Effect of Omega-3 Supplementation on Self-Regulation in Typically Developing Preschool-Aged Children: Results of the Omega Kid Pilot Study—A Randomised, Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participant Criteria
2.2. Recruitment and Randomisation
2.3. Intervention
2.4. Data Collection
2.5. Outcome Measures
2.5.1. Primary Outcome Measure
2.5.2. N-3 LCPUFA Erythrocyte Levels
2.6. Secondary Outcome Measures
2.6.1. Executive Function
2.6.2. Electroencephalographic (EEG) Measures
2.6.3. ADHD
2.7. Control Measures
2.7.1. PUFA Intake
2.7.2. Compliance to Study Protocol and Assessment of Blinding of Treatment Groups
2.8. Statistical Analysis
2.9. Sample Size
3. Results
3.1. Study Population
3.2. Outcome Measures at 12 Weeks Post-Intervention
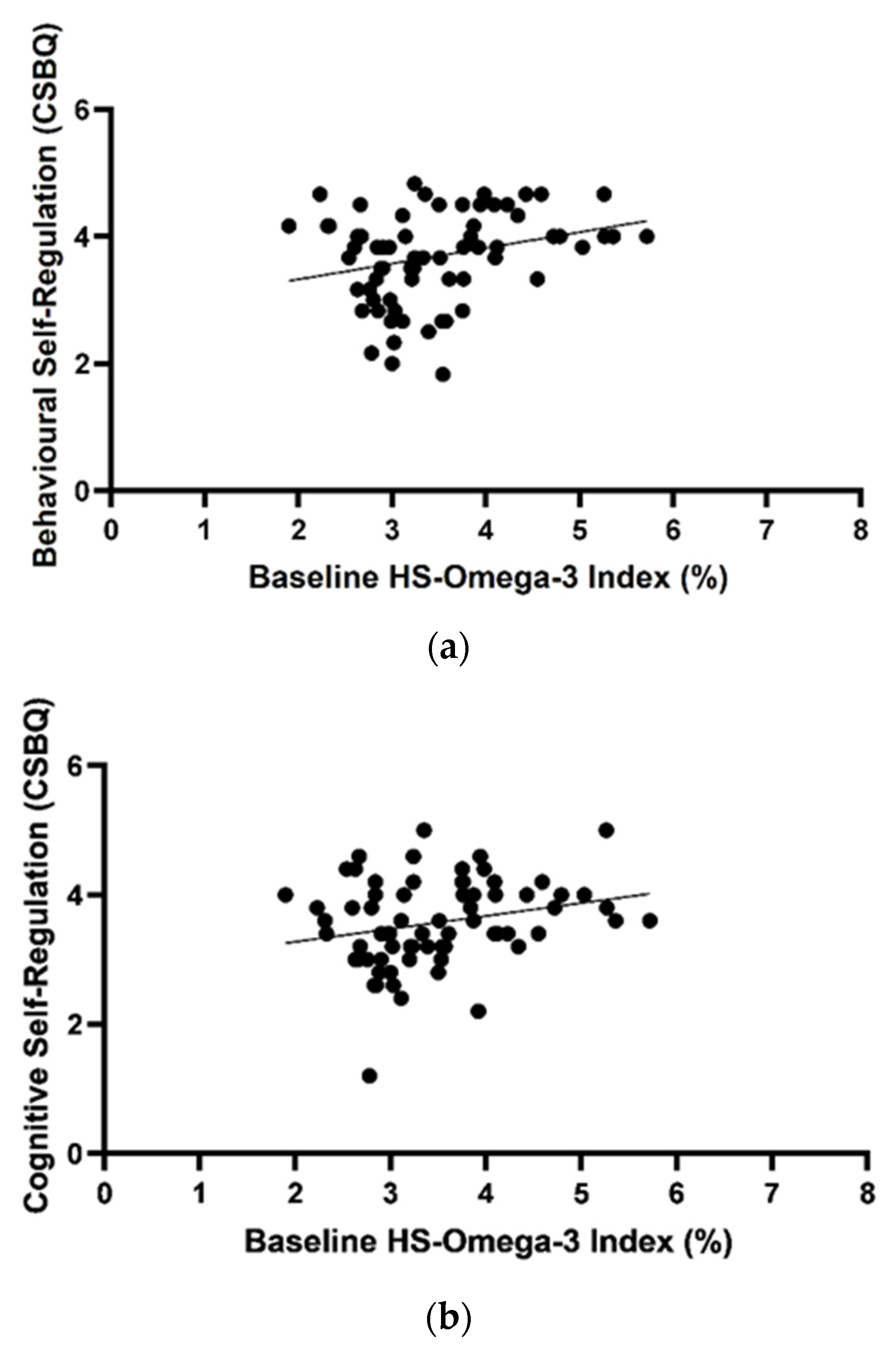
3.3. Omega-3 Index and Outcome Variables at Baseline
3.4. Early Years Toolbox Task Baseline Participant Scores
3.5. Dietary Intake of n-3 LCPUFA
3.6. Blinding of Omega-3 and Placebo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer, B.J.; Onyiaodike, C.C.; Brown, E.A.; Jordan, F.; Murray, H.; Nibbs, R.J.; Sattar, N.; Lyall, H.; Nelson, S.M.; Freeman, D.J. Maternal plasma DHA levels increase prior to 29 days post-LH surge in women undergoing frozen embryo transfer: A prospective, observational study of human pregnancy. J. Clin. Endocrinol. Metab. 2016, 101, 1745–1753. [Google Scholar] [CrossRef]
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560s–569s. [Google Scholar] [CrossRef] [Green Version]
- Dyerberg, J.; Bang, H. Dietary fat and thrombosis. Lancet 1978, 311, 152. [Google Scholar] [CrossRef]
- Sinn, N.; Howe, P. Mental health benefits of omega-3 fatty acids may be mediated by improvements in cerebral vascular function. Biosci. Hypotheses 2008, 1, 103–108. [Google Scholar] [CrossRef]
- Parletta, N.; Milte, C.M.; Meyer, B.J. Nutritional modulation of cognitive function and mental health. J. Nutr. Biochem. 2013, 24, 725–743. [Google Scholar] [CrossRef] [Green Version]
- Vlaardingerbroek, H.; Hornstra, G.; De Koning, T.; Smeitink, J.; Bakker, H.; de Klerk, H.; Rubio-Gozalbo, M. Essential polyunsaturated fatty acids in plasma and erythrocytes of children with inborn errors of amino acid metabolism. Mol. Genet. Metab. 2006, 88, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.J.; Kolanu, N. Australian children are not consuming enough long-chain omega-3 polyunsaturated fatty acids for optimal health. Nutrition 2011, 27, 1136–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haag, M. Essential fatty acids and the brain. Can. J. Psychiatry 2003, 48, 195–203. [Google Scholar] [CrossRef]
- Young, G.; Conquer, J. Omega-3 fatty acids and neuropsychiatric disorders. Reprod. Nutr. Dev. 2005, 45, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.J. Omega-3 fatty acids in ADHD and related neurodevelopmental disorders. Int. Rev. Psychiatry 2006, 18, 155–172. [Google Scholar] [CrossRef]
- Sinn, N. Nutritional and dietary influences on attention deficit hyperactivity disorder. Nutr. Rev. 2008, 66, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Bos, D.J.; Oranje, B.; Veerhoek, E.S.; Van Diepen, R.M.; Weusten, J.M.; Demmelmair, H.; Koletzko, B.; Eilander, A.; Hoeksma, M.; Durston, S. Reduced symptoms of inattention after dietary omega-3 fatty acid supplementation in boys with and without attention deficit/hyperactivity disorder. Neuropsychopharmacology 2015, 40, 2298–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, D.W.; Rosanbalm, K.; Christopoulos, C.; Hamoudi, A. Self-regulation and toxic stress: Foundations for understanding self-regulation from an applied developmental perspective. OPRE Rep. 2015, 21, 1–29. [Google Scholar]
- Hofmann, W.; Schmeichel, B.J.; Baddeley, A.D. Executive functions and self-regulation. Trends Cogn. Sci. 2012, 16, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Gogtay, N.; Giedd, J.N.; Lusk, L.; Hayashi, K.M.; Greenstein, D.; Vaituzis, A.C.; Nugent, T.F.; Herman, D.H.; Clasen, L.S.; Toga, A.W.; et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA 2004, 101, 8174–8179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robson, D.A.; Allen, M.S.; Howard, S.J. Self-regulation in childhood as a predictor of future outcomes: A meta-analytic review. Psychol. Bull. 2020, 146, 324. [Google Scholar] [CrossRef] [Green Version]
- Moffitt, T.E.; Arseneault, L.; Belsky, D.; Dickson, N.; Hancox, R.J.; Harrington, H.; Houts, R.; Poulton, R.; Roberts, B.W.; Ross, S. A gradient of childhood self-control predicts health, wealth, and public safety. Proc. Natl. Acad. Sci. USA 2011, 108, 2693–2698. [Google Scholar] [CrossRef] [Green Version]
- Widenhorn-Müller, K.; Schwanda, S.; Scholz, E.; Spitzer, M.; Bode, H. Effect of supplementation with long-chain ω-3 polyunsaturated fatty acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): A randomized placebo-controlled intervention trial. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 49–60. [Google Scholar] [CrossRef]
- Sinn, N.; Bryan, J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J. Dev. Behav. Pediatrics 2007, 28, 82–91. [Google Scholar] [CrossRef]
- Sorgi, P.J.; Hallowell, E.M.; Hutchins, H.L.; Sears, B. Effects of an open-label pilot study with high-dose EPA/DHA concentrates on plasma phospholipids and behavior in children with attention deficit hyperactivity disorder. Nutr. J. 2007, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Ryan, A.S.; Nelson, E.B. Assessing the effect of docosahexaenoic acid on cognitive functions in healthy, preschool children: A randomized, placebo-controlled, double-blind study. Clin. Pediatrics 2008, 47, 355–362. [Google Scholar] [CrossRef]
- Leutgeb, V.; Koechel, A.; Lang, L.; Koch, J. Effects of Omega-3 Fatty Acids on Cognitive, Emotional, and Social Behavioral Parameters in Kindergarten Children: A Pilot Study. Kindh. Entwickl. 2015, 24, 86–93. [Google Scholar] [CrossRef]
- Øyen, J.; Kvestad, I.; Midtbø, L.K.; Graff, I.E.; Hysing, M.; Stormark, K.M.; Markhus, M.W.; Baste, V.; Frøyland, L.; Koletzko, B. Fatty fish intake and cognitive function: FINS-KIDS, a randomized controlled trial in preschool children. BMC Med. 2018, 16, 41. [Google Scholar] [CrossRef] [Green Version]
- Heatherton, T.F.; Wagner, D.D. Cognitive neuroscience of self-regulation failure. Trends Cogn. Sci. 2011, 15, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, S.J.; Jiang, H.; Sun, L.; Rogers, J.M.; Valderrama, J.; Zhang, D. Development of frontal EEG differences between eyes-closed and eyes-open resting conditions in children: Data from a single-channel dry-sensor portable device. Clin. EEG Neurosci. 2021, 52, 235–245. [Google Scholar] [CrossRef]
- McNamara, R.K.; Able, J.; Jandacek, R.; Rider, T.; Tso, P.; Eliassen, J.C.; Alfieri, D.; Weber, W.; Jarvis, K.; DelBello, M.P. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: A placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am. J. Clin. Nutr. 2010, 91, 1060–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roach, L.A.; Byrne, M.K.; Howard, S.J.; Johnstone, S.J.; Batterham, M.; Wright, I.M.; Okely, A.D.; de Groot, R.H.; van der Wurff, I.S.; Jones, A. The Feasibility of the “Omega Kid” Study Protocol: A Double-Blind, Randomised, Placebo-Controlled Trial Investigating the Effect of Omega-3 Supplementation on Self-Regulation in Preschool-Aged Children. Nutrients 2021, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand 2014. Available online: https://www.nrv.gov.au/nutrients/fats-total-fat-fatty-acids (accessed on 29 March 2019).
- McClelland, M.M.; Cameron, C.E. Self-regulation in early childhood: Improving conceptual clarity and developing ecologically valid measures. Child Dev. Perspect. 2012, 6, 136–142. [Google Scholar] [CrossRef]
- Howard, S.J.; Melhuish, E. An early years toolbox for assessing early executive function, language, self-regulation, and social development: Validity, reliability, and preliminary norms. J. Psychoeduc. Assess. 2017, 35, 255–275. [Google Scholar] [CrossRef]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- Van der Wurff, I.; Von Schacky, C.; Berge, K.; Zeegers, M.; Kirschner, P.; De Groot, R. Association between blood omega-3 index and cognition in typically developing Dutch adolescents. Nutrients 2016, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Sherman, E.M.; Brooks, B.L. Behavior rating inventory of executive function–preschool version (BRIEF-P): Test review and clinical guidelines for use. Child Neuropsychol. 2010, 16, 503–519. [Google Scholar] [CrossRef]
- Rogers, J.M.; Johnstone, S.J.; Aminov, A.; Donnelly, J.; Wilson, P.H. Test-retest reliability of a single-channel, wireless EEG system. Int. J. Psychophysiol. 2016, 106, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin. Neurophysiol. 2003, 114, 171–183. [Google Scholar] [CrossRef]
- Johnstone, S.J.; Roodenrys, S.; Phillips, E.; Watt, A.J.; Mantz, S. A pilot study of combined working memory and inhibition training for children with AD/HD. ADHD Atten. Deficit Hyperact. Disord. 2010, 2, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, S.J.; Roodenrys, S.J.; Johnson, K.; Bonfield, R.; Bennett, S.J. Game-based combined cognitive and neurofeedback training using Focus Pocus reduces symptom severity in children with diagnosed AD/HD and subclinical AD/HD. Int. J. Psychophysiol. 2017, 116, 32–44. [Google Scholar] [CrossRef]
- Purpura, D.J.; Lonigan, C.J. Conners’ teacher rating scale for preschool children: A revised, brief, age-specific measure. J. Clin. Child Adolesc. Psychol. 2009, 38, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Swierk, M.; Williams, P.G.; Wilcox, J.; Russell, K.G.; Meyer, B.J. Validation of an Australian electronic food frequency questionnaire to measure polyunsaturated fatty acid intake. Nutrition 2011, 27, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Mann, N.J.; Sinclair, A.J.; Percival, P.; Lewis, J.L.; Meyer, B.J.; Howe, P.R. Development of a database of fatty acids in Australian foods. Nutr. Diet. 2003, 60, 42–45. [Google Scholar]
- Thorell, L.B.; Lindqvist, S.; Bergman Nutley, S.; Bohlin, G.; Klingberg, T. Training and transfer effects of executive functions in preschool children. Dev. Sci. 2009, 12, 106–113. [Google Scholar] [CrossRef]
- Van der Wurff, I.S.; Meyer, B.J.; de Groot, R.H. Effect of omega-3 long chain polyunsaturated fatty acids (N-3 LCPUFA) supplementation on cognition in children and adolescents: A systematic literature review with a focus on n-3 LCPUFA blood values and dose of DHA and EPA. Nutrients 2020, 12, 3115. [Google Scholar] [CrossRef]
- Ghasemi Fard, S.; Loh, S.P.; Turchini, G.M.; Wang, B.; Elliott, G.; Sinclair, A.J. Microencapsulated tuna oil results in higher absorption of DHA in toddlers. Nutrients 2020, 12, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groot, R.H.; Emmett, R.; Meyer, B.J. Non-dietary factors associated with n-3 long-chain PUFA levels in humans–a systematic literature review. Br. J. Nutr. 2019, 121, 793–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K.E.; Howard, S.J. Proximal and distal predictors of self-regulatory change in children aged 4 to 7 years. BMC Pediat. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Richardson, A.J.; Burton, J.R.; Sewell, R.P.; Spreckelsen, T.F.; Montgomery, P. Docosahexaenoic acid for reading, cognition and behavior in children aged 7–9 years: A randomized, controlled trial (the DOLAB Study). PLoS ONE 2012, 7, e43909. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Fransson, G.; Östlund, S.; Areskoug, B.; Gillberg, C. Omega 3/6 fatty acids for reading in children: A randomized, double-blind, placebo-controlled trial in 9-year-old mainstream schoolchildren in Sweden. J. Child Psychol. Psychiatry 2017, 58, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Emery, S.; Häberling, I.; Berger, G.; Walitza, S.; Schmeck, K.; Albert, T.; Baumgartner, N.; Strumberger, M.; Albermann, M.; Drechsler, R. Omega-3 and its domain-specific effects on cognitive test performance in youths: A meta-analysis. Neurosci. Biobehav. Rev. 2020, 112, 420–436. [Google Scholar] [CrossRef]
- Dumont, F.M.; Tarabulsy, G.M.; Sylvestre, A.; Voisin, J. Children’s emotional self-regulation in the context of adversity and the association with academic functioning. Child Psychiatry Hum. Dev. 2019, 50, 856–867. [Google Scholar] [CrossRef]
- Edossa, A.K.; Schroeders, U.; Weinert, S.; Artelt, C. The development of emotional and behavioral self-regulation and their effects on academic achievement in childhood. Int. J. Behav. Dev. 2018, 42, 192–202. [Google Scholar] [CrossRef]
- Cooper, R.E.; Tye, C.; Kuntsi, J.; Vassos, E.; Asherson, P. Omega-3 polyunsaturated fatty acid supplementation and cognition: A systematic review and meta-analysis. J. Psychopharmacol. 2015, 29, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Vesco, A.T.; Young, A.S.; Arnold, L.E.; Fristad, M.A. Omega-3 supplementation associated with improved parent-rated executive function in youth with mood disorders: Secondary analyses of the omega 3 and therapy (OATS) trials. J. Child Psychol. Psychiatry 2018, 59, 628–636. [Google Scholar] [CrossRef]
- Richardson, A.J.; Puri, B.K. A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2002, 26, 233–239. [Google Scholar] [CrossRef]
- Richardson, A.J.; Montgomery, P. The Oxford-Durham study: A randomized, controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics 2005, 115, 1360–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groot, R.H.; Meyer, B.J. ISSFAL Official Statement Number 6: The importance of measuring blood omega-3 long chain polyunsaturated fatty acid levels in research. Prostaglandins Leukot. Essent. Fat. Acids 2020, 157, 102029. [Google Scholar] [CrossRef]



| Baseline Omega-3 Group | Post Intervention Omega-3 Group | Baseline Placebo Group | Post Intervention Placebo Group | Mean Difference | Effect Size (ηp2) | p | p Adjusted for Age, Gender, EEG | Compliant Only (n Omega-3 Group/Placebo Group) | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 4.0 (3.5–4.8) | 4.4 (3.6–5.1) | 0.175 | ||||||
| Gender | 20 M, 18 F | 20 M, 18 F | 1.00 | ||||||
| HTKS | n = 26 | n = 25 | |||||||
| HTKS | 15.23 (16.53) | 21.04 (18.99) | 16.29 (16.93) | 26.61 (20.83) | −4.58 (−11.34, 2.19) | 0.035 | 0.181 | 0.189 | 0.115 (n = 17/23) |
| CSBQ | n = 29 | n = 28 | |||||||
| CSBQ Behavioural SR | 3.76 (0.63) | 3.64 (0.81) | 3.99 (0.71) | 4.03 (0.91) | −0.347 (−0.60, −0.10) | 0.125 | 0.007 | 0.018 | 0.013 (n = 20/23) |
| CSBQ Cognitive SR | 3.59 (0.56) | 3.52 (0.62) | 3.59 (0.81) | 3.67 (0.78) | −0.15 (−0.43, 0.13) | 0.022 | 0.279 | 0.429 | 0.465 (n = 20/23) |
| CSBQ Emotional SR | 3.30 (0.78) | 3.57 (0.78) | 3.40 (0.71) | 3.49 (0.64) | 0.14 (−0.12, 0.40) | 0.022 | 0.285 | 0.278 | 0.099 (n = 20/23) |
| HS-Omega-3 Index® | n = 25 | n = 25 | |||||||
| HS-Omega-3 Index® | 3.45 (0.54) | 9.65 (3.12) | 3.31 (0.98) | 3.32 (1.12) | 6.16 (4.96, 7.38) | 0.689 | <0.001 | <0.001 | <0.001 (n = 17/20) |
| Baseline Omega-3 Group | Post Intervention Omega-3 Group | Baseline Placebo | Post Intervention Placebo | Mean Difference | Effect Size (ηp2) | p | p Adjusted for Age, Gender, EEG | Compliant Only | |
|---|---|---|---|---|---|---|---|---|---|
| iPad Tasks | n = 27 | n = 28 | |||||||
| Mr Ant | 1.63 (0.69) | 1.59 (0.72) | 1.67 (0.86) | 1.86 (0.83) | −0.242 (−0.621, 0.136) | 0.031 | 0.205 | 0.472 | 0.270 (n = 19/23) |
| Go No Go | 0.49 (0.24) | 0.56 (0.21) | 0.63 (0.24) | 0.65 (0.19) | 0.022 (−0.079, 0.122) | 0.004 | 0.664 | 0.635 | 0.990 |
| BRIEF * | n = 27 | n = 27 | |||||||
| BRIEF Inhibit | 26.63 (5.75) | 25.37 (7.47) | 25.00 (5.25) | 24.07 (6.25) | −0.38 (−2.56, 1.80) | 0.002 | 0.725 | 0.960 | 0.734 |
| BRIEF Shift | 14.96 (3.59) | 14.56 (3.33) | 14.52 (3.68) | 14.41 (3.85) | −0.158 (−1.64, 1.33) | 0.001 | 0.831 | 0.807 | 0.779 |
| BRIEF emotional control | 16.89 (3.42) | 16.22 (4.56) | 16.37 (3.64) | 16.63 (3.33) | 0.178 (−1.38, 1.74) | 0.001 | 0.820 | 0.588 | 0.573 |
| BRIEF working memory | 26.89 (6.67) | 25.70 (7.12) | 24.93 (5.55) | 23.22 (6.35) | 0.77 (−1.52, 3.05) | 0.009 | 0.504 | 0.617 | 0.665 |
| BRIEF Plan/organise | 16.44 (3.95) | 15.81 (4.14) | 15.96 (3.56) | 14.89 (3.70) | 0.526 (−0.84, 1.89) | 0.012 | 0.444 | 0.209 | 0.624 |
| BRIEF ISCI | 43.52 (8.24) | 41.59 (10.68) | 41.37 (7.44) | 39.70 (8.81) | −0.26 (−3.51, 2.98) | 0.001 | 0.870 | 0.803 | 0.637 |
| BRIEF FI | 31.85 (5.93) | 30.78 (6.64) | 30.89 (6.17) | 30.04 (6.29) | 0.01 (−2.50, 2.51) | <0.001 | 0.997 | 0.843 | 0.880 |
| BRIEF EMI | 43.33 (10.22) | 41.52 (10.93) | 40.89 (8.60) | 38.11 (9.61) | 1.21 (−2.02, 4.43) | 0.011 | 0.456 | 0.412 | 0.598 |
| BRIEF GEC | 101.81 (18.59) | 97.67 (21.63) | 96.78 (16.72) | 92.22 (19.35) | 0.76 (−6.08, 7.60) | 0.001 | 0.825 | 0.663 | 0.941 (n = 19/22) |
| EEG measures ¥ | n = 24 | n = 23 | (n = 18/19) | ||||||
| EEG EC theta/beta | 12.34 (7.22) | 10.64 (6.65) | 11.54 (4.78) | 10.19 (3.39) | 0.28 (−2.80, 3.35) | 0.001 | 0.857 | 0.768 | 0.825 |
| (EC-EO) Delta (24/22) | 51.61 (68.54) | 17.43 (69.87) | 61.69 (81.57) | 20.39 (34.62) | −1.39 (−33.71, 30.94) | <0.001 | 0.932 | 0.756 | 0.265 |
| (EC-EO) Theta | 38.99 (45.03) | 23.59 (39.98) | 36.76 (31.61) | 21.91 (27.01) | 2.38 (−18.11, 22.87) | 0.001 | 0.816 | 0.884 | 0.417 |
| (EC-EO) Alpha | −1.14 (8.19) | −0.59 (6.31) | 3.15 (7.87) | 1.17 (6.71) | −1.59 (−5.50, 2.32) | 0.014 | 0.428 | 0.515 | 0.672 |
| (EC-EO) Beta | −6.29 (13.14) | −2.74 (6.57) | −2.68 (5.70) | −3.82 (5.00) | 1.72 (−1.66, 5.10) | 0.023 | 0.310 | 0.371 | 0.525 |
| CTRS | n = 29 | n = 28 | n = 26/27 | n = 20/23 | |||||
| CTRS-15 Hyperactivity/Impulsivity | 4.79 (3.90) | 4.83 (4.38) | 4.00 (3.06) | 4.18 (3.28) | 0.002 (−1.41, 1.41) | <0.001 | 0.998 | 0.896 | 0.767 |
| CTRS-15 Inattention | 4.66 (3.73) | 4.55 (3.68) | 3.14 (3.14) | 3.29 (2.87) | 0.214 (−1.11, 1.54) | 0.002 | 0.748 | 0.766 | 0.537 |
| CTRS-15 Opposition | 4.66 (2.88) | 3.90 (2.47) | 3.61 (2.51) | 3.54 (2.80) | −0.40 (−1.36, 0.56) | 0.013 | 0.406 | 0.330 | 0.188 |
| CSBQ Behavioural SR | CSBQ Cognitive SR | CSBQ Emotional SR | Go No-Go iPad Task | Mr Ant iPad Task | |
|---|---|---|---|---|---|
| Quintile | Number (%) | Number (%) | Number (%) | Number (%) | Number (%) |
| 1 | 9 (11.8) | 10 (13.2) | 21 (28.0) | 13 (18.1) | 11 (14.9) |
| 2 | 10 (13.2) | 7 (9.2) | 16 (21.3) | 14 (19.4) | 12 (16.2) |
| 3 | 25 (32.9) | 28 (36.8) | 12 (16.0) | 16 (22.2) | 10 (13.5) |
| 4 | 17 (22.4) | 7 (9.2) | 6 (8.0) | 18 (25.0) | 30 (40.5) |
| 5 | 15 (19.7) | 24 (31.6) | 20 (26.7) | 11 (15.3) | 11 (14.9) |
| Total | 76 (100) | 76 (100) | 75 (100) | 72 (100) | 74 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roach, L.A.; Byrne, M.K.; Howard, S.J.; Johnstone, S.J.; Batterham, M.; Wright, I.M.R.; Okely, A.D.; de Groot, R.H.M.; van der Wurff, I.S.M.; Jones, A.L.; et al. Effect of Omega-3 Supplementation on Self-Regulation in Typically Developing Preschool-Aged Children: Results of the Omega Kid Pilot Study—A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 3561. https://doi.org/10.3390/nu13103561
Roach LA, Byrne MK, Howard SJ, Johnstone SJ, Batterham M, Wright IMR, Okely AD, de Groot RHM, van der Wurff ISM, Jones AL, et al. Effect of Omega-3 Supplementation on Self-Regulation in Typically Developing Preschool-Aged Children: Results of the Omega Kid Pilot Study—A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients. 2021; 13(10):3561. https://doi.org/10.3390/nu13103561
Chicago/Turabian StyleRoach, Lauren A., Mitchell K. Byrne, Steven J. Howard, Stuart J. Johnstone, Marijka Batterham, Ian M. R. Wright, Anthony D. Okely, Renate H. M. de Groot, Inge S. M. van der Wurff, Alison L. Jones, and et al. 2021. "Effect of Omega-3 Supplementation on Self-Regulation in Typically Developing Preschool-Aged Children: Results of the Omega Kid Pilot Study—A Randomised, Double-Blind, Placebo-Controlled Trial" Nutrients 13, no. 10: 3561. https://doi.org/10.3390/nu13103561
APA StyleRoach, L. A., Byrne, M. K., Howard, S. J., Johnstone, S. J., Batterham, M., Wright, I. M. R., Okely, A. D., de Groot, R. H. M., van der Wurff, I. S. M., Jones, A. L., & Meyer, B. J. (2021). Effect of Omega-3 Supplementation on Self-Regulation in Typically Developing Preschool-Aged Children: Results of the Omega Kid Pilot Study—A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients, 13(10), 3561. https://doi.org/10.3390/nu13103561





_Okely.png)



