Milk Formula Diet Alters Bacterial and Host Protein Profile in Comparison to Human Milk Diet in Neonatal Piglet Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Metaproteome
2.3. Data Accessibility
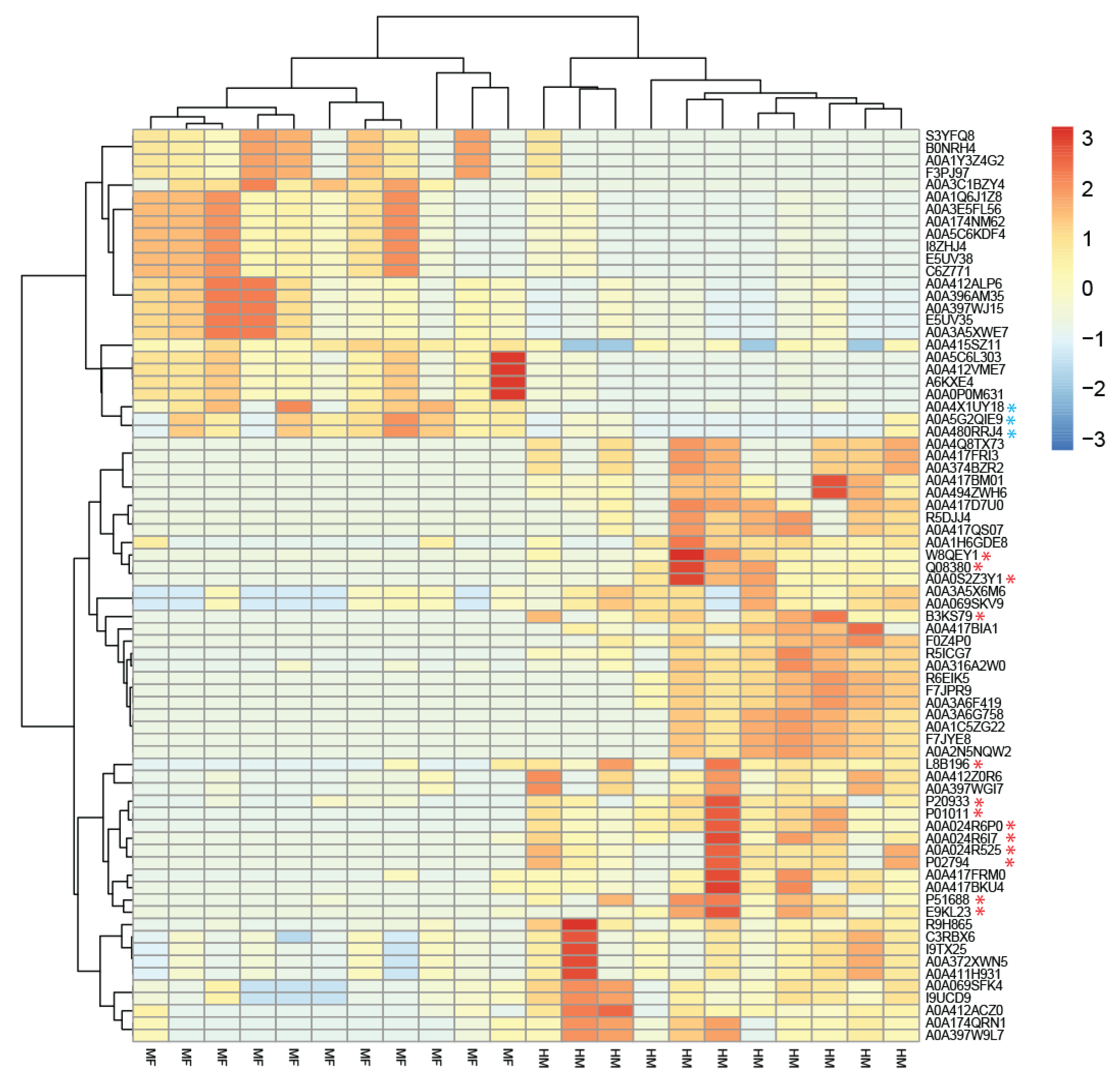
3. Results
3.1. Microbial Taxonomy Identification in the Cecal Lumen
3.2. Bacterial Proteins Impacted by Diet Groups in the Lumen of Cecum at PND 21
3.3. Host Proteins Identified in the Cecal Contents at PND 21
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, J.A.; Baumgartel, K.; Morowitz, M.J.; Giangrasso, V.; Demirci, J.R. The Role of Human Milk in Decreasing Necrotizing Enterocolitis Through Modulation of the Infant Gut Microbiome: A Scoping Review. J. Hum. Lact 2020, 36, 647–656. [Google Scholar] [CrossRef]
- Bering, S.B. Human Milk Oligosaccharides to Prevent Gut Dysfunction and Necrotizing Enterocolitis in Preterm Neonates. Nutrients 2018, 10, 1461. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Xiu, W.; Dai, Y.; Yang, C. Protective effects of different doses of human milk on neonatal necrotizing enterocolitis. Medicine 2020, 99, e22166. [Google Scholar] [CrossRef]
- Cañizo Vázquez, D.; Salas García, S.; Izquierdo Renau, M.; Iglesias-Platas, I. Availability of Donor Milk for Very Preterm Infants Decreased the Risk of Necrotizing Enterocolitis without Adversely Impacting Growth or Rates of Breastfeeding. Nutrients 2019, 11, 1895. [Google Scholar] [CrossRef] [Green Version]
- Balmer, S.E.; Wharton, B.A. Diet and faecal flora in the newborn: Breast milk and infant formula. Arch. Dis. Child. 1989, 64, 1672–1677. [Google Scholar] [CrossRef] [Green Version]
- Bezirtzoglou, E.; Tsiotsias, A.; Welling, G.W. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe 2011, 17, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Fallani, M.; Amarri, S.; Uusijarvi, A.; Adam, R.; Khanna, S.; Aguilera, M.; Gil, A.; Vieites, J.M.; Norin, E.; Young, D.; et al. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 2011, 157, 1385–1392. [Google Scholar] [CrossRef] [Green Version]
- Fanaro, S.; Chierici, R.; Guerrini, P.; Vigi, V. Intestinal microflora in early infancy: Composition and development. Acta Paediatr. Suppl. 2003, 91, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, H.J.; Wildeboer-Veloo, A.C.; Raangs, G.C.; Wagendorp, A.A.; Klijn, N.; Bindels, J.G.; Welling, G.W. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Mackie, R.I.; Sghir, A.; Gaskins, H.R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 1999, 69, 1035S–1045S. [Google Scholar] [CrossRef]
- Miklavcic, J.J.; Badger, T.M.; Bowlin, A.K.; Matazel, K.S.; Cleves, M.A.; LeRoith, T.; Saraf, M.K.; Chintapalli, S.V.; Piccolo, B.D.; Shankar, K.; et al. Human Breast-Milk Feeding Enhances the Humoral and Cell-Mediated Immune Response in Neonatal Piglets. J. Nutr. 2018, 148, 1860–1870. [Google Scholar] [CrossRef] [Green Version]
- Davila, A.M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.H.; Sanz, Y.; Tomé, D. Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host. Pharm. Res. 2013, 68, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Neis, E.P.; Dejong, C.H.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of Human Milk Bioactives on Infants’ Gut and Immune Health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [Green Version]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, F.; Matazel, K.S.; Bowlin, A.K.; Williams, K.D.; Elolimy, A.A.; Adams, S.H.; Bode, L.; Yeruva, L. Neonatal Diet Impacts the Large Intestine Luminal Metabolome at Weaning and Post-Weaning in Piglets Fed Formula or Human Milk. Front. Immunol. 2020, 11, 607609. [Google Scholar] [CrossRef]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Bjorksten, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Albenberg, L.G.; Wu, G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, A.; Akbari, P.; Difilippo, E.; Schols, H.A.; Ulfman, L.H.; Schoterman, M.H.; Garssen, J.; Fink-Gremmels, J.; Braber, S. The piglet as a model for studying dietary components in infant diets: Effects of galacto-oligosaccharides on intestinal functions. Br. J. Nutr. 2016, 115, 605–618. [Google Scholar] [CrossRef] [Green Version]
- Ardeshir, A.; Narayan, N.R.; Mendez-Lagares, G.; Lu, D.; Rauch, M.; Huang, Y.; Van Rompay, K.K.; Lynch, S.V.; Hartigan-O’Connor, D.J. Breast-fed and bottle-fed infant rhesus macaques develop distinct gut microbiotas and immune systems. Sci. Transl. Med. 2014, 6, 252ra120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, T.; Seyfried, F.; Docherty, N.G.; Tremaroli, V.; le Roux, C.W.; Perkins, R.; Backhed, F. Diabetes-associated microbiota in fa/fa rats is modified by Roux-en-Y gastric bypass. ISME J. 2017, 11, 2035–2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avershina, E.; Storro, O.; Oien, T.; Johnsen, R.; Pope, P.; Rudi, K. Major faecal microbiota shifts in composition and diversity with age in a geographically restricted cohort of mothers and their children. FEMS Microbiol. Ecol. 2014, 87, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L.; et al. Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. CMAJ 2013, 185, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Brink, L.R.; Matazel, K.; Piccolo, B.D.; Bowlin, A.K.; Chintapalli, S.V.; Shankar, K.; Yeruva, L. Neonatal Diet Impacts Bioregional Microbiota Composition in Piglets Fed Human Breast Milk or Infant Formula. J. Nutr. 2019, 149, 2236–2246. [Google Scholar] [CrossRef] [PubMed]
- Brink, L.R.; Mercer, K.E.; Piccolo, B.D.; Chintapalli, S.V.; Elolimy, A.; Bowlin, A.K.; Matazel, K.S.; Pack, L.; Adams, S.H.; Shankar, K.; et al. Neonatal diet alters fecal microbiota and metabolome profiles at different ages in infants fed breast milk or formula. Am. J. Clin. Nutr. 2020, 111, 1190–1202. [Google Scholar] [CrossRef]
- Wilmes, P.; Bond, P.L. Metaproteomics: Studying functional gene expression in microbial ecosystems. Trends Microbiol. 2006, 14, 92–97. [Google Scholar] [CrossRef]
- Verberkmoes, N.C.; Russell, A.L.; Shah, M.; Godzik, A.; Rosenquist, M.; Halfvarson, J.; Lefsrud, M.G.; Apajalahti, J.; Tysk, C.; Hettich, R.L.; et al. Shotgun metaproteomics of the human distal gut microbiota. ISME J. 2009, 3, 179–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elolimy, A.A.; Washam, C.; Byrum, S.; Chen, C.; Dawson, H.; Bowlin, A.K.; Randolph, C.E.; Saraf, M.K.; Yeruva, L. Formula Diet Alters the Ileal Metagenome and Transcriptome at Weaning and during the Postweaning Period in a Porcine Model. mSystems 2020, 5, e00457-20. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012; p. 420. [Google Scholar]
- Karaduta, O.; Glazko, G.; Dvanajscak, Z.; Arthur, J.; Mackintosh, S.; Orr, L.; Rahmatallah, Y.; Yeruva, L.; Tackett, A.; Zybailov, B. Resistant starch slows the progression of CKD in the 5/6 nephrectomy mouse model. Physiol. Rep. 2020, 8, e14610. [Google Scholar] [CrossRef] [PubMed]
- Zybailov, B.L.; Glazko, G.V.; Rahmatallah, Y.; Andreyev, D.S.; McElroy, T.; Karaduta, O.; Byrum, S.D.; Orr, L.; Tackett, A.J.; Mackintosh, S.G.; et al. Metaproteomics reveals potential mechanisms by which dietary resistant starch supplementation attenuates chronic kidney disease progression in rats. PLoS ONE 2019, 14, e0199274. [Google Scholar] [CrossRef] [Green Version]
- Mesuere, B.; Debyser, G.; Aerts, M.; Devreese, B.; Vandamme, P.; Dawyndt, P. The Unipept metaproteomics analysis pipeline. Proteomics 2015, 15, 1437–1442. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, L.; Shan, B.; Chen, W.; Xie, M.; Yuen, D.; Zhang, W.; Zhang, Z.; Lajoie, G.A.; Ma, B. PEAKS DB: De novo sequencing assisted database search for sensitive and accurate peptide identification. Mol. Cell Proteom. 2012, 11, M111.010587. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [Green Version]
- Esnaola, M.; Puig, P.; Gonzalez, D.; Castelo, R.; Gonzalez, J.R. A flexible count data model to fit the wide diversity of expression profiles arising from extensively replicated RNA-seq experiments. BMC Bioinform. 2013, 14, 254. [Google Scholar] [CrossRef] [Green Version]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R.; Ullrey, D.E. The pig as a model for human nutrition. Annu Rev. Nutr. 1987, 7, 361–382. [Google Scholar] [CrossRef]
- Moughan, P.J.; Birtles, M.J.; Cranwell, P.D.; Smith, W.C.; Pedraza, M. The piglet as a model animal for studying aspects of digestion and absorption in milk-fed human infants. World Rev. Nutr. Diet. 1992, 67, 40–113. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huo, G.; Li, X.; Yang, L.; Duan, C. Impact of diet in shaping gut microbiota revealed by a comparative study in infants during the six months of life. J. Microbiol. Biotechnol. 2014, 24, 133–143. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Persaud, R.R.; Guttman, D.S.; Chari, R.S.; Field, C.J.; Sears, M.R.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: A prospective cohort study. Bjog 2016, 123, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Heinzmann, S.S.; Schmitt-Kopplin, P. Deep metabotyping of the murine gastrointestinal tract for the visualization of digestion and microbial metabolism. J. Proteome Res. 2015, 14, 2267–2277. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Chassard, C. New insights in gut microbiota establishment in healthy breast fed neonates. PLoS ONE 2012, 7, e44595. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M.; et al. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe 2018, 24, 133–145.e135. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, M.; Lokmic, A.; Angell, I.L.; Lødrup Carlsen, K.C.; Carlsen, K.H.; Haugen, G.; Hedlin, G.; Jonassen, C.M.; Marsland, B.J.; Nordlund, B.; et al. Fecal Microbiota Nutrient Utilization Potential Suggests Mucins as Drivers for Initial Gut Colonization of Mother-Child-Shared Bacteria. Appl. Environ. Microbiol. 2021, 87, e02201-20. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Wexler, A.G.; Goodman, A.L. An insider’s perspective: Bacteroides as a window into the microbiome. Nat. Microbiol. 2017, 2, 17026. [Google Scholar] [CrossRef] [Green Version]
- Ward, R.E.; Niñonuevo, M.; Mills, D.A.; Lebrilla, C.B.; German, J.B. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Appl. Environ. Microbiol. 2006, 72, 4497–4499. [Google Scholar] [CrossRef] [Green Version]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of human milk oligosaccharides by gut-related microbes. J. Agric. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef] [Green Version]
- Cortes, L.; Wopereis, H.; Tartiere, A.; Piquenot, J.; Gouw, J.W.; Tims, S.; Knol, J.; Chelsky, D. Metaproteomic and 16S rRNA Gene Sequencing Analysis of the Infant Fecal Microbiome. Int. J. Mol. Sci. 2019, 20, 1430. [Google Scholar] [CrossRef] [Green Version]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef] [Green Version]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.X.; Li, Y.H.; Dai, W.K.; Li, X.S.; Qiu, C.Z.; Ruan, M.L.; Zou, B.; Dong, C.; Liu, Y.H.; He, J.Y.; et al. Fecal microbiota transplantation induces remission of infantile allergic colitis through gut microbiota re-establishment. World J. Gastroenterol. 2017, 23, 8570–8581. [Google Scholar] [CrossRef]
- Tailford, L.E.; Owen, C.D.; Walshaw, J.; Crost, E.H.; Hardy-Goddard, J.; Le Gall, G.; de Vos, W.M.; Taylor, G.L.; Juge, N. Discovery of intramolecular trans-sialidases in human gut microbiota suggests novel mechanisms of mucosal adaptation. Nat. Commun. 2015, 6, 7624. [Google Scholar] [CrossRef] [Green Version]
- Devlin, A.S.; Fischbach, M.A. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat. Chem. Biol. 2015, 11, 685–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsen, M.; Madelen Saunders, C.; Leena Angell, I.; Arntzen, M.; Lødrup Carlsen, K.C.; Carlsen, K.H.; Haugen, G.; Hagen, L.H.; Carlsen, M.H.; Hedlin, G.; et al. Butyrate Levels in the Transition from an Infant- to an Adult-Like Gut Microbiota Correlate with Bacterial Networks Associated with Eubacterium Rectale and Ruminococcus Gnavus. Genes 2020, 11, 1245. [Google Scholar] [CrossRef] [PubMed]
- Kentache, T.; Thabault, L.; Deumer, G.; Haufroid, V.; Frédérick, R.; Linster, C.L.; Peracchi, A.; Veiga-da-Cunha, M.; Bommer, G.T.; Van Schaftingen, E. The metalloprotein YhcH is an anomerase providing N-acetylneuraminate aldolase with the open form of its substrate. J. Biol. Chem. 2021, 296, 100699. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Sugawara, M.; Kawakami, H. Sialic acid in human milk: Composition and functions. Acta Paediatr. Taiwan 2001, 42, 11–17. [Google Scholar]
- Marcobal, A.; Sonnenburg, J.L. Human milk oligosaccharide consumption by intestinal microbiota. Clin. Microbiol. Infect. 2012, 18, 12–15. [Google Scholar] [CrossRef] [Green Version]
- Kitaoka, M. Bifidobacterial enzymes involved in the metabolism of human milk oligosaccharides. Adv. Nutr. 2012, 3, 422s–429s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiner, M. Metaproteomics: Much More than Measuring Gene Expression in Microbial Communities. mSystems 2019, 4, e00115-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Bacterial Organism 1 | HM 2 | SD | MF 2 | SD | log2 FC 3 | p-Value 4 |
|---|---|---|---|---|---|---|
| Bacteroides clarus | 6.9 | 27.1 | 95.8 | 68.4 | −3.8 | <0.0001 |
| Bacteroides stercoris CAG:120 | 3.4 | 13.3 | 44.5 | 33.9 | −3.7 | 0.0028 |
| Bacteroides sp. AF35-22 | 39.2 | 49.2 | 266.5 | 194.2 | −2.8 | 0.0076 |
| Clostridium clostridioforme CAG:132 | 3.0 | 6.2 | 21.7 | 21.7 | −2.9 | 0.0076 |
| Bacteroides stercoris ATCC 43183 | 4.0 | 15.7 | 35.9 | 30.4 | −3.2 | 0.0112 |
| Bacteroides sp. AM44-19 | 57.6 | 62.1 | 312.0 | 229.9 | −2.4 | 0.0131 |
| Firmicutes bacterium CAG:129 | 1.8 | 6.9 | 18.0 | 12.8 | −3.3 | 0.0159 |
| Firmicutes bacterium CAG:137 | 2.5 | 11.2 | 78.9 | 82.5 | −5.0 | 0.0164 |
| Firmicutes bacterium CAG:176 | 2.2 | 3.8 | 17.0 | 16.0 | −3.0 | 0.0197 |
| Bacteroides clarus YIT 12056 | 3.4 | 13.3 | 32.0 | 30.7 | −3.2 | 0.0375 |
| (Clostridium) clostridioforme 90A7 | 6.7 | 7.9 | 26.9 | 27.2 | −2.0 | 0.0375 |
| Clostridium bolteae (strain ATCC BAA-613/WAL 16351) | 2.5 | 6.4 | 16.2 | 15.0 | −2.7 | 0.0375 |
| (Ruminococcus) lactaris | 135.1 | 113.0 | 3.6 | 10.9 | 5.2 | <0.0001 |
| Firmicutes bacterium OM07-11 | 82.1 | 51.3 | 2.5 | 5.4 | 5.1 | 0.0001 |
| Ruminococcus gnavus | 303.5 | 202.5 | 7.1 | 15.4 | 5.4 | 0.0001 |
| Firmicutes bacterium AM41-5BH | 199.0 | 119.8 | 15.6 | 15.9 | 3.7 | 0.0001 |
| Mediterraneibacter sp. gm002 | 47.7 | 39.0 | 4.5 | 7.7 | 3.4 | 0.0009 |
| Firmicutes bacterium AM43-11BH | 275.4 | 158.2 | 19.5 | 18.6 | 3.8 | 0.0012 |
| (Ruminococcus) torques ATCC 27756 | 73.2 | 67.4 | 3.2 | 11.2 | 4.5 | 0.0028 |
| Firmicutes bacterium AM31-12AC | 209.2 | 169.6 | 6.4 | 10.8 | 5.0 | 0.0067 |
| Firmicutes bacterium CAG:212 | 116.8 | 57.1 | 3.5 | 8.3 | 5.0 | 0.0087 |
| Faecalicatena orotica | 50.3 | 38.1 | 6.2 | 12.6 | 3.0 | 0.0131 |
| Selenomonas ruminantium | 233.3 | 140.5 | 41.9 | 106.8 | 2.5 | 0.0152 |
| Dorea sp. CAG:317 | 13.1 | 11.0 | 2.0 | 5.1 | 2.7 | 0.0485 |
| Hespellia stercorisuis DSM 15480 | 12.8 | 11.7 | 0.0 | 0.0 | NA | 0.0152 |
| Lachnospiraceae bacterium AM40-2BH | 139.2 | 116.6 | 33.0 | 51.5 | 2.1 | 0.0152 |
| Faecalicatena contorta | 164.5 | 122.5 | 33.1 | 41.5 | 2.3 | 0.0174 |
| Lachnospiraceae bacterium 2_1_58FAA | 88.4 | 73.1 | 0.3 | 0.9 | 8.3 | 0.0197 |
| Lachnospiraceae bacterium TM07-2AC | 117.5 | 112.5 | 0.0 | 0.0 | NA | 0.0197 |
| Dorea sp. OM07-5 | 44.0 | 36.5 | 0.0 | 0.0 | NA | 0.0389 |
| Lachnospiraceae bacterium OF09-33XD | 18.8 | 20.5 | 0.0 | 0.0 | NA | 0.0482 |
| Uniprot_ID | Organism | Protein Name | HM 1 (sum) | MF 1 (sum) | HM 2 | MF 2 | Log2 FC 3 | p-Value 4 |
|---|---|---|---|---|---|---|---|---|
| A0A412VME7 | Phocaeicola vulgatus (Bacteroides vulgatus) | RagB/SusD family nutrient uptake outer membrane protein | 51 | 573 | 3.4 | 47.5 | −3.8 | 0.002 |
| A0A412VME7 | Phocaeicola dorei | RagB/SusD family nutrient uptake outer membrane protein | 51 | 573 | 3.4 | 45.9 | −3.8 | 0.008 |
| R9H865 | Bacteroides vulgatus CL09T03C04 | Uncharacterized protein | 1783 | 111 | 3.4 | 32.9 | −3.3 | 0.03 |
| C6Z771 | Bacteroides sp. 4_3_47FAA | SusD family protein | 51 | 353 | 3.4 | 32.9 | −3.3 | 0.03 |
| A0A1Y3Z4G2 | Bacteroides clarus | Polyribonucleotide nucleotidyltransferase (EC 2.7.7.8) (polynucleotide phosphorylase) (PNPase) | 44 | 386 | 3.7 | 35.4 | −3.2 | 0.05 |
| A0A397WJ15 | Phocaeicola vulgatus (Bacteroides vulgatus) | Beta-galactosidase (EC 3.2.1.23) | 107 | 614 | 8.4 | 52.2 | −2.6 | 0.02 |
| A0A415SZ11 | Phocaeicola vulgatus (Bacteroides vulgatus) | Malate dehydrogenase (EC 1.1.1.37) | 1008 | 1997 | 92.3 | 180.3 | −1 | 0.03 |
| C3RBX6 | Bacteroides vulgatus CL09T03C04 | 60 kDa chaperonin (GroEL protein) (protein Cpn60) | 3254 | 1195 | 245.9 | 118.3 | 1.1 | 0.03 |
| C3RBX6 | Bacteroides sp. AM18-9 | 60 kDa chaperonin (GroEL protein) (protein Cpn60) | 3254 | 1195 | 245.9 | 118.3 | 1.1 | 0.03 |
| A0A069SFK4 | Bacteroides vulgatus str. 3975 RP4 | TonB-linked outer membrane, SusC/RagA family protein | 1370 | 468 | 103.2 | 37.4 | 1.5 | 0.02 |
| A0A069SKV9 | Bacteroides vulgatus str. 3975 RP4 | Tetracycline resistance protein TetQ | 3792 | 1001 | 302.9 | 96.4 | 1.7 | 0.03 |
| A0A069SKV9 | Bacteroides sp. AF32-15BH | Tetracycline resistance protein TetQ | 3792 | 1001 | 302.9 | 96.4 | 1.7 | 0.03 |
| A0A1H6GDE8 | Selenomonas ruminantium | Phosphoenolpyruvate carboxykinase (ATP) (PCK) (PEP carboxykinase) (PEPCK) (EC 4.1.1.49) | 2383 | 519 | 238.1 | 43.5 | 2.5 | 0.04 |
| A0A174QRN1 | Phocaeicola vulgatus (Bacteroides vulgatus) | DUF1735 domain-containing protein (galactose oxidase) (EC 3.2.1.18) | 171 | 29 | 13.1 | 2 | 2.7 | 0.03 |
| I9UCD9 | Phocaeicola vulgatus (Bacteroides vulgatus) | SusC/RagA family TonB-linked outer membrane protein | 1370 | 469 | 32.3 | 4.7 | 2.8 | 0.03 |
| A0A417FRM0 | Firmicutes bacterium AM31-12AC | LacI family transcriptional regulator | 251 | 35 | 24.5 | 2.8 | 3.1 | 0.02 |
| A0A397WGI7 | Phocaeicola vulgatus (Bacteroides vulgatus) | RagB/SusD family nutrient uptake outer membrane protein (starch-binding associating with outer membrane) | 1087 | 111 | 96.8 | 10.5 | 3.2 | 0.02 |
| A0A316A2W0 | Faecalicatena contorta | Phosphoglycerate kinase (EC 2.7.2.3) | 1142 | 96 | 107.1 | 8.7 | 3.6 | 0.002 |
| R9H865 | Bacteroides vulgatus dnLKV7 | Uncharacterized protein | 1783 | 111 | 128.5 | 10.5 | 3.6 | 0.01 |
| A0A417FRM0 | Firmicutes bacterium AM43-11BH | LacI family transcriptional regulator | 251 | 35 | 21.6 | 1.5 | 3.8 | 0.05 |
| R5ICG7 | Firmicutes bacterium CAG:124 | L-fucose isomerase (FucIase) (EC 5.3.1.25) (6-deoxy-L-galactose isomerase) | 937 | 0 | 84.9 | 0 | NA | <0.0001 |
| A0A3C1BZY4 | Clostridiales bacterium | Carbon monoxide dehydrogenase (EC 1.2.7.4) | 0 | 535 | 0 | 53.7 | NA | 0.007 |
| F0Z4P0 | Clostridium sp. D5 | Acetate kinase (EC 2.7.2.1) (acetokinase) | 296 | 0 | 25.9 | 0 | NA | 0.008 |
| A0A374BZR2 | Firmicutes bacterium AM31-12AC | D-ribose pyranase (EC 5.4.99.62) | 155 | 0 | 13.1 | 0 | NA | 0.02 |
| A0A417BM01 | Firmicutes bacterium AM43-11BH | UPF0210 protein DW928_02850 | 148 | 0 | 12.9 | 0 | NA | 0.02 |
| A0A2N5NQW2 | Ruminococcus gnavus | Class II fructose-1,6-bisphosphate aldolase (EC 4.1.2.13) (fructose-1,6-bisphosphate aldolase, class II) | 777 | 0 | 78.2 | 0 | NA | 0.02 |
| A0A494ZWH6 | Ruminococcus sp. B05 | UPF0210 protein D8Q48_03220 | 148 | 0 | 12.9 | 0 | NA | 0.02 |
| A0A374BZR2 | Mediterraneibacter sp. gm002 | D-ribose pyranase (EC 5.4.99.62) | 155 | 0 | 13.1 | 0 | NA | 0.02 |
| A0A316A2W0 | Dorea sp. CAG:105 | Phosphoglycerate kinase (EC 2.7.2.3) | 1142 | 96 | 47.3 | 0 | NA | 0.02 |
| F0Z4P0 | Lachnospiraceae bacterium 1_4_56FAA | Acetate kinase (EC 2.7.2.1) (acetokinase) | 296 | 0 | 18.6 | 0 | NA | 0.03 |
| A0A3A6G758 | Lachnospiraceae bacterium TM07-2AC | Class II fructose-1,6-bisphosphate aldolase (EC 4.1.2.13) | 776 | 0 | 78.1 | 0 | NA | 0.03 |
| A0A374BZR2 | Ruminococcus sp. AM42-11 | D-ribose pyranase (EC 5.4.99.62) | 155 | 0 | 12 | 0 | NA | 0.04 |
| C3RBX6 | Firmicutes bacterium AM43-11BH | 60 kDa chaperonin (GroEL protein) (protein Cpn60) | 3254 | 1195 | 107.2 | 0 | NA | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, F.; Zybailov, B.L.; Glazko, G.V.; Rahmatallah, Y.; Byrum, S.; Mackintosh, S.G.; Bowlin, A.K.; Yeruva, L. Milk Formula Diet Alters Bacterial and Host Protein Profile in Comparison to Human Milk Diet in Neonatal Piglet Model. Nutrients 2021, 13, 3718. https://doi.org/10.3390/nu13113718
Rosa F, Zybailov BL, Glazko GV, Rahmatallah Y, Byrum S, Mackintosh SG, Bowlin AK, Yeruva L. Milk Formula Diet Alters Bacterial and Host Protein Profile in Comparison to Human Milk Diet in Neonatal Piglet Model. Nutrients. 2021; 13(11):3718. https://doi.org/10.3390/nu13113718
Chicago/Turabian StyleRosa, Fernanda, Boris L. Zybailov, Galina V. Glazko, Yasir Rahmatallah, Stephanie Byrum, Samuel G. Mackintosh, Anne K. Bowlin, and Laxmi Yeruva. 2021. "Milk Formula Diet Alters Bacterial and Host Protein Profile in Comparison to Human Milk Diet in Neonatal Piglet Model" Nutrients 13, no. 11: 3718. https://doi.org/10.3390/nu13113718
APA StyleRosa, F., Zybailov, B. L., Glazko, G. V., Rahmatallah, Y., Byrum, S., Mackintosh, S. G., Bowlin, A. K., & Yeruva, L. (2021). Milk Formula Diet Alters Bacterial and Host Protein Profile in Comparison to Human Milk Diet in Neonatal Piglet Model. Nutrients, 13(11), 3718. https://doi.org/10.3390/nu13113718






