Copper and Copper/Zn Ratio in a Series of Children with Chronic Diseases: A Cross-Sectional Study
Abstract
:1. Introduction
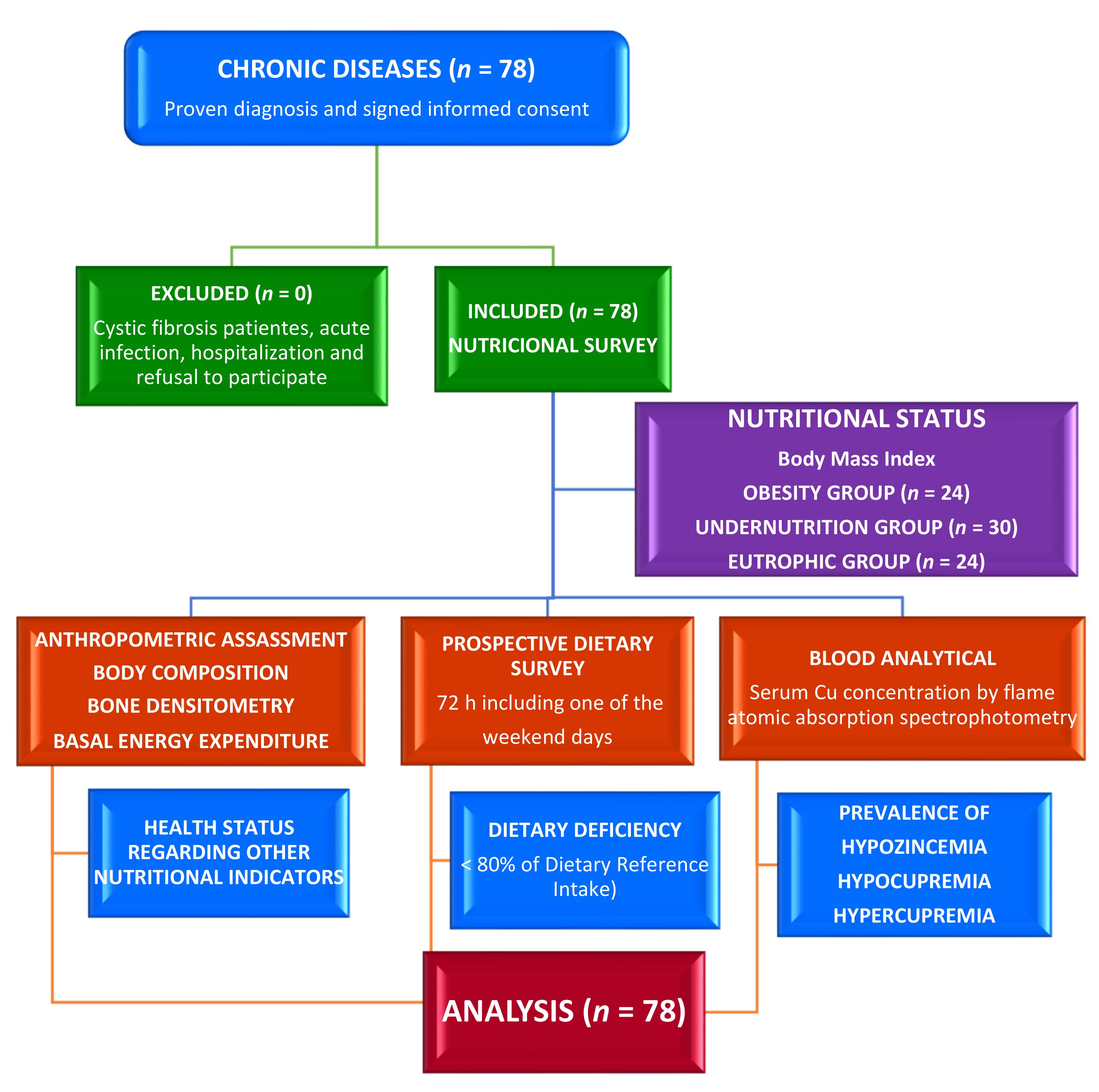
2. Materials and Methods
2.1. Study Site, Design, and Participants
2.2. Ethical Consideration
2.3. Assessment of Phenotypical Characteristics
2.4. Dietary Assessment
2.5. Clinical Evaluation
2.6. Laboratory Exploration
2.7. Statistical Analysis
3. Results
4. Discussion
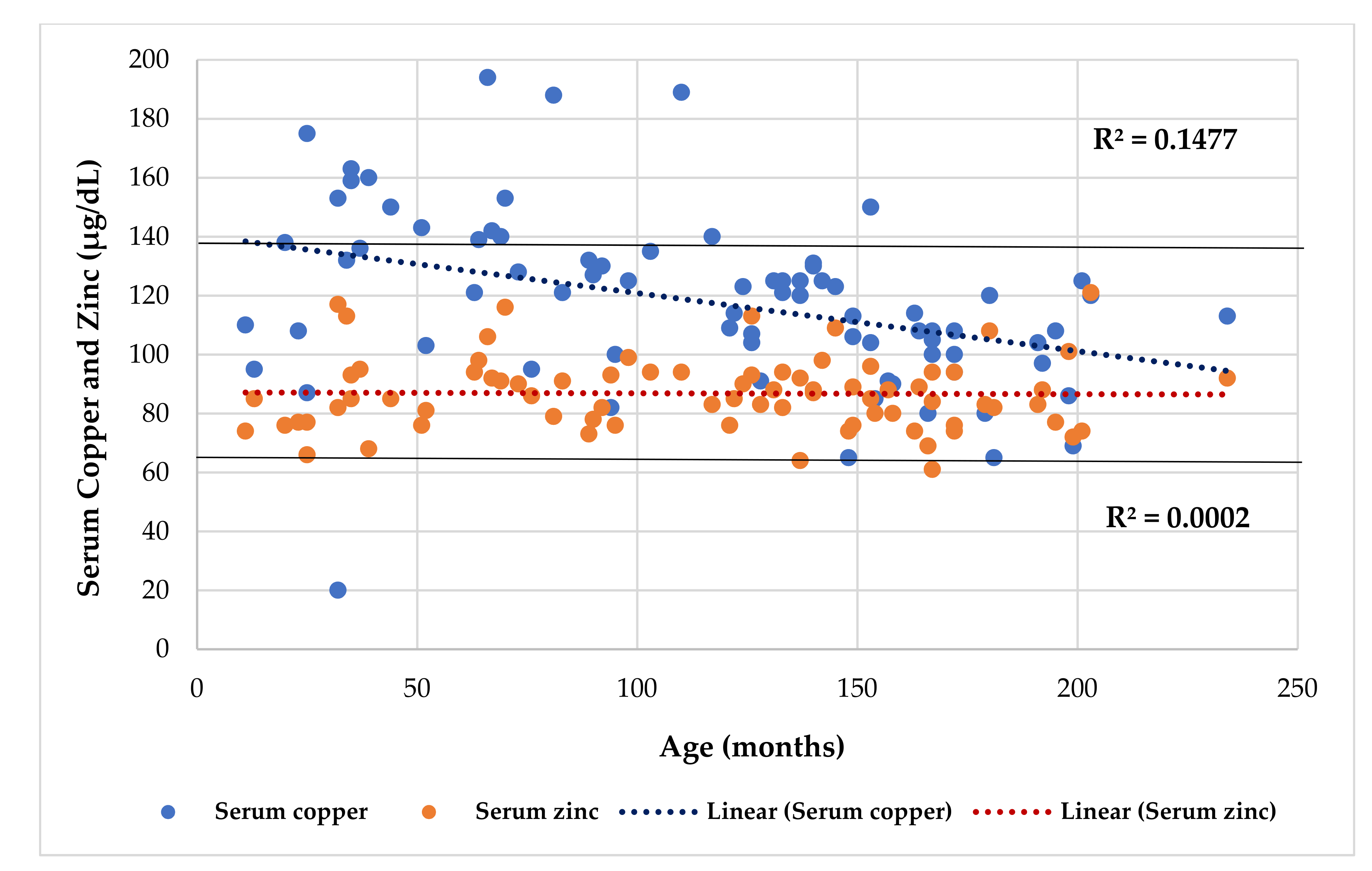
4.1. Serum Cu Levels
4.2. Phenotypical Characteristics
4.3. Bone Densitometry
4.4. Dietary Intake Survey
4.5. Biochemical Analysis
4.6. Blood Analysis and Inflammatory Response
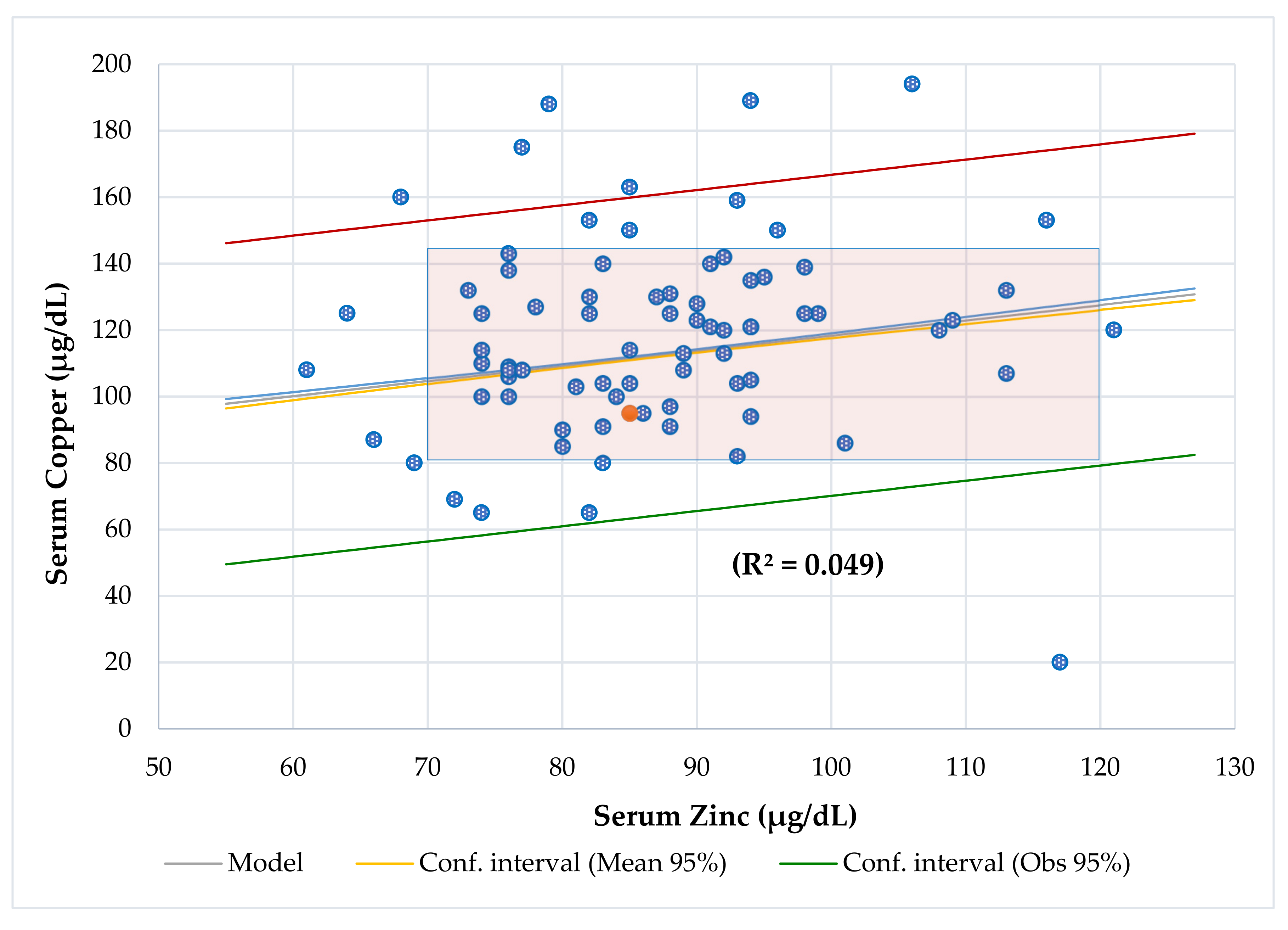
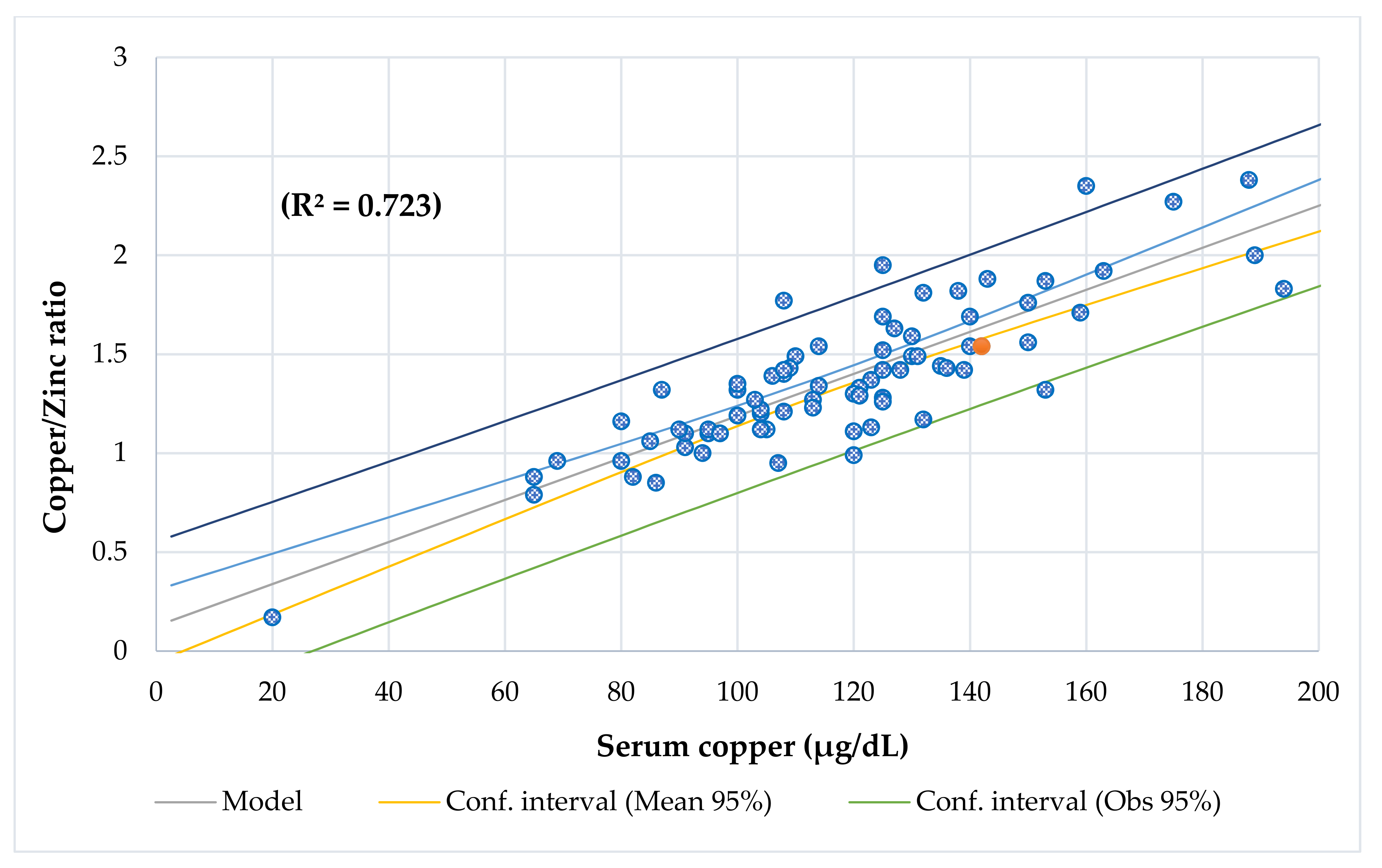
4.7. Copper-to-Zinc Ratio
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | Cystic Fibrosis |
| BIA | Bioelectrical impedance analysis |
| BCS | Bone conduction speed |
| %DRI | Dietary Reference Intake |
| Cu/Zn | Copper/Zinc |
| Zn/Cu | Zinc/Copper |
| CRP | C-reactive protein |
| ESR | Erythrocyte sedimentation rate |
| IGF-1 | Insulin-like growth factor-1 |
| IGFBP3 | Insulin-like growth factor-binding protein 3 |
| OR | Odds ratio |
| GGT | Gamma-glutamyl transferase |
| ALT | Alanine aminotransferase |
| IDA | Iron deficiency anemia |
| ADHD | Attention deficit/hyperactivity disorder |
| ZICD | Zinc-induced Cu deficiency |
| MT | Metallothioneins |
| CDM | Copper deficiency myelopathy |
| BUN | Blood urea nitrogen |
| ASD | Autism spectrum disorder |
References
- Johnson, R.C.; Schoeni, R.F. Early-life origins of adult disease: National longitudinal population-based study of the United States. Am. J. Public Health 2011, 101, 2317–2324. [Google Scholar] [CrossRef]
- Mokkink., L.; van der Lee, J.; Grootenhuis, M.; Offringa, M.; Heymans, H. Defining chronic diseases and health conditions in childhood (0–18 years of age): National consensus in the Netherlands, The Dutch National Consensus Comittee. Chronic Diseases and Health Conditions in Childhood. Eur. J. Pediatr. 2008, 167, 1441–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janse, A.J.; Uiterwaal, C.S.P.M.; Genke, R.J.B.J.; Kimpen, J.L.L.; Sinnema, G. A difference in perception of quality of life in chronically ill children was found between parents and pediatricians. J. Clin. Epidemiol. 2005, 58, 495–502. [Google Scholar] [CrossRef]
- Turkel, S.; Pao, M. Late Consequences of Pediatric Chronic Illness. Psychiatr. Clin. N. Am. 2007, 30, 819–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compas, B.; Jaser, S.; Dunn, M.; Rodriguez, E. Coping with Chronic Illness in Childhood and Adolescence. Annu. Rev. Clin. Psychol. 2012, 8, 455–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, I.; Thompson, M.; Gill, P.; Tamburlini, G.; Blair, M.; van den Bruel, A.; Ehrich, J.; Pettoello-Mantovani, M.; Janson, S.; Karanikolos, M.; et al. Health services for children in western Europe. Lancet 2013, 381, 1224–1234. [Google Scholar] [CrossRef]
- Gibson, R.S. Principles of Nutritional Assessment, 2nd ed.; Oxford University: New York, NY, USA, 2005; pp. 697–711. [Google Scholar]
- Emsley, J. Nature’s Building Blocks: An A-Z Guide to the Elements; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Domellöf, M.; Szitanyi, P.; Simchowitz, V.; Franz, A.; Mimouni, F. ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Iron and trace minerals. Clin. Nutr. 2018, 37, 2354–2359. [Google Scholar] [CrossRef] [Green Version]
- Rucker, R.B.; Kosonen, T.; Clegg, M.S.; Mitchell, A.E.; Rucker, B.R.; Uriu-Hare, J.Y.; Keen, C.L. Copper, lysyl oxidase, and extracellular matrix protein cross-linking. Am. J. Clin. Nutr. 1998, 67, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Collins, J.F. Copper: Basic Physiological and Nutritional Aspects. In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Academic Press: Cambridge, MA, USA, 2017; Chapter 7. [Google Scholar]
- Bogden, J.D.; Kemp, F.W.; Han, S.; Li, W.; Bruening, K.; Denny, T.; Oleske, J.M.; Lloyd, J.; Baker, H.; Perez, G.; et al. Status of selected nutrients and progression of human immunodeficiency virus type 1 infection. Am. J. Clin. Nutr. 2000, 72, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Laine, J.T.; Tuomainen, T.P.; Salonen, J.T.; Virtanen, J.K. Serum copper-to-zinc-ratio and risk of incident infection in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Eur. J. Epidemiol. 2020, 35, 1149–1156. [Google Scholar] [CrossRef]
- Luterotti, S.; Kordic, T.V.; Letoja, I.Z.; Dodig, S. Contribution to diagnostics/prognostics of tuberculosis in children. Indicative value of metal ions and biochemical parameters in serum. Acta Pharm. 2015, 65, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Koltas, I.S.; Ozcan, K.; Tamer, L.; Aksungru, P. Serum copper, zinc, and magnesium levels in children with enterobiosis. J. Trace Elem. Med. Biol. 1997, 11, 40–52. [Google Scholar] [CrossRef]
- Karakas, Z.; Demirel, N.; Tarakcioglu, M.; Mete, M. Serum Zn and Cu Levels in Southeastern Turkish Children with Giardiasis or Amebiasis. Biol. Trace Elem. Res. 2001, 84, 11–18. [Google Scholar] [CrossRef]
- Wisniewska, M.; Cremer, M.; Wiehe, L.; Becker, N.P.; Rijntjes, E.; Martitz, J.; Renko, K.; Bührer, C.; Schomburg, L. Cu to Zn ratio as disease biomarker in neonates with early-onset congenital infections. Nutrients 2017, 9, 343. [Google Scholar] [CrossRef] [Green Version]
- M’boh, G.M.; Boyvin, L.; Beourou, S.; Djama, A.J. Blood Cu/Zn Ratio in Children of School Age, Living in Malaria Endemic Area in Abidjan (Côte D’ivoire). Int. J. Child Health Nutr. 2013, 2, 29–33. [Google Scholar] [CrossRef]
- Boloursaz, M.R.; Khalilzadeh, S.; Milanifar, A.R.; Hakimi, S.S.; Khodayari, A.A.E.; Velayati, A.A. Evaluation of Copper, Zinc and Copper/Zinc Ratio in the Serum of Pulmonary Tuberculosis Children. Pediatric Oncall 2007, 4, 10–15. Available online: http://www.pediatriconcall.com (accessed on 18 December 2020).
- Malavolta, M.; Giacconi, R.; Piacenza, F.; Santarelli, L.; Cipriano, C.; Costarelli, L.; Tesei, S.; Pierpaoli, S.; Basso, A.; Galeazzi, R.; et al. Plasma copper/zinc ratio: An inflammatory/nutritional biomarker as predictor of all-cause mortality in elderly population. Biogerontology 2010, 11, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Leone, N.; Courbon, D.; Ducimetiere, P.; Zureik, M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology 2006, 17, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Emokpae, M.A.; Fatimehin, E.B. Cu/ZnRatio as an Inflammatory Marker in Patients with Sickle Cell Disease. Sci 2020, 2, 89. [Google Scholar] [CrossRef]
- Guo, C.H.; Chen, P.C.; Yeh, M.S.; Hsiung, D.Y.; Wang, C.L. Cu/Zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clin. Biochem. 2011, 44, 275–280. [Google Scholar] [CrossRef]
- Strain, J.J. Newer aspects of micronutrients in chronic disease: Copper. Proc. Nutr. Soc. 1994, 53, 583–598. [Google Scholar] [CrossRef] [Green Version]
- Escobedo-Monge, M.F.; Torres-Hinojal, M.C.; Barrado, E.; Escobedo-Monge, M.A.; Marugán-Miguelsanz, J.M. Zn Nutritional Status in a Series of Children with Chronic Diseases: A Cross-Sectional Study. Nutrients 2021, 13, 1121. [Google Scholar] [CrossRef] [PubMed]
- Monge, M.F.E.; Barrado, E.; Vicente, C.A.; del Río, M.P.R.; de Miguelsanz, J.M.M. Zn Nutritional Status in Patients with Cystic Fibrosis. Nutrients 2019, 11, 150. [Google Scholar] [CrossRef] [Green Version]
- Escobedo-Monge, M.F.; Barrado, E.; Alonso, C.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M.; Redondo del Río, M.P. Cu and Copper/Zn Ratio in a Series of Cystic Fibrosis Patients. Nutrients 2020, 12, 3344. [Google Scholar] [CrossRef]
- Frisancho, A.R. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am. J. Clin. Nutr. 1981, 34, 2540–2545. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Sobradillo, B.; Aguirre, A.; Aresti, U.; Bilbao, A.; Fernández-Ramos, C.; Lizárraga, A.; Lorenzo, H.; Madariaga, L.; Rica, I. Curvas y Tablas de Crecimiento (Estudios Longitudinal y Transversal); Fundación Faustino Orbegozo: Bilbao, Spain, 1985. [Google Scholar]
- Martínez, M.J.; Redondo, D.; Conde, F.; Redondo, P.; Franch, M.A. Gráficas Longitudinales de Velocidad de Conducción Media de Ultrasonidos en FALANGES. Estudio Nutricional de Castilla y León; de CyL, J., Ed.; Junta Castilla y León: Valladolid, Spain, 2009. [Google Scholar]
- Mataix Verdú, J.; García Diaz, J. Nutriber. V. 1.0; Fundación Universitaria Iberoamericana: Barcelona, Spain, 2005. [Google Scholar]
- Cuervo, M.; Corbalán, M.; Baladía, E.; Cabrerizo, L.; Formiguera, X.; Iglesias, C.; Lorenzo, H.; Polanco, I.; Quiles, J.; De Avila, M.D.R.; et al. Comparison of dietary reference intakes (DRI) between different countries of the European Union, The United States and the World Health Organization. Nutr. Hosp. 2009, 24, 384–414. [Google Scholar]
- Kaler, S.G. Inborn errors of copper metabolism. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 113, pp. 1745–1754. [Google Scholar] [CrossRef] [Green Version]
- Jaiser, S.R.; Winston, G.P. Cu deficiency myelopathy. J. Neurol. 2010, 257, 869–881. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.S. Lessons Learned from Experimental Human Model of Zinc Deficiency. J. Immunol. Res. 2020, 2020, 9207279. [Google Scholar] [CrossRef]
- Escobedo, M.F.; Barrado, E.; Alonso, C.; de Miguelsanz, J.M.M. Comparison study between colorimetric method and flame atomic absorption spectrophotometry in serum Zn status. Nutr. Clin. Dietética Hosp. 2018, 38, 128–133. [Google Scholar]
- Shenkin, A. Vitamins and trace elements. In Textbook of Clinical Chemistry and Molecular Diagnosis, 4th ed.; Bertis, S.A., Ashvud, E.R., Bruns, D., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2006. [Google Scholar]
- Walsh, W.J.; Isaacson, H.; Rehman, F.; Hall, A. Elevated blood copper/zinc ratios in assaultive young males. Physiol. Behav. 1997, 62, 327–329. [Google Scholar] [CrossRef]
- Dizdar, O.S.; Baspınar, O.; Kocer, D.; Dursun, Z.B.; Avcı, D.; Karakükcü, C.; Çelik, I.; Gundogan, K. Nutritional Risk, Micronutrient Status and Clinical Outcomes: A Prospective Observational Study in an Infectious Disease Clinic. Nutrients 2016, 8, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaslow, J.E. Copper/Zn Imbalance. Medical Board of California. Available online: http://www.mbc.ca.gov (accessed on 15 August 2020).
- Eck, P.; Wilson, L. Toxic Metals in Human Health and Disease; Eck Institute of Applied Nutrition and Bioenergetics, Ltd.: Phoenix, AZ, USA, 1989. [Google Scholar]
- Gibson, R.S.; Hess, S.Y.; Hotz, C.; Brown, K.H. Indicators of Zn status at the population level: A review of the evidence. Br. J. Nutr. 2008, 99, S14–S23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-N.; Wilson, A.; Church, B.B.; Ehman, S.; Roberts, W.L.; McMillin, G.A. Pediatric reference intervals for serum Cu and zinc. Clin. Chim. Acta 2012, 413, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Rükgauer, M.; Klein, J.; Kruse-Jarres, J.D. Reference values for the trace elements copper, manganese, selenium, and Zn in the serum/plasma of children, adolescents, and adults. J. Trace Elem. Med. Biol. 1997, 11, 92–98. [Google Scholar] [CrossRef]
- Milne, D.B.; Johnson, P.E. Assessment of Cu status: Effect of age and gender on reference ranges in healthy adults. Clin. Chem. 1993, 39, 883–887. [Google Scholar] [CrossRef]
- Skalny, A.V.; Mazaletskaya, A.L.; Ajsuvakova, O.P.; Bjørklund, G.; Skalnaya, M.G.; Chao, J.C.-J.; Chernova, L.; Shakieva, R.A.; Kopylov, P.Y.; Skalny, A.A.; et al. Serum zinc, copper, zinc-to-Cu ratio, and other essential elements and minerals in children with attention deficit/hyperactivity disorder (ADHD). J. Trace Elements Med. Biol. 2020, 58, 126445. [Google Scholar] [CrossRef]
- Acosta, E.; Galdona, E.; Barón, M.A.; Páez, M.C.; Velásquez, E.; Solano, L. Zn and Cu in serum and zinc/Cu ratio in a group of children from South Valencia, Venezuela. Acta Bioquímica Clín. Latinoam. 2010, 44, 25–31. [Google Scholar]
- Arvanitidou, V.; Voskaki, I.; Tripsianis, G.; Athanasopoulou, H.; Tsalkidis, A.; Filippidis, S.; Schulpis, K.; Androulakis, I. Serum Cu and Zn concentrations in healthy children aged 3–14 years in Greece. Biol. Trace Elem. Res. 2007, 115, 1–12. [Google Scholar] [CrossRef]
- Rakhra, G.; Masih, D.; Vats, A.; Verma, S.K.; Singh, V.K.; Rana, R.T.; Kirar, V.; Singh, S.N. Effect of physical activity and age on plasma copper, zinc, iron, and magnesium concentration in physically active healthy males. Nutrition 2017, 43, 75–82. [Google Scholar] [CrossRef]
- Brunetto, M.D.R.; Alarcón, O.; Davila, E.; Contreras, Y.; Gallignani, M.; Rondón, C.; Burguera, J.; Burguera, M.; Angarita, C. Serum trace elements and fat-soluble vitamins A and E in healthy pre-school children from a Venezuelan rural community. J. Trace Elem. Med. Biol. 1999, 13, 40–50. [Google Scholar] [CrossRef]
- Romero, C.D.; Henríquez, P.; López, F.; Rodríguez, E.; Serra, L. Serum Cu and Zn concentrations in a representative sample of the Canarian population. J. Trace Elem. Med. Biol. 2002, 16, 75–81. [Google Scholar] [CrossRef]
- Cao, J.; Gao, Z.; Yan, J.; Li, M.; Su, J.; Xu, J.; Yan, C.-H. Evaluation of Trace Elements and Their Relationship with Growth and Development of Young Children. Biol. Trace Elem. Res. 2016, 171, 270–274. [Google Scholar] [CrossRef]
- Abiaka, C.; Olusi, S.; Al-Awadhi, A. Reference Ranges of Cu and Zn and the Prevalence of Their Deficiencies in an Arab Population Aged 15–80 Years. Biol. Trace Elem. Res. 2003, 91, 33–43. [Google Scholar] [CrossRef]
- Bui, V.Q.; Stein, A.; DiGirolamo, A.M.; Ramakrishnan, U.; Flores-Ayala, R.C.; Ramirez-Zea, M.; Grant, F.K.; Villalpando, S.; Martorell, R. Associations between serum C-reactive protein and serum zinc, ferritin, and Cu in Guatemalan school children. Biol. Trace Elem. Res. 2012, 148, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Duncan, A.; Talwar, D.; Morrison, I. The predictive value of low plasma Cu and high plasma Zn in detecting zinc-induced Cu deficiency. Ann. Clin. Biochem. 2015, 53, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Cordano, A. Clinical manifestations of nutritional Cu deficiency in infants and children. Am. J. Clin. Nutr. 1998, 67, 1012S–1016S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of Cu in humans. Am. J. Clin. Nutr. 1998, 67, 952S–959S. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, X.; Li, X.; Wang, W.; Wang, X.; Liu, L.; Deng, Q.; Bai, G.; Wang, J.; Feng, H.; et al. Effects of copper on proliferation and autocrine secretion of insulin-like growth factor-1 (IGF-1) and IGF-binding protein-3 (IGFBP-3) in chondrocytes from newborn pigs in vitro. Biol. Trace Elem. Res. 2011, 144, 588–596. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Guo, Y.; Wang, Z.; Zhao, B.; Yin, Y.; Liu, G. Influence of Dietary Cu on Serum Growth-Related Hormone Levels and Growth Performance of Weanling Pigs. Biol. Trace Elem. Res. 2016, 172, 134–139. [Google Scholar] [CrossRef]
- Giv, Z.J.; Avan, A.; Hamidi, F.; Tayefi, M.; Khayyatzadeh, S.S.; Javandoost, A.; Nematy, M.; Ferns, G.A.; Mobarhan, M.G. Nutrients intake, and serum calcium and phosphorus levels: An evidence-based study. J. Clin. Lab. Anal. 2018, 32, e22235. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Yan, W.; Zhao, Q.; Ji, B.; Ban, B.; Zhang, M. Association Between Serum Calcium and Phosphorus Levels and Insulin-Like Growth Factor-1 in Chinese Children and Adolescents with Short Stature. Int. J. Gen. Med. 2020, 13, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, G.; Alves, J.; Toro, V.; Robles, M.C.; Muñoz, D.; Maynar, M. Association between Trace Elements and Body Composition Parameters in Endurance Runners. Int. J. Environ. Res. Public Health 2020, 17, 6563. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.C.V.; Costa, N.M.B.; Sant’Anna, H.M.P.; de Luces Fortes Ferreira, C.L.; de Castro Franceschini, S.d.C. Improvement the nutritional status of pre-school children following intervention with a supplement containing iron, zinc, copper, vitamin a, vitamin c and prebiotic. Ciência Saúde Coletiva 2017, 22, 359–368. [Google Scholar] [CrossRef]
- Laitinen, R.; Vuori, E.; Dahlström, S.; Åkerblom, H.K. Zinc, copper, and growth status in children and adolescents. Pediatr. Res. 1989, 25, 323–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amare, B.; Moges, B.; Fantahun, B.; Tafess, K.; Woldeyohannes, D.; Yismaw, G.; Ayane, T.; Yabutani, T.; Mulu, A.; Ota, F.; et al. Micronutrient level and nutritional status of school children living in Northwest Ehiopia. Nutr. J. 2012, 11, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonoodi, K.; Moslem, A.; Darroudi, S.; Ahmadnezhad, M.; Mazloum, Z.; Tayefi, M.; Zadeh, S.A.T.; Eslami, S.; Shafiee, M.; Khashayarmanesh, Z.; et al. Serum and dietary zinc and copper in Iranian girls. Clin. Biochem. 2018, 54, 25–31. [Google Scholar] [CrossRef]
- Sorokman, T.V.; Sokolnyk, S.V.; Popelyuk, O.V.; Bezruk, T.O.; Makarova, O.V. The copper content in the blood plasma of children with different types of short stature. Wiad Lek 2020, 73, 266–271. [Google Scholar] [CrossRef]
- Lima, S.C.V.C.; Arrais, R.F.; Sales, C.H.; Almeida, M.G.; De Sena, K.C.M.; Oliveira, V.T.L.; De Andrade, A.S.; Pedrosa, L.F.C. Assessment of Cu and Lipid Profile in Obese Children and Adolescents. Biol. Trace Elem. Res. 2006, 114, 19–29. [Google Scholar] [CrossRef]
- Osredkar, J.; Susta, N. Cu and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, 2161, 0495. [Google Scholar] [CrossRef] [Green Version]
- Gu., K.; Li, X.; Xiang, W.; Jiang, X. The Relationship between Serum Cu and Overweight/Obesity: A Meta-analysis. Biol. Trace Elem. Res. 2020, 194, 336–347. [Google Scholar] [CrossRef]
- Golden, N.H.; Abrams, S.A. Committee on Nutrition. Optimizing bone health in children and adolescents. Pediatrics 2014, 134, e1229–e1243. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Saini, N.; Mathias, A.; Arya, A.; Jain, S.; Yachha, S.K. Prevalence and predictive factors of undernutrition and low bone mineral density in children with chronic pancreatitis. Pancreatology 2021, 21, 74–80. [Google Scholar] [CrossRef]
- Li., S.; Wang, M.; Chen, X.; Li, S.F.; Li-Ling, J.; Xie, H.Q. Inhibition of osteogenic differentiation of mesenchymal stem cells by copper supplementation. Cell Prolif. 2014, 47, 81–90. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Yao, Q. Copper-based biomaterials for bone and cartilage tissue engineering. J. Orthop. Transl. 2021, 29, 60–71. [Google Scholar] [CrossRef]
- Opsahl, W.; Zeronian, H.; Ellison, M.; Lewis, D.; Rucker, R.B.; Riggins, R.S. Role of Cu in collagen cross-linking and its influence on selected mechanical properties of chick bone and tendon. J. Nutr. 1982, 112, 708–716. [Google Scholar] [CrossRef]
- Danks, D.M. Cu deficiency in humans. Annu. Rev. Nutr. 1988, 8, 235–257. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.; Infantino, V.; Gasparri, C.; Iannello, G.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A.; Peroni, G. Copper as Dietary Supplement for Bone Metabolism: A Review. Nutrients 2021, 13, 2246. [Google Scholar] [CrossRef]
- Fan, Y.; Ni, S.; Zhang, H. Associations of Cu Intake with Bone Mineral Density and Osteoporosis in Adults: Data from the National Health and Nutrition Examination Survey. Biol. Trace Elem. Res. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow, C.K. Cu toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Lonnerdal, B. Cu nutrition during infancy and childhood. Am. J. Clin. Nutr. 1998, 67, 1046S–1053S. [Google Scholar] [CrossRef] [PubMed]
- Mertz, W. Trace elements and the needs of the elderly. In Nutrition and Aging; Hutchinson, M., Munro, H.N., Eds.; Academic Press: New York, NY, USA, 1986; pp. 71–82. [Google Scholar]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zn External Link Disclaimer; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Turnlund, J.R.; Keyes, W.R.; Kim, S.K.; Domek, J.M. Long-term high Cu intake: Effects on Cu absorption, retention, and homeostasis in men. Am. J. Clin. Nutr. 2005, 81, 822–828. [Google Scholar] [CrossRef]
- Munie, S.; Pintavorn, P. Erythropoietin-Resistant Anemia Secondary to Zinc-Induced Hypocupremia in a Hemodialysis Patient. Case Rep. Nephrol. Dial. 2021, 11, 167–175. [Google Scholar] [CrossRef]
- Angelova, M.; Asenova, S.; Nedkova, V.; Koleva-Kolarova, R. Cu in the Human organism. Trakia J. Sci. 2011, 9, 88–98. [Google Scholar]
- Anuk, A.T.; Polat, N.; Akdas, S.; Erol, S.A.; Tanacan, A.; Biriken, D.; Keskin, H.L.; Tekin, O.M.; Yazihan, N.; Sahin, D. The Relation Between Trace Element Status (Zinc, Copper, Magnesium) and Clinical Outcomes in COVID-19 Infection During Pregnancy. Biol. Trace Elem. Res. 2021, 199, 3608–3617. [Google Scholar] [CrossRef]
- Peng, Y.F.; Wang, C.F.; Pan, G.G. Relation of serum γ-glutamyl transferase activity with copper in an adult population. Clin. Chem. Lab. Med. 2017, 55, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, A.; Terazawa-Watanabe, M.; Kazumi, T.; Fukuo, K. Associations of decreased serum transthyretin with elevated high-sensitivity CRP, serum copper and decreased hemoglobin in ambulatory elderly women. Asia Pac. J. Clin. Nutr. 2015, 24, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Huang, H.; Zhuang, Z.; Chen, R.; Xie, Z.; Xu, C.; Mo, X. The association between serum copper concentrations and cardiovascular disease risk factors in children and adolescents in NHANES. Environ. Sci. Pollut. Res. 2018, 25, 16951–16958. [Google Scholar] [CrossRef]
- Tamai, Y.; Iwasa, M.; Eguchi, A.; Shigefuku, R.; Sugimoto, K.; Hasegawa, H.; Takei, Y. Serum copper, zinc and metallothionein serve as potential biomarkers for hepatocellular carcinoma. PLoS ONE 2020, 15, e0237370. [Google Scholar] [CrossRef]
- Johnson, M.A.; Fischer, J.G.; Kays, S.E. Is Cu an antioxidant nutrient? Crit. Rev. Food Sci. Nutr. 1992, 32, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Ece, A.; Uyanik, B.S.; İşcan, A.; Ertan, P.; Yiğitoğlu, R. Increased Serum Cu and Decreased Serum Zn Levels in Children with Iron Deficiency Anemia. Biol. Trace Elem. Res. 1997, 59, 31–39. [Google Scholar] [CrossRef]
- Collins, J.F.; Prohaska, J.R.; Knutson, M.D. Metabolic crossroads of iron and copper. Nutr. Rev. 2010, 68, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Dharmalingam, K.; Birdi, A.; Tomo, S.; Sreenivasulu, K.; Charan, J.; Yadav, D.; Purohit, P.; Sharma, P. Trace Elements as Immunoregulators in SARS-CoV-2 and Other Viral Infections. Indian J. Clin. Biochem. 2021, 36, 416–426. [Google Scholar] [CrossRef]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Maggini, S.; Wintergerst, E.S.; Beveridge, S.; Hornig, D.H. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br. J. Nutr. 2007, 98, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metab. 2007, 51, 301–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, T.; Caviezel, D.; Korcan Ayata, C.; Kiss, C.; Niess, J.H.; Hruz, P. The Copper/Zinc Ratio Correlates with Markers of Disease Activity in Patients with Inflammatory Bowel Disease. Crohn’s Colitis 2020, 360, otaa001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percival, S.S.; Bowser, E.; Wagner, M. Reduced copper enzyme activities in blood cells of children with cystic fibrosis. Am. J. Clin. Nutr. 1995, 62, 633–638. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Kawashima, T.; Miyazawa, M.; Ohshiro, T. Validity of the copper/Zn ratio as a diagnostic marker for taste disorders associated with Zn deficiency. J. Trace Elem. Med. Biol. 2016, 36, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Bahi, G.A.; Boyvin, L.; Méité, S.; M’Boh, G.M.; Yeo, K.; N’Guessan, K.R.; Bidié, A.D.P.; Djaman, A.J. Assessments of serum Cu and Zn concentration, and the Cu/Zn ratio determination in patients with multidrug resistant pulmonary tuberculosis (MDR-TB) in Côte d’Ivoire. BMC Infect. Dis. 2017, 17, 257. [Google Scholar] [CrossRef] [Green Version]
- Bogden, J.D. Blood Zn in health and disease. In Zn in Environment, Part II—Health Effects; Jerome, O.N., Ed.; Wiley: New York, NY, USA, 1980; pp. 137–169. [Google Scholar]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zn and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef]
- Shenkin, A. Trace elements and inflammatory response: Implications for nutritional support. Nutrition 1995, 11 (Suppl. 1), 100–105. [Google Scholar]
- Mariani, E.; Cattini, L.; Neri, S.; Malavolta, M.; Mocchegiani, E.; Ravaglia, G.; Facchini, A. Simultaneous evaluation of circulating chemokine and cytokine profiles in elderly subjects by multiplex technology: Relationship with Zn status. Biogerontology 2006, 7, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, M.; Piacenza, F.; Basso, A.; Giacconi, R.; Costarelli, L.; Mocchegiani, E. Serum Cu to Zn ratio: Relationship with aging and health status. Mech. Ageing Dev. 2015, 151, 93–100. [Google Scholar] [CrossRef] [PubMed]
- de Romaña, D.L.; Olivares, M.; Uauy, R.; Araya, M. Risks and benefits of Cu in light of new insights of Cu homeostasis. J. Trace Elem. Med. Biol. 2011, 25, 3–13. [Google Scholar] [CrossRef]
- Lee, M.J.; Alvarez, J.A.; Smith, E.M.; Killilea, D.W.; Chmiel, J.F.; Joseph, P.M.; Grossmann, R.E.; Gaggar, A.; Ziegler, T.R.; Tangpricha, V.; et al. Vitamin D for Enhancing the Immune System in Cystic Fibrosis Investigators. Changes in Mineral Micronutrient Status during and after Pulmonary Exacerbation in Adults with Cystic Fibrosis. Nutr. Clin. Pract. 2015, 30, 838–843. [Google Scholar] [CrossRef] [Green Version]
- Mwangala, L.M.J.; Waihenya, R.; Mwatha, J.; Mwamburi, D.M. Association of Body Mass Index, Waist-Hip Ratio, Zinc, Copper, CD4+ T Cells Count and Viral Load in Early HIV Infection in ART Naïve HIV Infected Adults in Taita Taveta County, Kenya. ASRJETS 2017, 38, 126–137. [Google Scholar]
- Emokpae, M.A.; Aruomaren, A.; Osime, E. Relationship between neutrophil-to-lymphocyte ratio and inflammatory markers in Sickle cell anaemia patients with proteinuria. Med. Sci. 2016, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Poo, J.L.; Romero, R.R.; Robles, J.A.; Montemayor, A.C.; Isoard, F.; Estanes, A.; Uribe, M. Diagnostic value of the copper/zinc ratio in digestive cancer: A case control study. Arch. Med. Res. 1997, 28, 259–263. [Google Scholar] [PubMed]
- Gupta, S.K.; Singh, S.P.; Shukla, V.K. Copper, zinc, and Cu/Zn ratio in carcinoma of the gallbladder. J. Surg. Oncol. 2005, 91, 204–208. [Google Scholar] [CrossRef]
- Stepien, M.; Hughes, D.J.; Hybsier, S.; Bamia, C.; Tjønneland, A.; Overvad, K.; Affret, A.; His, M.; Boutron-Ruault, M.C.; Katzke, V.; et al. Circulating Cu and Zn levels and risk of hepatobiliary cancers in Europeans. Br. J. Cancer 2017, 116, 688–696. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shukla, V.K.; Gupta, V.; Gupta, S. Serum trace elements and Cu/Zn ratio in malignant lymphomas in children. J. Trop. Pediatrics 1994, 40, 185–187. [Google Scholar] [CrossRef]
- Feng, Y.; Zeng, J.W.; Ma, Q.; Zhang, S.; Tang, J.; Feng, J.F. Serum Cu and Zn levels in breast cancer: A meta-analysis. J. Trace Elem. Med. Biol. 2020, 62, 126629. [Google Scholar] [CrossRef]
- Zabłocka-Słowińska, K.; Prescha, A.; Płaczkowska, S.; Porębska, I.; Kosacka, M.; Pawełczyk, K. Serum and Whole Blood Cu and Zn Status in Predicting Mortality in Lung Cancer Patients. Nutrients 2020, 13, 60. [Google Scholar] [CrossRef]
- Hamasaki, H.; Kawashima, Y.; Yanai, H. Serum Zn/Cu Ratio Is Associated with Renal Function, Glycemic Control, and Metabolic Parameters in Japanese Patients with and without Type 2 Diabetes: A Cross-sectional Study. Front. Endocrinol. 2016, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Ashwood, E.R. (Ed.) ARUP’s Guide to Pediatric Clinical Laboratory Testing: Test Interpretation, Utilization, and Age-Specific Reference Intervals, 3rd ed.; ARUP Laboratories: Salt Lake City, UT, USA, 2004. [Google Scholar]
- Faber, S.; Zinn, G.M.; Kern, J.C., 2nd; Kingston, H.M. The plasma zinc/serum Cu ratio as a biomarker in children with autism spectrum disorders. Biomarkers 2009, 14, 171–180. [Google Scholar] [CrossRef]
- Barceloux, D.G. Copper. J. Toxicol. Clin. Toxicol. 1999, 37, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, Y.; Man, Q.; Li, Y.; Lu, J.; Yang, L. Study on Reference Range of Zinc, Copper and Copper/Zinc Ratio in Childbearing Women of China. Nutrients 2021, 13, 946. [Google Scholar] [CrossRef] [PubMed]
- Klevay, L. Hypercholesterolemia in rats produced by an increase in the ratio of Zn to Cu ingested. Am. J. Clin. Nutr. 1973, 26, 1060–1068. [Google Scholar] [CrossRef]
- Belbraouet, S.; Biaudet, H.; Tébi, A.; Chau, N.; Gray-Donald, K.; Debry, G. Serum zinc and copper status in hospitalized vs. healthy elderly subjects. J. Am. Coll. Nutr. 2007, 26, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis; Cengage Learning: Boston, MA, USA, 2018. [Google Scholar]
- Laur, N.; Kinscherf, R.; Pomytkin, K.; Kaiser, L.; Knes, O.; Deigner, H.P. ICP-MS trace element analysis in serum and whole blood. PLoS ONE 2020, 15, e0233357. [Google Scholar] [CrossRef] [PubMed]
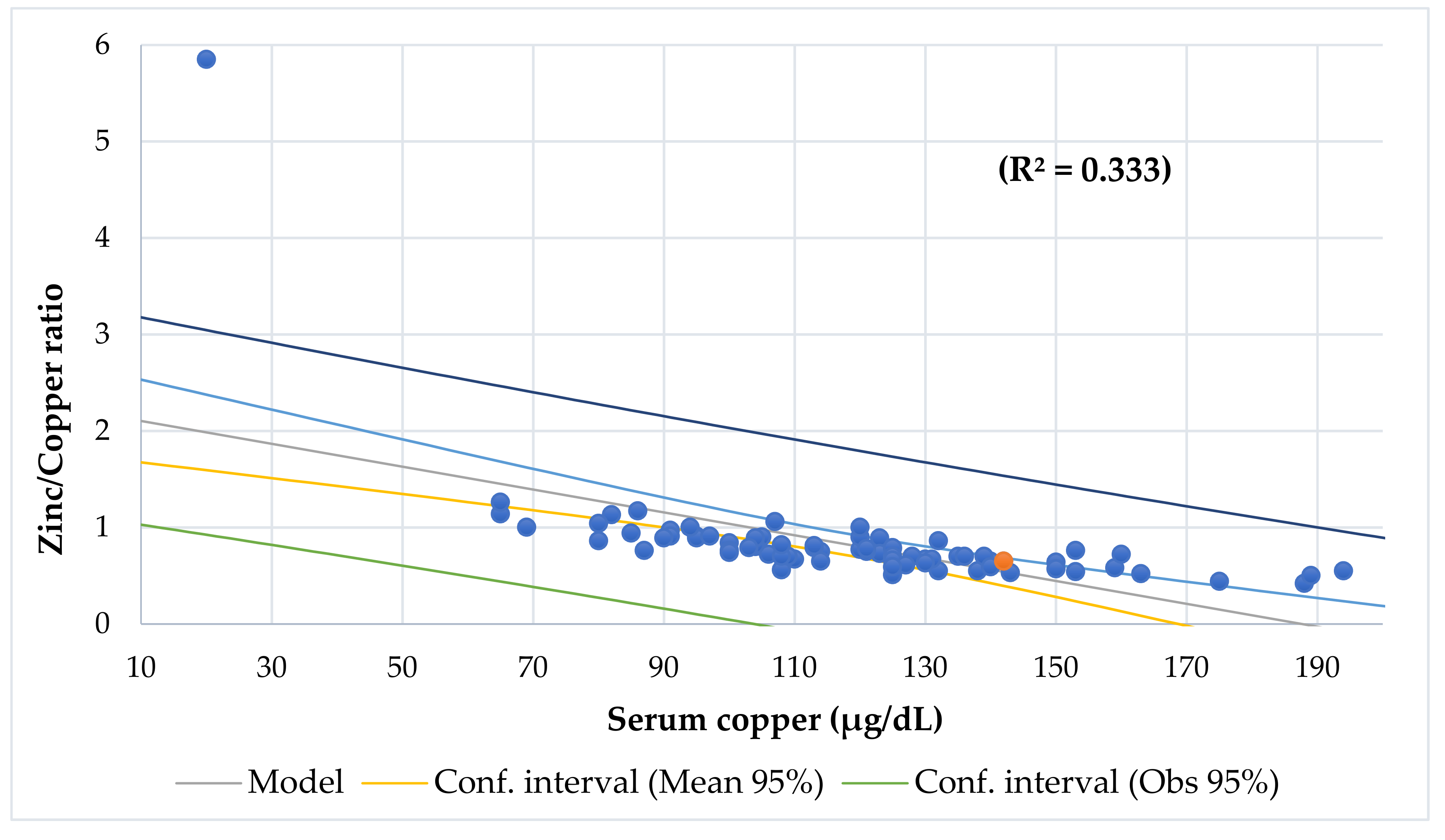
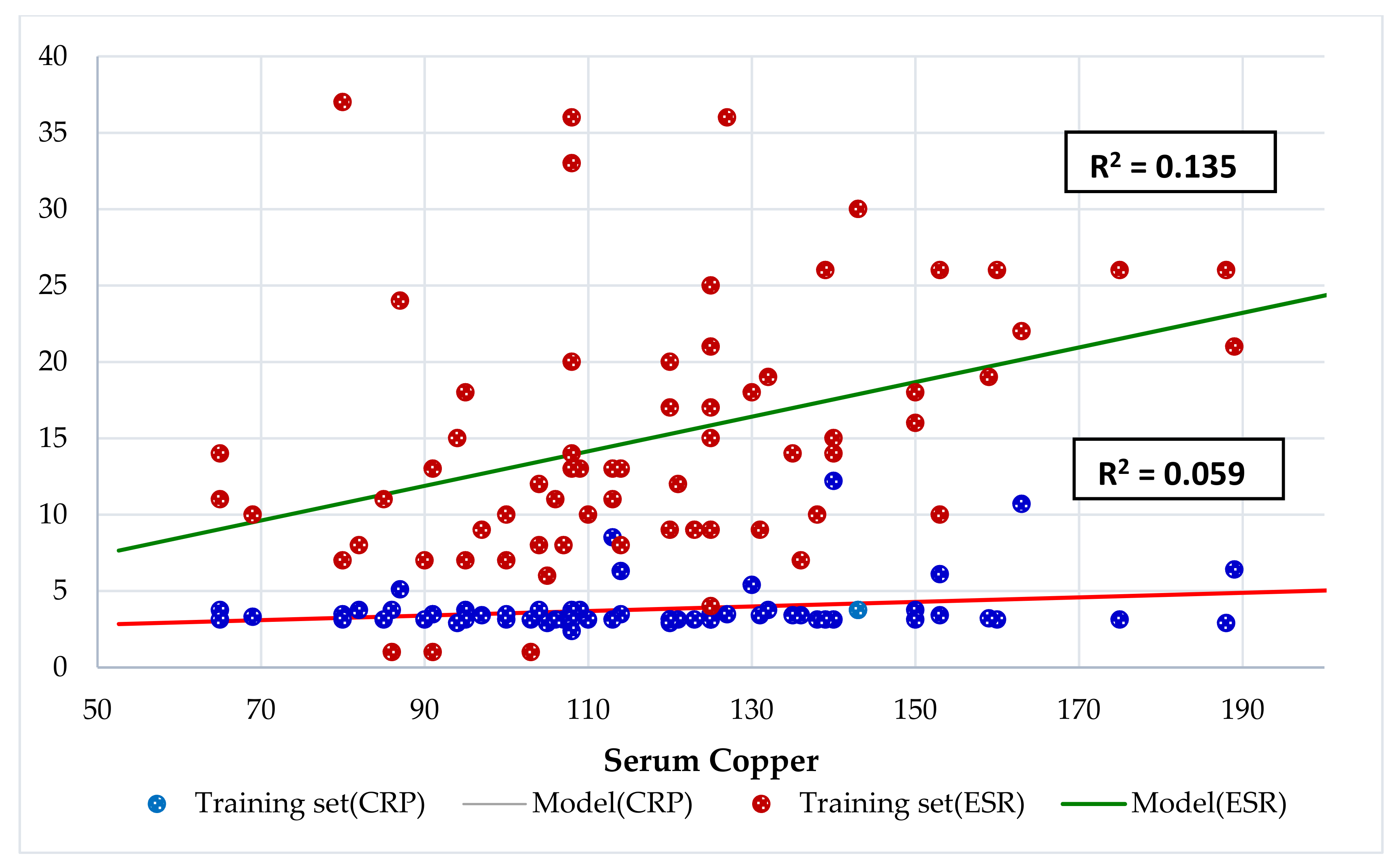
| Characteristics | Total | Obesity | Undernutrition | Eutrophic | p-Value |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| n | 78 | 24 | 30 | 24 | |
| Female (%) | 43 (55) | 15 (62.5) | 17 (56.7) | 11 (45.8) | 0.472 |
| Age (years) | 9.6 ± 4.8 | 11 ± 4 | 7 ± 5 | 10 ± 5 | 0.003 * |
| Age-for-50° Height (months) | 115 ± 93 | 142 ± 62 | 72 ± 70 | 142 ± 122 | 0.005 * |
| Children (age in years) | 6 ± 3 | 7 ± 3 | 4 ± 3 | 7 ± 3 | 0.026 * |
| Adolescent age in years) | 13 ± 2 | 13 ± 2 | 13 ± 2 | 14 ± 2 | 0.363 |
| Time of chronic disease (months) | 66 ± 47 | 55 ± 38 | 65 ± 53 | 77 ± 47 | 0.282 |
| Weight-for-age (kg) | 38 ± 26 | 63 ± 24 | 18 ± 12 | 38 ± 18 | 0.000 * |
| Height-for-age (cm) | 131 ± 31 | 147 ± 21 | 112 ± 30 | 139 ± 28 | 0.000 * |
| Height-for-age Z-score | −0.76 ± 1.5 | −0.7 ± 1.3 | −1.6 ± 1.6 | −0.4 ± 1.2 | 0.000 * |
| Weight-for-Height Z-score | 0.2 ± 2.1 | 2.5 ± 1.5 | −1.7 ± 1.1 | 0.3 ± 1.2 | 0.000 * |
| Body mass index(kg/cm2) | 19 ± 7.2 | 28 ± 5 | 13 ± 1.4 | 18 ± 2.8 | 0.000 * |
| Triceps skinfold (mm) | 13 ± 9 | 24 ± 7 | 6 ± 2 | 11 ± 1 | 0.000 * |
| Muscle mass by anthropometry (Kg) | 28 ± 15 | 40 ± 15 | 16 ± 9 | 29 ± 12 | 0.000 * |
| Fat mass by anthropometry (Kg) | 11 ±11 | 23 ± 11 | 2 ± 2 | 9 ± 7 | 0.000 * |
| Muscle mass by BIA | 30 ± 16 | 41 ± 15 | 19 ± 18 | 29 ± 13 | 0.000 * |
| Fat mass by BIA | 12 ± 11 | 22 ± 12 | 4 ± 4 | 9 ± 6 | 0.000 * |
| Bone conduction speed | 1923 ± 88 | 1931 ± 71 | 1909 ± 99 | 1933 ± 90 | 0.561 |
| Blood Analytic | |||||
| Prealbumin (mg/dL) | 22 ± 0.6 | 23 ± 6 | 21 ± 6 | 21 ± 6 | 0.185 |
| Albumin (g/dL) | 4.3 ± 0.3 | 4.2 ± 0.3 | 4.3 ± 0.3 | 4.3 ± 0.3 | 0.533 |
| Transferrin (mg/dL) | 254 ± 34 | 258 ± 27 | 257 ± 40 | 248 ± 33 | 0.538 |
| Leucocytes (cell/mm3) | 7465 ± 2239 | 7025 ± 2373 | 8344 ± 2349 | 6806 ± 1593 | 0.020 * |
| Lymphocytes (cell/mm3) | 3080 ± 1432 | 2887 ± 312 | 3606 ± 1641 | 2615 ± 719 | 0.028 * |
| Total cholesterol (mg/dL) | 174 ± 38 | 161 ± 29 | 174 ± 40 | 187 ± 40 | 0.072 |
| LDL-cholesterol (mg/dL) | 104 ± 30 | 94 ± 31 | 104 ± 28 | 111 ± 32 | 0.187 |
| Blood urea nitrogen | 30 ± 9 | 30 ± 6 | 28 ± 8 | 33 ± 11 | 0.163 |
| Calcium (mg/dL) | 10 ± 0.5 | 9.8 ± 0.5 | 10 ± 0.5 | 10 ± 0.4 | 0.129 |
| Phosphorus (mg/dL) | 4.8 ± 0.6 | 4.7 ± 0.6 | 4.9 ± 0.6 | 4.6 ± 0.5 | 0.233 |
| IGF-1 (ng/mL) | 212 ± 136 | 264 ± 119 | 149 ± 115 | 241 ± 152 | 0.004 * |
| IGFBP3 (μg/mL) | 2.8 ± 0.8 | 3.2 ± 0.8 | 2.4 ± 0.8 | 2.9 ± 0.8 | 0.004 * |
| Iron (µg/dL) | 78.6 ± 31 | 80.8 ± 20 | 75.7 ± 40 | 80.4 ± 28 | 0.806 |
| C-reactive protein (U/L) | 3.8 ± 1.6 | 3.8 ± 1.3 | 3.6 ± 0.9 | 3.9 ± 2.4 | 0.795 |
| Erythrocyte sedimentation rate (mm/h) | 15 ± 8 | 15 ± 9 | 15 ± 8 | 14 ± 8 | 0.943 |
| Gamma-glutamyl transferase | 16 ± 24 | 15 ± 6 | 18 ± 38 | 13 ± 4.6 | 0.000 * |
| Alanine aminotransferase | 28 ± 10 | 25 ± 8 | 34 ± 11 | 24 ± 7 | 0.725 |
| Zinc (µg/dL) | 87 ± 12 | 87 ± 12 | 85 ± 13 | 88 ± 13 | 0.761 |
| Copper (µg/dL) | 118 ± 29 | 119 ± 23 | 114 ± 35 | 122 ± 3 | 0.622 |
| Copper/Zinc ratio | 1.4 ± 0.4 | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.4 | 0.845 |
| Zinc/Copper ratio | 0.8 ± 0.6 | 0.7 ± 1.4 | 0.9 ± 0.9 | 0.8 ± 0.2 | 0.480 |
| Hypocupremia cases (%) | 4 (5) | 1 (4) | 3 (10) | 0 | 0.762 |
| Hypercupremia cases (%) | 13 (17) | 4 (17) | 5 (17) | 4 (17) | 0.762 |
| Hypozincemia cases (%) | 5 (6) | 2 (8) | 2 (6) | 1 (4) | 0.840 |
| Copper/Zinc ratio > 1 (%) | 68 (87) | 23 (99) | 25 (83) | 20 (83) | 0.318 |
| Copper/Zinc ratio > 2 (%) | 4 (5) | 0 | 2 (7) | 2 (8) | 0.382 |
| Zinc/Copper ratio > 4 (%) | 1 (1) | 0 | 1 (3) | 0 | 0.449 |
| Prospective Dietary Survey | |||||
| Dietary Zn intake (%DRI) | 69 ± 35 | 81 ± 40 | 60 ± 30 | 67 ± 35 | 0.110 |
| Dietary Zn intake (mg/day) | 10 ± 5 | 12 ± 6 | 9 ± 4 | 10 ± 5 | 0.109 |
| Protein (%DRI) | 276 ± 176 | 248 ± 140 | 307 ± 222 | 266 ± 140 | 0.453 |
| Carbohydrates (%DRI) | 79 ± 35 | 81 ± 50 | 79 ± 26 | 77 ± 27 | 0.934 |
| Total lipids(%DRI) | 111 ± 40 | 100 ± 28 | 108 ± 43 | 126 ± 45 | 0.078 |
| Cholesterol (%DRI) | 265 ± 131 | 317 ± 113 | 231 ± 120 | 257 ± 149 | 0.053 |
| Vitamin A (%DRI) | 248 ± 960 | 87 ±78 | 165 ± 135 | 508 ± 1706 | 0.268 |
| Folic acid (%DRI) | 167 ± 86 | 189 ± 83 | 133 ± 69 | 187 ± 96 | 0.024 * |
| Fiber (%DRI) | 16 ± 7 | 17 ± 7 | 15 ± 8 | 18 ± 5 | 0.319 |
| Kilocalories (%DRI) | 94 ± 24 | 87 ± 23 | 96 ± 25 | 98 ± 23 | 0.276 |
| Dietary Zn deficiency (%) | 53 (68) | 14 (58) | 22 (73) | 17 (71) | 0.388 |
| Gender by Group | Age (Years) | Serum Zn (µg/dL) | Serum Cu (µg/dL) | Cu/Zn Ratio | Zn/Cu Ratio | Dietary Zn Intake (%DRI) | CRP | ESR |
|---|---|---|---|---|---|---|---|---|
| Obesity | ||||||||
| Female | 2 | 93 | 159 ** | 1.7 † | 0.6 | 54 ‡ | 3.2 | 19 ## |
| Male | 5 | 92 | 142 ** | 1.5 † | 0.6 | 111 | 3.1 | 14 |
| Female | 5 | 116 | 153 ** | 1.3 † | 0.8 | 69 ‡ | 3.4 | 26 ## |
| Female | 12 | 74 | 65 *** | 0.9 | 1.1 | 77 ‡ | 3.1 | 14 |
| Male | 12 | 96 | 150 ** | 1.6 † | 0.6 | 118 | 3.1 | 16 ## |
| Female | 13 | 69 * | 80 | 1.2 † | 0.9 | 74 ‡ | 3.5 | 37 ## |
| Male | 13 | 61 * | 108 | 1.8 † | 0.6 | 74 ‡ | 3.75 | 36 ## |
| Undernutrition | ||||||||
| Male | 2 | 66 * | 87 | 1.3 † | 0.8 | 20 ‡ | 5.1 # | 24 ## |
| Male | 2 | 117 | 20 *** | 0.2 | 5.8 †† | 18 ‡ | 3.2 | 14 |
| Female | 2 | 82 | 153 ** | 1.9 † | 0.5 | 140 | 6.1 # | 10 |
| Male | 3 | 85 | 150 ** | 1.8 † | 0.6 | 72 ‡ | 3.7 | 18 ## |
| Male | 4 | 76 | 143 ** | 1.9 † | 0.5 | 96 | 3.7 | 30 ## |
| Male | 6 | 79 | 188 ** | 2.4 † | 0.4 | 20 ‡ | 2.9 | 26 ## |
| Male | 9 | 94 | 189 ** | 2 † | 0.5 | 47 ‡ | 6.4 # | 21 ## |
| Female | 11 | 64 * | 125 | 1.9 † | 0.5 | 52 ‡ | 3.5 | 25 ## |
| Male | 15 | 82 | 65 *** | 0.8 | 1.3 | 50 ‡ | 3.7 | 11 |
| Female | 16 | 72 | 69 *** | 0.9 | 1 | 51 ‡ | 3.3 | 10 |
| Eutrophic | ||||||||
| Male | 2 | 77 | 175 ** | 2.3 † | 0.4 | 31 ‡ | 3.1 | 26 ## |
| Male | 2 | 85 | 163 ** | 1.9 † | 0.5 | 24 ‡ | 10.7 # | 22 ## |
| Male | 3 | 68 * | 160 ** | 2.3 † | 0.7 | 76 ‡ | 3.1 | 26 ## |
| Male | 5 | 106 | 194 ** | 1.8 † | 0.5 | 58 ‡ | 3.1 | 14 |
| Characteristics | Male | Female | p-Value |
|---|---|---|---|
| Children age (years) | 6 ± 3 | 6 ± 3 | 0.851 |
| Adolescent age (years) | 13 ± 2 | 14 ± 2 | 0.250 |
| Serum copper level (µg/dL) | 123 ± 37 | 113 ± 21 | 0.138 |
| Copper/zinc ratio | 1.4 ± 0.5 | 1.3 ± 0.3 | 0.108 |
| Zinc/copper ratio | 0.9 ± 0.9 | 0.8 ± 0.1 | 0.501 |
| Serum zinc concentration (µg/dL) | 87 ± 12 | 87 ± 13 | 0.967 |
| Dietary zinc intake (%Dietary Reference Intake) | 67 ± 29 | 70 ± 41 | 0.783 |
| Dietary zinc intake (mg/day) | 10 ± 4 | 10 ± 6 | 0.773 |
| Age Group | Children | Adolescent | |
| Serum copper level (µg/dL) | 128 ± 32 | 106 ± 20 | 0.001 * |
| Copper/zinc ratio | 1.5 ± 0.4 | 1.2 ± 0.3 | 0.004 * |
| Zinc/copper ratio | 0.8 ± 0.8 | 0.8 ± 0.6 | 0.953 |
| Serum zinc concentration (µg/dL) | 88 ± 12 | 85 ± 13 | 0.393 |
| Dietary zinc intake (%Dietary Reference Intake) | 74 ± 44 | 63 ± 23 | 0.206 |
| Dietary zinc intake (mg/day) | 11 ± 6 | 9 ± 3 | 0.201 |
| C-Reactive Protein | Normal | High | |
| Serum copper level (µg/dL) | 115 ± 25 | 136 ± 32 | 0.036 * |
| Copper/zinc ratio | 1.3 ± 0.3 | 1.6 ± 0.3 | 0.047 * |
| Zinc/copper ratio | 0.8 ± 0.2 | 0.6 ± 0.1 | 0.029 * |
| Serum zinc concentration (µg/dL) | 87 ± 12 | 84 ± 8 | 0.604 |
| Dietary zinc intake (%Dietary Reference Intake) | 70 ± 36 | 70 ± 39 | 0.992 |
| Dietary zinc intake (mg/day) | 10 ± 5 | 10 ± 6 | 0.996 |
| Erythrocyte Sedimentation Rate | Normal | High | |
| Serum copper level (µg/dL) | 110 ± 27 | 136 ± 31 | 0.001 * |
| Copper/zinc ratio | 1.2 ± 0.3 | 1.7 ± 0.4 | 0.000 * |
| Zinc/copper ratio | 0.9 ± 0.7 | 0.6 ± 0.1 | 0.084 |
| Serum zinc concentration (µg/dL) | 89 ± 11.9 | 81 ± 13 | 0.011 * |
| Dietary zinc intake (%Dietary Reference Intake) | 69 ± 29 | 66 ± 37 | 0.707 |
| Dietary zinc intake (mg/day) | 10 ± 4 | 10 ± 5 | 0.698 |
|
Fisher’s Exact Test | Odds Ratio |
95% Confidence Interval | Cochran’s | Mantel–Haensze | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Abnormal copper levels | ||||||
| Males | 0.016 | 3.965 | 1.237 | 12.78 | 0.016 | 0.034 |
| Children | 0.031 | 3.59 | 1.050 | 12.247 | 0.034 | 0.067 |
| Children < 5 years | 0.005 | 5.889 | 1.759 | 19.712 | 0.002 | 0.007 |
| Age-for-50° Height | 0.010 | 4.381 | 1.418 | 13.536 | 0.007 | 0.018 |
| Low weight-for-age | 0.014 | 4.154 | 1.338 | 12.891 | 0.010 | 0.025 |
| Low folic acid intake | 0.011 | 7.917 | 1.662 | 37.707 | 0.004 | 0.014 |
| High erythrocyte sedimentation rate | 0.013 | 4.950 | 1.577 | 15.538 | 0.005 | 0.014 |
| Leukocytosis | 0.013 | 8.056 | 1.847 | 35.132 | 0.003 | 0.013 |
| Hypercupremia | ||||||
| Males | 0.012 | 5.333 | 1.337 | 21.276 | 0.011 | 0.026 |
| Children | 0.022 | 14 | 2.405 | 81.486 | 0.002 | 0.006 |
| Children < 5 years | 0.004 | 7.259 | 1.983 | 26.580 | 0.001 | 0.004 |
| Age-for-50° Height | 0.003 | 6.891 | 1.867 | 25.436 | 0.002 | 0.005 |
| High vitamin A intake | 0.017 | 4.607 | 1.340 | 15.840 | 0.014 | 0.033 |
| High erythrocyte sedimentation rate | 0.000 | 10.8 | 2.929 | 39.828 | 0.000 | 0.001 |
| Total Series | Obesity | Undernutrition | Eutrophic | |||||
|---|---|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| Age (months) | 0.773 ** | 0.000 | 0.761 ** | 0.000 | 0.732 ** | 0.000 | 0.876 ** | 0.000 |
| Age-for-50° height | 0.638 ** | 0.000 | 0.539 * | 0.012 | 0.799 ** | 0.000 | 0.639 ** | 0.001 |
| Weight-for-age | 0.525 ** | 0.000 | 0.589 ** | 0.005 | 0.786 ** | 0.000 | 0.834 ** | 0.000 |
| Height-for-age | 0.742 ** | 0.000 | 0.700 ** | 0.000 | 0.791 ** | 0.000 | 0.834 ** | 0.000 |
| Weight-for-height | 0.287 * | 0.016 | - | - | - | - | 0.576 ** | 0.004 |
| Body mass index | 0.261 * | 0.029 | - | - | - | - | 0.626 ** | 0.001 |
| Muscle mass by A. (Kg) | 0.622 ** | 0.000 | 0.653 ** | 0.001 | 0.765 ** | 0.000 | 0.889 ** | 0.000 |
| Fat mass by A. (Kg) | 0.354 ** | 0.003 | 0.453 * | 0.039 | 0.737 ** | 0.000 | 0.598 ** | 0.003 |
| Muscle mass by BIA | 0.490 ** | 0.000 | 0.492 * | 0.023 | - | - | 0.871 ** | 0.000 |
| Fat mass by BIA | 0.330 ** | 0.008 | 0.572 ** | 0.007 | - | - | 0.561 ** | 0.005 |
| Serum Copper | Cu/Zn Ratio | Zn/Cu Ratio |
|---|---|---|
| r = 0.493 Height-for age p = 0.000 Height-for-age Z score p = 0.024 Triceps skinfold p = 0.032 | r = 0.387 Height-for age p = 0.000 Triceps skinfold p = 0.045 | r = 0.356 Height-for age p = 0.000 Triceps skinfold p = 0.012 |
| r = 0.295 BCS absolute value p = 0.000 | r = 0.260 BCS absolute value p = 0.000 | r = 0.224 BCS absolute value p = 0.000 |
| r = 0.230 Fiber %DRI p = 0.000 Magnesium %DRI p = 0.016 Vitamin B6 %DRI p = 0.021 | r = 0.054 Fiber %DRI p = 0.042 | r = 0.534 Vitamin B1 %DRI p = 0.000 Vitamin B2 %DRI p = 0.002 Vitamin B6 %DRI p = 0.034 |
| r = 0.409 Prealbumin p = 0.000 GGT p = 0.002 Cardiovascular risk index p = 0.005 | r = 0.401 Prealbumin p = 0.003 GGT p = 0.002 Total bilirubin p = 0.007 | r = 0.940 GGT p = 0.000 ALT p = 0.000 Prealbumin p = 0.012 Total bilirubin p = 0.024 |
| r = 0.250 IGF-1 p = 0.000 | r = 0.218 IGF-1 p = 0.000 | r = 0.157 IGF-1 p = 0.001 |
| r = 0.400 Leucocytes p = 0.049 Hemoglobin p = 0.002 MCHC p = 0.028 Neutrophils p = 0.036 | r = 0.435 Hemoglobin p = 0.000 Neutrophils p = 0.000 | r = 0.350 ESR p = 0.000 MCV p = 0.016 CRP p = 0.036 |
| r = 0.365 IgG4 p = 0.000 IgG1 p = 0.012 T-lymphocytes CD4 p = 0.041 | r = 0.207 IgG3 p = 0.014 IgG4 p = 0.019 | r = 0.075 IgG4 p = 0.045 |
| r = 0.056 Time of illness p = 0.036 |
| Obesity | Undernutrition | Eutrophic | ||||||
|---|---|---|---|---|---|---|---|---|
| Serum Copper | Cu/Zn Ratio | Zn/Cu Ratio | Serum Copper | Cu/Zn Ratio | Zn/Cu Ratio | Serum Copper | Cu/Zn Ratio | Zn/Cu Ratio |
| r = 0.591 Height-for age p = 0.000 Height-for-age Z score p = 0.013 | r = 0.418 Height-for age p = 0.005 Height-for-age Z score p = 0.047 | r = 0.686 Kg mass muscular p = 0.001 Suprailiac skinfold Z-score p = 0.005 Waist/hip ratio p = 0.024 | r = 0.318 Wrist perimeter p = 0.010 | r = 0.545 Kg mass muscular p = 0.005 Hip perimeter. p = 0.030 | r = 0.724 Waist/Height ratio p = 0.0001 Arm muscular area p = 0.0002 | r = 0.747 Height-for age p = 0.000 Wrist perimeter p = 0.000 Body mass index Z-score p = 0.031 | r = 0.400 Kg mass muscular p = 0.002 | |
| r = 0.421 BCS absolute value p = 0.002 | r = 0.316 BCS absolute value p = 0.010 | r = 0.214 BCS absolute value p = 0.040 | r = 0.582 BCS absolute value p = 0.000 | r = 0.494 BCS absolute value p = 0.000 | r = 0.463 BCS absolute value p = 0.000 | |||
| r = 0.672 Fiber %DRI p = 0.012 Folic acid %DRI p = 0.002 Magnesium %DRI p = 0.001 | r = 0.384 Folic acid %DRI p = 0.001 | r = 0.409 Folic acid %DRI p = 0.001 | r = 0.364 Vitamin B1 %DRI p = 0.002 Fiber %DRI p = 0.017 | r = 0.319 Vitamin B1 %DRI p = 0.003 Fiber %DRI p = 0.035 | r = 0.917 Vitamin B1 %DRI p = 0.000 Vitamin B6 %DRI p = 0.000 Cholesterol %DRI p = 0.029 | r = 0.506 Carbohydrates %DRI p = 0.006 Fiber %DRI p = 0.006 Protein %DRI p = 0.042 | r = 0.362 Vitamin B1 %DRI p = 0.003 Magnesium %DRI p = 0.035 | r = 0.166 Vitamin B1 %DRI p = 0.048 |
| r = 0.399 Creatinine p = 0.012 | r = 0.416 BUN p = 0.009 | r = 0.426 BUN p = 0.008 | r = 0.363 Transferrin saturation index p = 0.002 | r = 0.602 Prealbumin p = 0.000 Cholesterol p = 0.029 Alkaline phosphatase p = 0.043 | r = 0.990 GGT p = 0.000 Prealbumin p = 0.000 BUN p = 0.015 | r = 0.618 Total bilirubin p = 0.000 Transferrin p = 0.003 | r = 0.518 Total bilirubin p = 0.004 Transferrin p = 0.010 | r = 0.511 Total bilirubin p = 0.001 Transferrin p = 0.044 |
| r = 0.512 Phosphorus p = 0.027 IGF-1 p = 0.028 | r = 0.241 Phosphorus p = 0.017 | r = 0.213 Phosphorus p = 0.027 | r = 0.307 IGF-1 p = 0.006 | r = 0.308 IGF-1 p = 0.006 | r = 0.284 IGF-1 p = 0.009 | r = 0.350 IGF-1 p = 0.012 | r = 0.610 IGF-1 p = 0.002 Phosphorus p = 0.016 | r = 0.454 IGF-1 p = 0.008 IGBP3 p = 0.039 |
| r = 0.242 Hemoglobin p = 0.032 | r = 0.398 Neutrophils p = 0.002 | r = 0.569 Neutrophils p = 0.002 MCV p = 0.030 | r = 0.409 Neutrophils p = 0.007 Eosinophils p = 0.046 | r = 0.726 ESR p = 0.000 MCV p = 0.008 CRP p = 0.024 | r = 0.850 ESR p = 0.000 Leucocytes p = 0.000 MCV p = 0.007 Basophils p = 0.015 | r = 0.656 ESR p = 0.003 Leucocytes p = 0.009 CRP p = 0.023 | ||
| r = 0.882 IgG3 p = 0.000 IgG2 p = 0.000 IgG4 p = 0.006 | r = 0.393 Lymphocytes CD16 + 56 p = 0.003 IgA p = 0.023 | r = 0.382 Lymphocytes CD16 + 56 p = 0.005 IgA p = 0.020 | r = 0.573 Complement C4 p = 0.000 | r = 0.493 IgG2 p = 0.016 Complement C3 p = 0.024 | r = 0.443 IgG2 p = 0.012 IgG1 p = 0.046 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Copper and Copper/Zn Ratio in a Series of Children with Chronic Diseases: A Cross-Sectional Study. Nutrients 2021, 13, 3578. https://doi.org/10.3390/nu13103578
Escobedo-Monge MF, Barrado E, Parodi-Román J, Escobedo-Monge MA, Torres-Hinojal MC, Marugán-Miguelsanz JM. Copper and Copper/Zn Ratio in a Series of Children with Chronic Diseases: A Cross-Sectional Study. Nutrients. 2021; 13(10):3578. https://doi.org/10.3390/nu13103578
Chicago/Turabian StyleEscobedo-Monge, Marlene Fabiola, Enrique Barrado, Joaquín Parodi-Román, María Antonieta Escobedo-Monge, María Carmen Torres-Hinojal, and José Manuel Marugán-Miguelsanz. 2021. "Copper and Copper/Zn Ratio in a Series of Children with Chronic Diseases: A Cross-Sectional Study" Nutrients 13, no. 10: 3578. https://doi.org/10.3390/nu13103578






