The Optimal Strategy of Vitamin D for Sarcopenia: A Network Meta-Analysis of Randomized Controlled Trials
Abstract
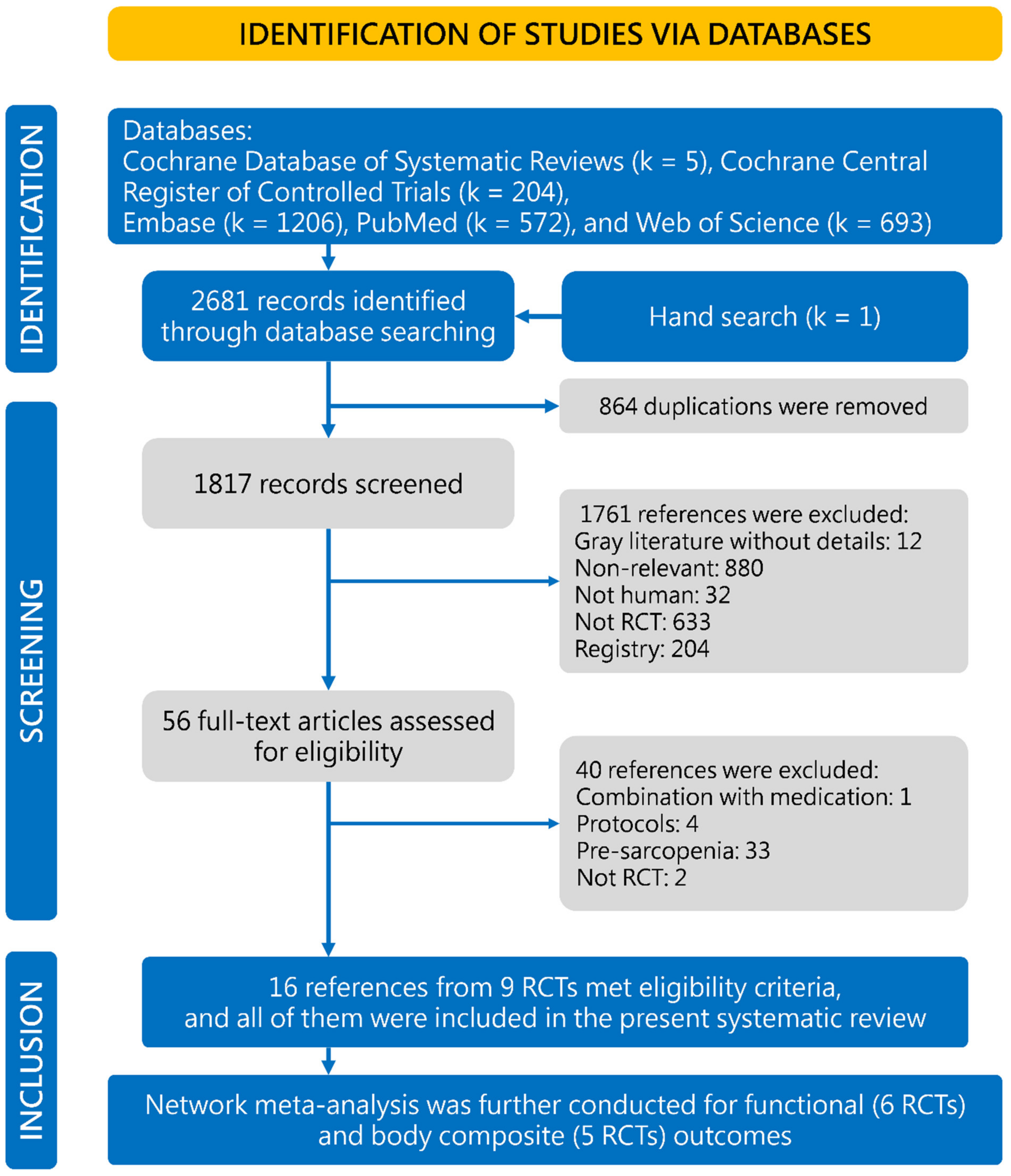
:1. Introduction
2. Methods
2.1. Data Sources and Evidence Selection
2.2. Data Extraction
2.3. Quality Evaluation
2.4. Data Synthesis and Analysis
3. Results
3.1. Characteristics and Quality of Included Studies
3.2. Functional Outcomes
3.3. Body Compositional Outcomes
4. Discussion
4.1. Key Findings
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| EWGSOP | European Working Group of Sarcopenia in Older People |
| RCT | randomized controlled trial |
| RoB | risk of bias |
| RSMI | relative skeletal muscle index |
| SE | standard error |
| WMD | weighted mean differences |
References
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.-S.; Krairit, O.; et al. Sarcopenia in asia: Consensus report of the asian working group for sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised european consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Lips, P.; van Schoor, N.M. The effect of vitamin d on bone and osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 585–591. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Mokbel, N.; Cheng, K.; Gunton, J.E. Vitamin d signaling regulates proliferation, differentiation, and myotube size in c2c12 skeletal muscle cells. Endocrinology 2014, 155, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Barker, T.; Henriksen, V.T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Schneider, E.D.; Dixon, B.M.; Weaver, L.K. Higher serum 25-hydroxyvitamin d concentrations associate with a faster recovery of skeletal muscle strength after muscular injury. Nutrients 2013, 5, 1253–1275. [Google Scholar] [CrossRef] [Green Version]
- Houston, D.K.; Cesari, M.; Ferrucci, L.; Cherubini, A.; Maggio, D.; Bartali, B.; Johnson, M.A.; Schwartz, G.G.; Kritchevsky, S.B. Association between vitamin d status and physical performance: The inchianti study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 440–446. [Google Scholar] [CrossRef]
- Gerdhem, P.; Ringsberg, K.A.; Obrant, K.J.; Akesson, K. Association between 25-hydroxy vitamin d levels, physical activity, muscle strength and fractures in the prospective population-based opra study of elderly women. Osteoporos. Int. 2005, 16, 1425–1431. [Google Scholar] [CrossRef]
- Tomlinson, P.B.; Joseph, C.; Angioi, M. Effects of vitamin d supplementation on upper and lower body muscle strength levels in healthy individuals. A systematic review with meta-analysis. J. Sci. Med. Sport 2015, 18, 575–580. [Google Scholar] [CrossRef]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyere, O. The effects of vitamin d on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, M.; Begerow, B.; Minne, H.W.; Suppan, K.; Fahrleitner-Pammer, A.; Dobnig, H. Effects of a long-term vitamin d and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos. Int. 2009, 20, 315–322. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Nikolakopoulou, A.; Higgins, J.P.; Papakonstantinou, T.; Chaimani, A.; Del Giovane, C.; Egger, M.; Salanti, G. Cinema: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020, 17, e1003082. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.M.; Mikušová, L.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; Mets, T.; Wijers, S.L.J.; Garthoff, J.A.; et al. Safety and tolerability of 6-month supplementation with a vitamin d, calcium and leucine-enriched whey protein medical nutrition drink in sarcopenic older adults. Aging Clin. Exp. Res. 2020, 32, 1501–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin d and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the provide study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Björkman, M.P.; Suominen, M.H.; Kautiainen, H.; Jyväkorpi, S.K.; Finne-Soveri, H.U.; Strandberg, T.E.; Pitkälä, K.H.; Tilvis, R.S. Effect of protein supplementation on physical performance in older people with sarcopenia—A randomized controlled trial. J. Am. Med. Dir. Assoc. 2020, 21, 226–232.e1. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.C.; Liu, C.F.; Ji, Z.; Yang, R.H.; An, Q.Q.; Zhang, X.Y.; You, J.; Duan, D.D.; Sun, Y.F.; Zhu, Y.W.; et al. A high whey protein, vitamin d and e supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: A double-blind randomized controlled trial. Clin. Nutr. 2019, 38, 159–164. [Google Scholar] [CrossRef]
- Chang, K.-V.; Wu, W.-T.; Huang, K.-C.; Han, D.-S. Effectiveness of early versus delayed exercise and nutritional intervention on segmental body composition of sarcopenic elders—A randomized controlled trial. Clin. Nutr. 2020, 40, 1052–1059. [Google Scholar] [CrossRef]
- Cramer, J.T.; Cruz-Jentoft, A.J.; Landi, F.; Hickson, M.; Zamboni, M.; Pereira, S.L.; Hustead, D.S.; Mustad, V.A. Impacts of high-protein oral nutritional supplements among malnourished men and women with sarcopenia: A multicenter, randomized, double-blinded, controlled trial. J. Am. Med. Dir. Assoc. 2016, 17, 1044–1055. [Google Scholar] [CrossRef] [Green Version]
- Hill, T.R.; Verlaan, S.; Biesheuvel, E.; Eastell, R.; Bauer, J.M.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; Mets, T.; et al. A vitamin d, calcium and leucine-enriched whey protein nutritional supplement improves measures of bone health in sarcopenic non-malnourished older adults: The provide study. Calcif. Tissue Int. 2019, 105, 383–391. [Google Scholar] [CrossRef] [Green Version]
- Kemmler, W.; Kohl, M.; Fröhlich, M.; Jakob, F.; Engelke, K.; von Stengel, S.; Schoene, D. Effects of high-intensity resistance training on osteopenia and sarcopenia parameters in older men with osteosarcopenia-one-year results of the randomized controlled franconian osteopenia and sarcopenia trial (frost). J. Bone Miner. Res. 2020, 35, 1634–1644. [Google Scholar] [CrossRef]
- Kemmler, W.; Kohl, M.; Jakob, F.; Engelke, K.; von Stengel, S. Effects of high intensity dynamic resistance exercise and whey protein supplements on osteosarcopenia in older men with low bone and muscle mass. Final results of the randomized controlled frost study. Nutrients 2020, 12, 2341. [Google Scholar] [CrossRef]
- Kemmler, W.; Weineck, M.; Kohl, M.; von Stengel, S.; Giessing, J.; Fröhlich, M.; Schoene, D. High intensity resistance exercise training to improve body composition and strength in older men with osteosarcopenia. Results of the randomized controlled franconian osteopenia and sarcopenia trial (frost). Front. Sports Act. Living 2020, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, M.; Kojima, N.; Fujino, K.; Hosoi, E.; Kobayashi, H.; Somekawa, S.; Niki, Y.; Yamashiro, Y.; Yoshida, H. Exercise and nutritional supplementation on community-dwelling elderly Japanese women with sarcopenic obesity: A randomized controlled trial. J. Am. Med. Dir. Assoc. 2016, 17, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Liberman, K.; Njemini, R.; Luiking, Y.; Forti, L.N.; Verlaan, S.; Bauer, J.M.; Memelink, R.; Brandt, K.; Donini, L.M.; Maggio, M.; et al. Thirteen weeks of supplementation of vitamin d and leucine-enriched whey protein nutritional supplement attenuates chronic low-grade inflammation in sarcopenic older adults: The provide study. Aging Clin. Exp. Res. 2019, 31, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenberg, T.; von Stengel, S.; Sieber, C.; Kemmler, W. The favorable effects of a high-intensity resistance training on sarcopenia in older community-dwelling men with osteosarcopenia: The randomized controlled frost study. Clin. Interv. Aging 2019, 14, 2173–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondanelli, M.; Klersy, C.; Terracol, G.; Talluri, J.; Maugeri, R.; Guido, D.; Faliva, M.A.; Solerte, B.S.; Fioravanti, M.; Lukaski, H.; et al. Whey protein, amino acids, and vitamin d supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am. J. Clin. Nutr. 2016, 103, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Yoshimura, Y.; Shimazu, S.; Jeong, S.; Yamaga, M.; Koga, H. Effects of branched-chain amino acids and vitamin d supplementation on physical function, muscle mass and strength, and nutritional status in sarcopenic older adults undergoing hospital-based rehabilitation: A multicenter randomized controlled trial. Geriatr. Gerontol. Int. 2019, 19, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Verlaan, S.; Maier, A.B.; Bauer, J.M.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.T.; Mets, T.; Seal, C.; et al. Sufficient levels of 25-hydroxyvitamin d and protein intake required to increase muscle mass in sarcopenic older adults—The provide study. Clin. Nutr. 2018, 37, 551–557. [Google Scholar] [CrossRef]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin d on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [Green Version]
- Ceglia, L.; Harris, S.S. Vitamin d and its role in skeletal muscle. Calcif. Tissue Int. 2013, 92, 151–162. [Google Scholar] [CrossRef]
- Koundourakis, N.E.; Avgoustinaki, P.D.; Malliaraki, N.; Margioris, A.N. Muscular effects of vitamin d in young athletes and non-athletes and in the elderly. Hormones 2016, 15, 471–488. [Google Scholar] [CrossRef] [Green Version]
- Uusi-Rasi, K.; Patil, R.; Karinkanta, S.; Kannus, P.; Tokola, K.; Lamberg-Allardt, C.; Sievanen, H. Exercise and vitamin d in fall prevention among older women: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 703–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. Vitamin d deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A. Vitamin d deficiency: A global perspective. Nutr. Rev. 2008, 66, S153–S164. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin d deficiency in adults: When to test and how to treat. Mayo Clin. Proc. 2010, 85, 752–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onambele-Pearson, G.L.; Breen, L.; Stewart, C.E. Influence of exercise intensity in older persons with unchanged habitual nutritional intake: Skeletal muscle and endocrine adaptations. Age 2010, 32, 139–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, K.-J.; Liao, C.-D.; Tsai, M.-W.; Chen, C.-N. Effects of exercise and nutritional intervention on body composition, metabolic health, and physical performance in adults with sarcopenic obesity: A meta-analysis. Nutrients 2019, 11, 2163. [Google Scholar] [CrossRef] [Green Version]



| Criteria for Sarcopenia | Type of Sarcopenia | Intervention Duration | ||||
| Author | Year | Area | Age | |||
| PROVIDE trial | 2015–2020 | Europe | ≥65 | SBBP 4~9 SMI < 37%/28% | Primary | 13 weeks |
| Cramer | 2016 | Europe + America | ≥65 | EWGSOP | Primary | 24 weeks |
| Kim | 2016 | Asia (Japan) | ≥70 | SMI < 5.67 kg/m2 | Primary | 3 months |
| Grip strength < 17 kg | ||||||
| Walk speed < 1 m/s | ||||||
| Rondanelli | 2016 | Europe (Italy) | ≥65 | Relative muscle mass | Primary | 12 weeks |
| <7.26/5.5 kg/m2 (M/F) | ||||||
| Bo | 2017 | Asia (China) | 60–85 | AWGS | Primary | 6 months |
| Takeuchi | 2018 | Asia (Japan) | ≥65 | AWGS | Primary | 8 weeks |
| Björkman | 2019 | Europe (Finland) | ≥74 | Complicated | Primary | 12 months |
| Chang | 2020 | Asia (Taiwan) | ≥65 | EWGSOP | Primary | 12 weeks |
| FrOST trial (Kemmler) | 2018–2020 | Europe (Germany) | ≥72 | EWGSOP | Primary | 18 months |
| Baseline Vitamin D | ||||||
| Author | Intervention | Sex (M/F) | Serum Vitamin D | Deficiency | ||
| PROVIDE trial | P + D (vitamin D 1600 IU/day, whey protein 40 g) | 64/120 | 25(OH)D 48 (nmol/L) | Deficient | ||
| Iso-caloric product | 67/129 | 25(OH)D 49 (nmol/L) | Deficient | |||
| Cramer | P + D (vitamin D3 998 IU/day, protein 40 g) | 63/102 | Vitamin D 65 (nmol/L) | Non-deficient | ||
| Protein (protein 28 g with non-therapeutic dose vitamin D3) | 63/102 | Vitamin D 60 (nmol/L) | Non-deficient | |||
| Kim | P + D + E (vitamin D 800 IU/day, leucine-enriched amino acid 3 g) | 0/36 | Vitamin D 23.2 (ng/mL) | Non-deficient | ||
| P + D (vitamin D 800 IU/day, leucine-enriched amino acid 3 g) | 0/34 | Vitamin D 22.5 (ng/mL) | Non-deficient | |||
| Exercise | 0/35 | Vitamin D 24.2 (ng/mL) | Non-deficient | |||
| No nutritional supplement (with health education only) | 0/34 | Vitamin D 27.0 (ng/mL) | Non-deficient | |||
| Rondanelli | P + E (with non-therapeutic dose vitamin D3, essential amino acids 32 g) | 29/40 | Not reported | Not reported | ||
| Exercise (with non-therapeutic dose vitamin D3) | 24/37 | Not reported | Not reported | |||
| Bo | P + D (vitamin D 1404 IU/day, protein 44 g) | 13/17 | Vitamin D3 21.29 (ng/mL) | Non-deficient | ||
| Iso-caloric product | 14/16 | Vitamin D3 20.85 (ng/mL) | Non-deficient | |||
| Takeuchi | P + D (vitamin D 12.5 μg/day, BCAA 10 g) | 12/20 | Not reported | Not reported | ||
| No nutritional supplement | 13/18 | Not reported | Not reported | |||
| Björkman | D + E (vitamin D 800 IU/day) | 16/56 | Not reported | Not reported | ||
| D + E + I (vitamin D 800 IU/day, iso-caloric product) | 27/46 | Not reported | Not reported | |||
| P + D + E (vitamin D 800 IU/day, whey protein 20 g) | 22/51 | Not reported | Not reported | |||
| Chang | Exercise | 6/22 | Not reported | Not reported | ||
| P + D + E (vitamin D3 1600 IU/day, BCAA 6 g) | 7/22 | Not reported | Not reported | |||
| FrOST trial | P + D + E (vitamin D 2500–5000 IU/week, whey protein 80 g) | 21/0 | 25(OH)D 21.6 (ng/mL) | Non-deficient | ||
| P + D (vitamin D 2500–5000 IU/week, whey protein 80 g) | 22/0 | 25(OH)D 17.5 (ng/mL) | Deficient | |||
| Changes in gait speed | Iso. | Nil | |||
| 0.02 (−0.07,0.10) | P | ||||
| 0.02 (−0.05,0.09) | 0 (−0.04,0.05) | P + D | |||
| 0.05 (−0.04,0.14) | 0.03 (−0.05,0.12) | 0.03 (−0.04,0.10) | P + D + E | ||
| Appendicular muscle mass | NNS | Nil | |||
| 0.04 (−0.8,0.88) | Ex | ||||
| −0.5 (−1.48,0.48) | 0.54 (−0.5,1.58) | P + D | |||
| −0.24 (−1.08,0.6) | 0.28 (−0.47,1.03) | −0.26 (−1.3,0.79) | P + D + E | ||
| Lower limbs muscle mass | NNS | 0.01 (−0.23,0.24) | −0.1 (−0.39,0.19) | −0.01 (−0.28,0.26) | Upper limbs muscle mass |
| 0.02 (−0.65,0.68) | Ex | −0.11 (−0.39,0.18) | −0.02 (−0.27,0.24) | ||
| −0.4 (−1.13,0.33) | 0.42 (−0.39,1.23) | P + D | 0.09 (−0.23,0.41) | ||
| −0.21 (−0.87,0.45) | 0.23 (−0.39,0.84) | −0.19 (−0.99,0.61) | P + D + E | ||
| Changes in body fat mass | Iso. | −0.17 (−0.33,−0.01) | – | −0.52 (−0.71,−0.33) | Changes in RSMI |
| 0.03 (−1.1,1.15) | P | – | −0.35 (−0.45,−0.25) | ||
| −0.4 (−1.44,0.64) | −0.43 (−0.86,0.00) | P + D | – | ||
| – | – | – | P + D + E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.-H.; Chen, K.-H.; Chen, C.; Chu, W.-C.; Kang, Y.-N. The Optimal Strategy of Vitamin D for Sarcopenia: A Network Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 3589. https://doi.org/10.3390/nu13103589
Cheng S-H, Chen K-H, Chen C, Chu W-C, Kang Y-N. The Optimal Strategy of Vitamin D for Sarcopenia: A Network Meta-Analysis of Randomized Controlled Trials. Nutrients. 2021; 13(10):3589. https://doi.org/10.3390/nu13103589
Chicago/Turabian StyleCheng, Shih-Hao, Kee-Hsin Chen, Chiehfeng Chen, Woei-Chyn Chu, and Yi-No Kang. 2021. "The Optimal Strategy of Vitamin D for Sarcopenia: A Network Meta-Analysis of Randomized Controlled Trials" Nutrients 13, no. 10: 3589. https://doi.org/10.3390/nu13103589
APA StyleCheng, S.-H., Chen, K.-H., Chen, C., Chu, W.-C., & Kang, Y.-N. (2021). The Optimal Strategy of Vitamin D for Sarcopenia: A Network Meta-Analysis of Randomized Controlled Trials. Nutrients, 13(10), 3589. https://doi.org/10.3390/nu13103589






