54 | |
| Infant sex (n = 83) | ||
| Male/female | 39/44 | |
| Gestational age (weeks) | 39.2 ± 0.1 | 36.6–41.2 |
| Infant birth weight (kg) (n = 78) | 3.49 ± 0.48 | 2.36–4.36 |
| Infant WFA z-score at birth (n = 78) | 0.38 ± 0.1 | −2.08–2.16 |
| Infant length at birth (cm) (n = 78) | 51.0 ± 0.28 | 45–59 |
| Infant LFA z-score at birth (n = 78) | 0.78 ± 0.15 | −2.23–4.82 |
| 1 Month (n = 74) | 2 Months (n = 72) | 3 Months (n = 73) | 4 Months (n = 66) | 5 Months (n = 57) | 6 Months (n = 60) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AI Value | Intake Median (Range) | Meeting AI n (%) | Intake Median (Range) | Meeting AI n (%) | Intake Median (Range) | Meeting AI n (%) | Intake Median (Range) | Meeting AI n (%) | Intake Median (Range) | Meeting AI n (%) | Intake Median (Range) | Meeting AI n (%) | |
| Ca (mg/day) | 210 | 201 (87.6–411) | 28 (45.2) | 204 (93.6–431) | 27 (45.8) | 195 (83.6–421) | 27 (44.3) | 195 (74.1–427) | 24 (42.1) | 190 (86.1–330) | 18 (34.6) | 175 (74.5–399) | 15 (29) |
| Na (mg/day) | 120 | 95.7 (34.5–293) | 14 (22.6) | 85.5 (31.3–288) | 13 (22.0) | 73.5 (38.2–259) | 9 (15.0) | 67.0 (26.2–238) | 9 (15.5) | 64.6 (24.5–358) | 6 (11.5) | 67.7 (23.4–168) | 4 (7.80) |
| K (mg/day) | 400 | 418.1 (160–673) | 33 (53.2) | 376 (132–624) | 26 (44.1) | 365 (119–704) | 21 (34.4) | 372 (109–673) | 22 (38.6) | 362 (116–638) | 16 (30.8) | 326 (116–638) | 15 (29.4) |
| P (mg/day) | 100 | 121 (50.4–246) | 44 (71.0) | 100 (42.8–211.9) | 29 (49.2) | 97.9 (48.4–206) | 27 (44.2) | 104 (47.3–173) | 32 (56.1) | 98.0 (44.9–191) | 24 (46.2) | 87.5 (44.6–168) | 17 (32.7) |
| Mg (mg/day) | 30 | 18.6 (9.13–39.8) | 3 (4.84) | 22.1 (8.35–44.9) | 9 (15.3) | 23.8 (8.03–49.0) | 7 (11.5) | 24.3 (8.03–49.0) | 12 (21.1) | 24.3 (10.2–50.1) | 13 (25) | 22.9 (10.1–48.0) | 9 (17.3) |
| I (µg/day) | 90 | 119 (26.0–528) | 37 (59.7) | 109 (25.1–557) | 35 (59.3) | 88.6 (21.8–617) | 29 (47.5) | 65.6 (14.5–303) | 21 (37.5) | 72.1 (21–304) | 16 (31.3) | 54.6 (8.82–286) | 15 (29.4) |
| Se (µg/day) | 12 | 13.3 (4.87–29.3) | 36 (58.1) | 11.1 (4.52–31.5) | 25 (42.3) | 10.8 (3.48–26.7) | 24 (39.3) | 10.2 (4.18–24.7) | 16 (28.1) | 10.1 (3.83–18.8) | 14 (26.9) | 10.6 (3.83–18.8) | 14 (26.9) |
| Cu (µg/day) | 300 | 299 (98.5–474) | 51 (82.2) | 218.7 (82.5–532) | 37 (62.7) | 195 (50.8–374) | 28 (45.9) | 179 (63.9–364) | 20 (35.1) | 157 (56.6–302) | 15 (28.8) | 159 (36.2–321) | 10 (19.2) |
| Mn (µg/day) | 3 | 1.00 (0.19–6.38) | 4 (6.45) | 1.00 (0.14–12.4) | 3 (5.01) | 0.89 (0.16–4.86) | 1 (1.64) | 0.95 (0.17–4.86) | 6 (10.5) | 1.22 (0.23–5.07) | 3 (5.77) | 1.03 (0.26–4.12) | 3 (3.92) |
| Mo (µg/day) | 2 | 0.56 (0.06–10.6) | 9 (14.5) | 0.42 (0.15–10.3) | 10 (16.9) | 0.42 (0.06–7.83) | 8 (13.1) | 0.31 (0.11–10.0) | 4 (7.02) | 0.35 (0.10–5.58) | 4 (7.69) | 0.30 (0.10–4.33) | 2 (3.85) |
| Fe (µg/day) | 200 | 185 (24.1–686) | 28 (45.1) | 145 (29.2–436) | 22 (37.3) | 138 (22.0–677) | 19 (31.1) | 123 (24.4–490) | 14 (26.9) | 138 (39.3–396) | 14 (26.9) | 126 (12.1–539) | 11 (21.2) |
| Zn (µg/day) | 2000 | 1569 (452-4058) | 16 (25.8) | 848 (300–2233) | 3 (5.08) | 768 (192–1908) | 0 (0) | 594 (240–1571) | 0 (0) | 573 (240–1471) | 0 (0) | 539 (203–1471) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilston-John, S.H.; Narayanan, A.; Lai, C.T.; Rea, A.; Joseph, J.; Geddes, D.T. Macro- and Trace-Element Intake from Human Milk in Australian Infants: Inadequacy with Respect to National Recommendations. Nutrients 2021, 13, 3548. https://doi.org/10.3390/nu13103548
Bilston-John SH, Narayanan A, Lai CT, Rea A, Joseph J, Geddes DT. Macro- and Trace-Element Intake from Human Milk in Australian Infants: Inadequacy with Respect to National Recommendations. Nutrients. 2021; 13(10):3548. https://doi.org/10.3390/nu13103548
Chicago/Turabian StyleBilston-John, Sabrina H., Ardra Narayanan, Ching T. Lai, Alethea Rea, John Joseph, and Donna T. Geddes. 2021. "Macro- and Trace-Element Intake from Human Milk in Australian Infants: Inadequacy with Respect to National Recommendations" Nutrients 13, no. 10: 3548. https://doi.org/10.3390/nu13103548
APA StyleBilston-John, S. H., Narayanan, A., Lai, C. T., Rea, A., Joseph, J., & Geddes, D. T. (2021). Macro- and Trace-Element Intake from Human Milk in Australian Infants: Inadequacy with Respect to National Recommendations. Nutrients, 13(10), 3548. https://doi.org/10.3390/nu13103548







