A Novel Multi-Ingredient Supplement Activates a Browning Program in White Adipose Tissue and Mitigates Weight Gain in High-Fat Diet-Fed Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Dietary Interventions
2.2. Preliminary Study to Examine Effect of Supplementation (Experimental Approach 1)
2.3. Study to Determine the Effect of Combinatory Supplementation (Experimental Approach 2)
2.4. Metabolic Measurements In Vivo Following Short-Term Feeding (Experimental Approach 3)
2.5. Food Consumption Tracking
2.6. In Vivo Measures of Adiposity and Relative Lean Mass
2.7. Maximal Running Capacity Exercise Test
2.8. Structured Endurance Exercise Intervention
2.9. Animal Euthanasia
2.10. Mitochondrial Respiration in Permeabilized Muscle Fiber Bundles
2.11. Immunoblotting
2.12. RNA Isolation and cDNA Synthesis
2.13. Real-Time Quantitative PCR (RT-qPCR)
2.14. High-Sensitivity Immunology Multiplex Assay
2.15. Cytochrome c Oxidase (COX), Citrate Synthase (CS), and Short Chain β-Hydroxy-Acyl-CoA-Dehydrogenase (β-HAD) Enzyme Activity Assays
2.16. Histological Analysis
2.17. Statistical Analysis
3. Results
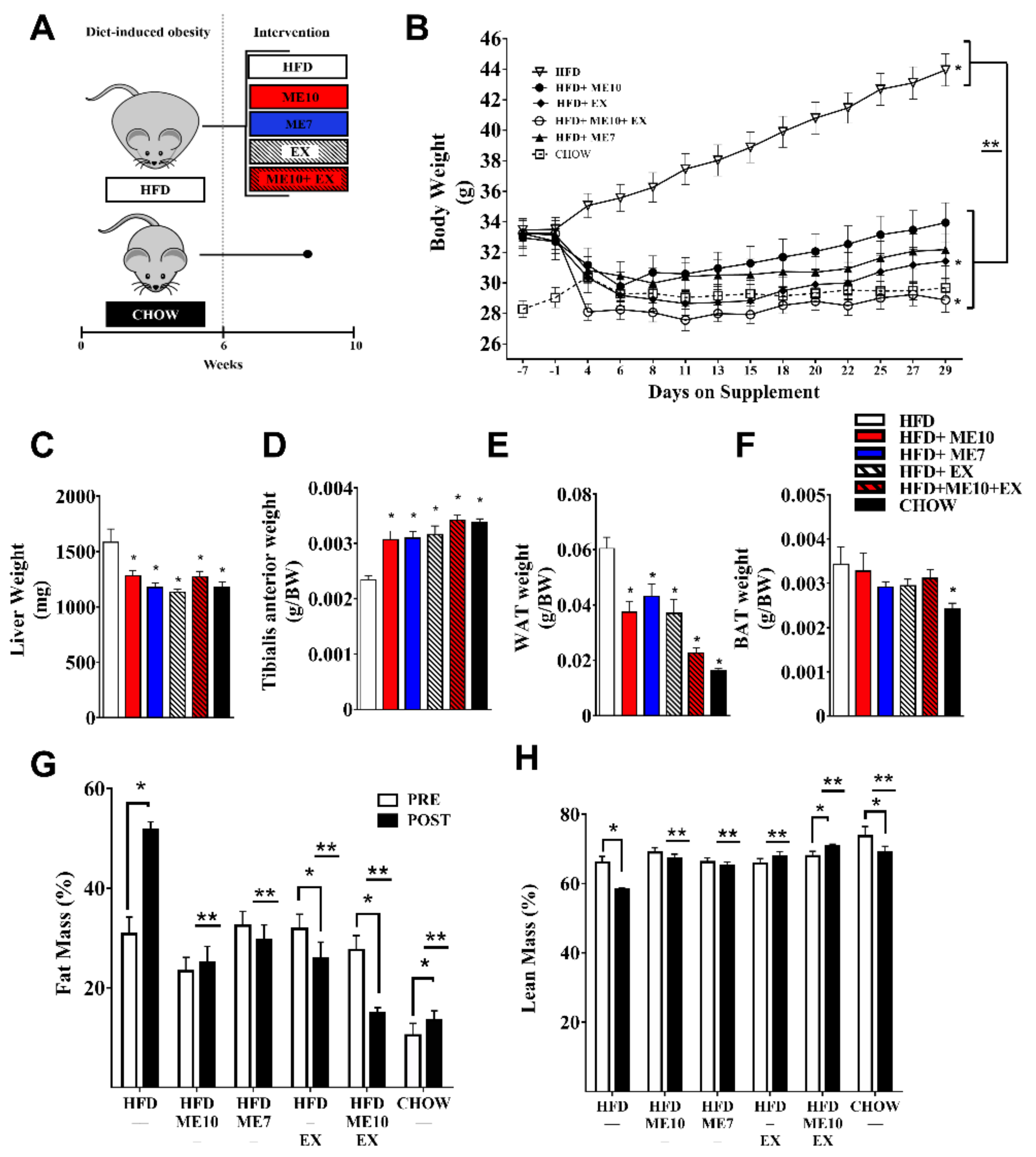
3.1. Supplementation with ME10 and/or ME7 Attenuated Accumulation of Fat Mass and Preserved Lean Body Mass, Which Was Further Enhanced When Combined with Exercise
3.2. Weight Loss Not Exclusively Dependent on Appetite Suppresion
3.3. Deleterious Impact of HFD-Induced Weight Gain on Exercise Ameliorated with Supplementation
3.4. Exercise and Supplementation Upregulated Mitochondrial Biogenesis and mRNA Abundance of Markers of the Browning Program in WAT
3.5. Supplementation and Exercise Increased SOD1 Antioxidant Defense in WAT
3.6. Exercise and Supplementation Improved WAT Lipid Transport and Metabolism mRNA Expression
3.7. OXPHOS Protein Expression Was Altered in a Complex-Specific Manner in BAT Following Exercise and Supplementation
3.8. ME7 Supplementation Improved OXHOS Protein Expression in Inguinal White Adipose Tissue Depots in a Similar Manner to Epidydimal WAT
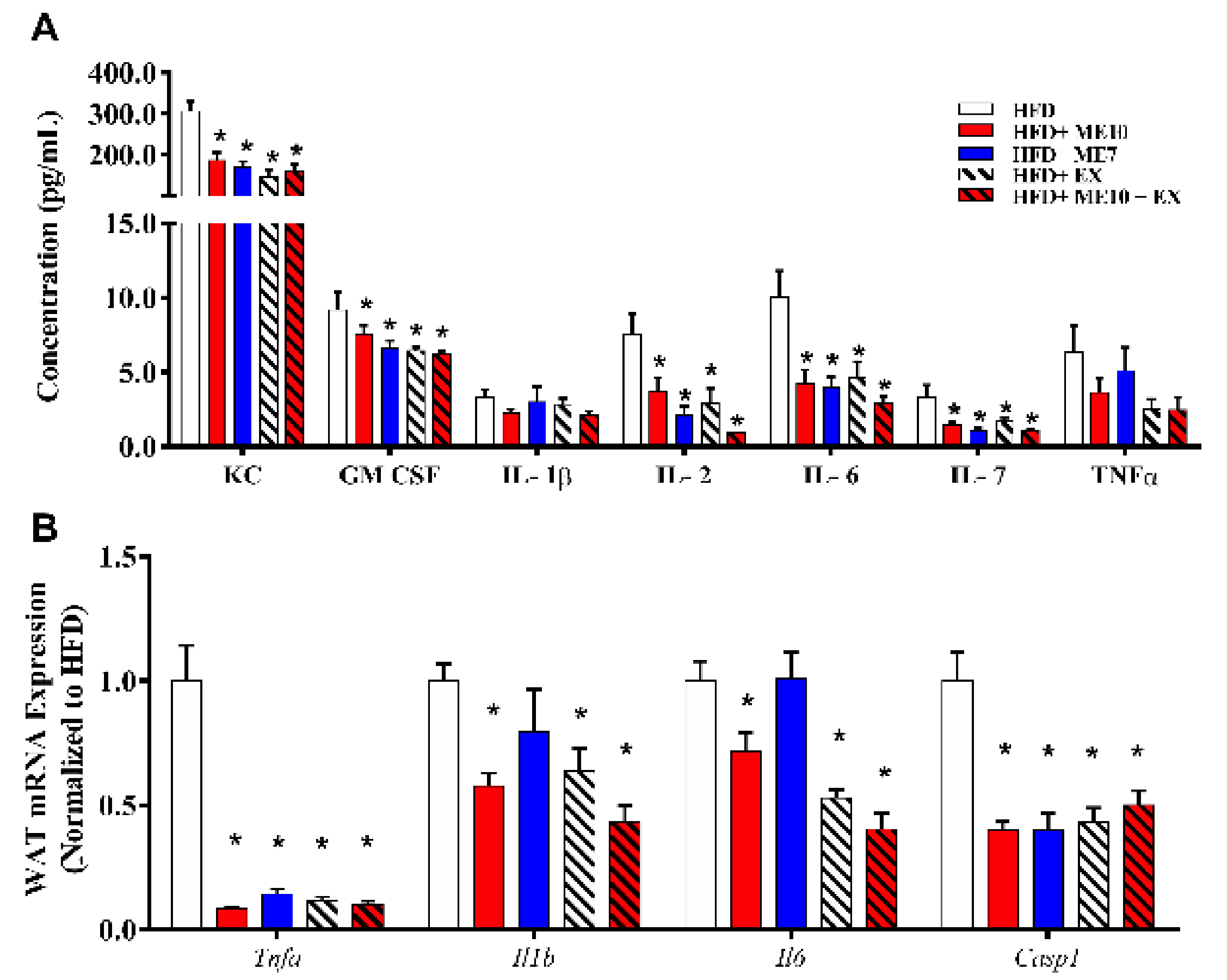
3.9. Exercise and Supplementation with ME7 and ME10 Limits Inflammation in WAT and Systemic Circulation
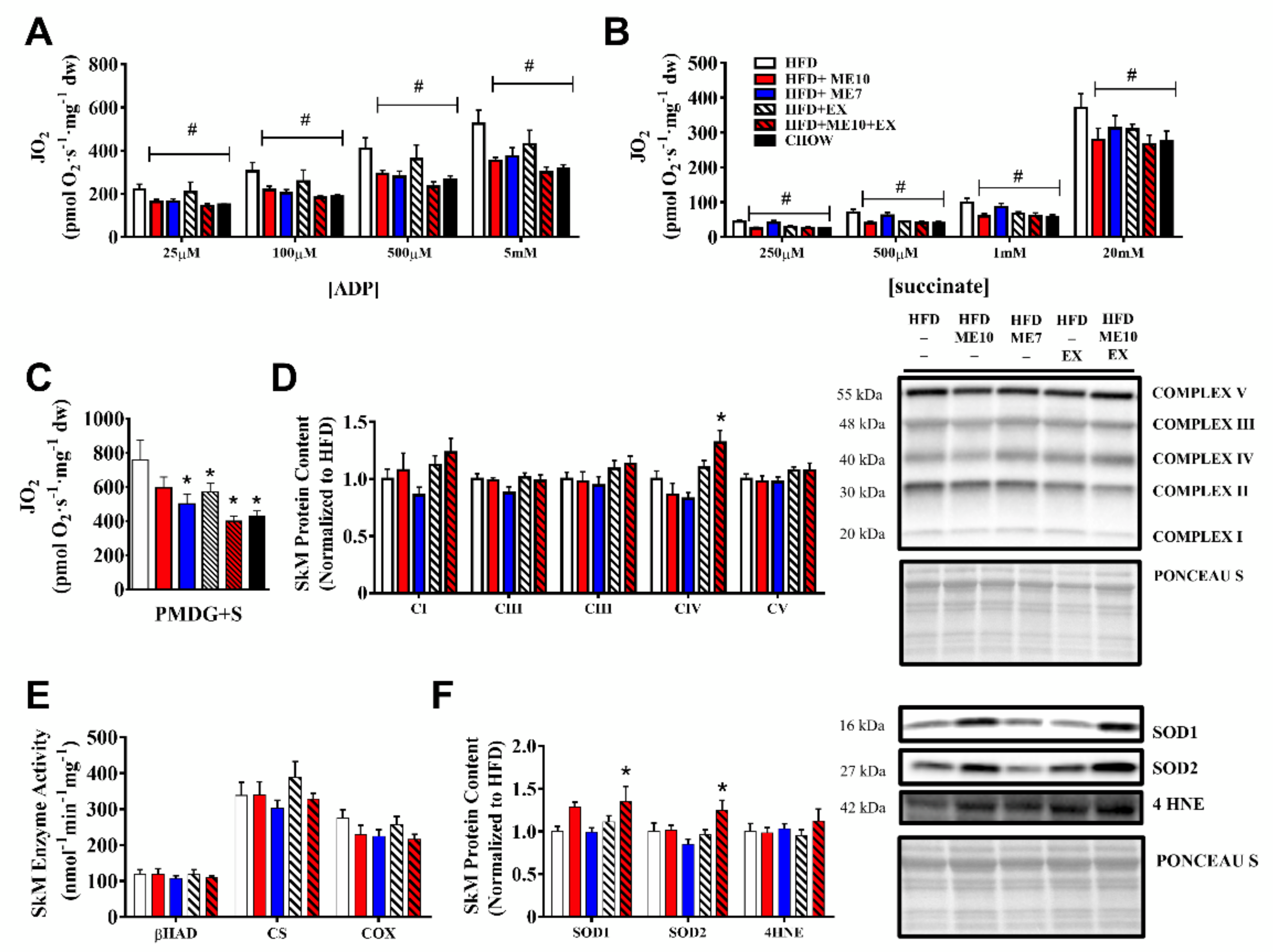
3.10. Exercise and Supplementation Ameliorated the Alteration of Mitochondrial Function in SkM Compared to HFD
3.11. Improvement in SkM Mitochondrial Respiration Independent of Mitochondrial Protein Expression Following Supplementation
3.12. Supplementation and Exercise Increased SOD1 Antioxidant Defense in SkM
3.13. In Vivo Lipid Oxidation Rates, Animal Activity, and Tissue-Specific AMPK Phosphorylation Increased Following a Short-Term (3d) Supplementation Period
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018 Key findings Data from the National Health and Nutrition Examination Survey. NCHS Data Brief 2020, 360, 1–8. [Google Scholar]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Lisheng, L.; et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet 2005, 366, 1640–1649. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webber, J. Energy balance in obesity. Proc. Nutr. Soc. 2003, 62, 539–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity & inflammation: The linking mechanism & the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Bournat, J.C.; Brown, C.W. Mitochondrial dysfunction in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 446–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisoli, E.; Clementi, E.; Carruba, M.O.; Moncada, S. Defective mitochondrial biogenesis: A hallmark of the high cardiovascular risk in the metabolic syndrome? Circ. Res. 2007, 100, 795–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Berg, A.H.; Iyengar, P.; Lam, T.K.T.; Giacca, A.; Combs, T.P.; Rajala, M.W.; Du, X.; Rollman, B.; Li, W.; et al. The hyperglycemia-induced inflammatory response in adipocytes: The role of reactive oxygen species. J. Biol. Chem. 2005, 280, 4617–4626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Justin Milner, J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedergaard, J.; Cannon, B. The Browning of White Adipose Tissue: Some Burning Issues. Cell Metab. 2014, 20, 396–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and beige fat: Physiological roles beyond heat generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, G.; Apovian, C.M. Current pharmacotherapy for obesity. Nat. Rev. Endocrinol. 2017, 14, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Cheung, B.M.Y.; Cheung, T.T.; Samaranayake, N.R. Safety of antiobesity drugs. Ther. Adv. Drug Saf. 2013, 4, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemstra, M.; Bird, Y.; Nwankwo, C.; Rogers, M.; Moraros, J. Weight loss intervention adherence and factors promoting adherence: A meta-analysis. Patient Prefer. Adherence 2016, 10, 1547–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, R.A.; Gannon, N.P.; Carriker, C.R. Nitrate-containing beetroot enhances myocyte metabolism and mitochondrial content. J. Tradit. Complement. Med. 2016, 6, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Tarnopolsky, M.A.; Roy, B.D.; MacDonald, J.R. A randomized, controlled trial of creatine monohydrate in patients with mitochondrial cytopathies. Muscle Nerve 1997, 20, 1502–1509. [Google Scholar] [CrossRef]
- Xing, L.W.; Rainwater, D.L.; Mahaney, M.C.; Stocker, R. Cosupplementation with vitamin E and coenzyme Q10 reduces circulating markers of inflammation in baboons. Am. J. Clin. Nutr. 2004, 80, 649–655. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, J.O.; Kim, J.H.; Kim, N.; You, G.Y.; Moon, J.W.; Sha, J.; Kim, S.J.; Lee, Y.W.; Kang, H.J.; et al. Coenzyme Q10 increases the fatty acid oxidation through AMPK-mediated PPARα induction in 3T3-L1 preadipocytes. Cell. Signal. 2012, 24, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Kucukgoncu, S.; Zhou, E.; Lucas, K.B.; Tek, C. Alpha-lipoic acid (ALA) as a supplementation for weight loss: Results from a meta-analysis of randomized controlled trials. Obes. Rev. 2017, 18, 594–601. [Google Scholar] [CrossRef] [Green Version]
- Litosch, I.; Hudson, T.H.; Mills, I.; Li, S.Y.; Fain, J.N. Forskolin as an activator of cyclic AMP accumulation and lipolysis in rat adipocytes. Mol. Pharmacol. 1982, 22, 109–115. [Google Scholar] [PubMed]
- Thom, E. The Effect of Chlorogenic Acid Enriched Coffee on Glucose Absorption in Healthy Volunteers and Its Effect on Body Mass When Used Long-term in Overweight and Obese People. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef]
- Chow, C.K. Vitamin E Regulation of Mitochondrial Superoxide Generation. Neurosignals 2001, 10, 112–124. [Google Scholar] [CrossRef]
- Park, Y.; Albright, K.J.; Liu, W.; Storkson, J.M.; Cook, M.E.; Pariza, M.W. Effect of conjugated linoleic acid on body composition in mice. Lipids 1997, 32, 853–858. [Google Scholar] [CrossRef]
- Hsu, C.H.; Tsai, T.H.; Kao, Y.H.; Hwang, K.C.; Tseng, T.Y.; Chou, P. Effect of green tea extract on obese women: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2008, 27, 363–370. [Google Scholar] [CrossRef]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallén, J.; Rønnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Østgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Park, J.Y.; Namkoong, C.; Jang, P.G.; Ryu, J.W.; Song, H.S.; Yun, J.Y.; Namgoong, I.S.; Ha, J.; Park, I.S.; et al. Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat. Med. 2004, 10, 727–733. [Google Scholar] [CrossRef]
- Chen, N.; Bezzina, R.; Hinch, E.; Lewandowski, P.A.; Cameron-Smith, D.; Mathai, M.L.; Jois, M.; Sinclair, A.J.; Begg, D.P.; Wark, J.D.; et al. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutr. Res. 2009, 29, 784–793. [Google Scholar] [CrossRef]
- Vinson, J.A.; Burnham, B.R.; Nagendran, M. V to evaluate the efficacy and safety of a green coffee bean extract in overweight subjects. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 5, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Mottillo, E.P.; Desjardins, E.M.; Crane, J.D.; Smith, B.K.; Green, A.E.; Ducommun, S.; Henriksen, T.I.; Rebalka, I.A.; Razi, A.; Sakamoto, K.; et al. Lack of Adipocyte AMPK Exacerbates Insulin Resistance and Hepatic Steatosis through Brown and Beige Adipose Tissue Function. Cell Metab. 2016, 24, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, R. Reduction in Obesity and Related Comorbid Conditions after Diet-Induced Weight Loss or Exercise-Induced Weight Loss in Men. Ann. Intern. Med. 2000, 133, 92. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, M.D.; Ford, R.J.; McGregor, C.P.; LeBlond, N.D.; Snider, S.A.; Stypa, S.A.; Day, E.A.; Lhoták, Š.; Schertzer, J.D.; Austin, R.C.; et al. Salicylate improves macrophage cholesterol homeostasis via activation of Ampk. J. Lipid Res. 2015, 56, 1025–1033. [Google Scholar] [CrossRef] [Green Version]
- Perry, C.G.R.; Kane, D.A.; Lanza, I.R.; Neufer, P.D. Methods for assessing mitochondrial function in diabetes. Diabetes 2013, 62, 1041–1053. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Gong, H.; Sun, L.; Chen, B.; Han, Y.; Pang, J.; Wu, W.; Qi, R.; Zhang, T.M. Evaluation of candidate reference genes for RT-qPCR studies in three metabolism related tissues of mice after caloric restriction. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, S.L.; Rennie, C.; Tarnopolsky, M.A. Substrate utilization during endurance exercise in men and women after endurance training. Am. J. Physiol. Endocrinol. Metab. 2001, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2015, 16, 378–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samjoo, I.A.; Safdar, A.; Hamadeh, M.J.; Raha, S.; Tarnopolsky, M.A. The effect of endurance exercise on both skeletal muscle and systemic oxidative stress in previously sedentary obese men. Nutr. Diabetes 2013, 3, e88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, H.K.; Powers, S.K.; Demirel, H.A.; Coombes, J.S.; Naito, H. Exercise training protects against contraction-induced lipid peroxidation in the diaphragm. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Wanders, R.J.A. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowell, B.B.; Susulic, V.S.; Hamann, A.; Lawitts, J.A.; Himms-Hagen, J.; Boyer, B.B.; Kozak, L.P.; Flier, J.S. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993, 366, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Sepa-Kishi, D.M.; Ceddia, R.B. Exercise-Mediated Effects on White and Brown Adipose Tissue Plasticity and Metabolism. Exerc. Sport Sci. Rev. 2016, 44, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Surmi, B.K.; Hasty, A.H. Macrophage infiltration into adipose tissue: Initiation, propagation and remodeling. Future Lipidol. 2008, 3, 545–556. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, M.S.; Miyoshi, H.; Souza, S.C.; Cacicedo, J.M.; Saha, A.K.; Greenberg, A.S.; Ruderman, N.B. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: Potential mechanism and physiological relevance. J. Biol. Chem. 2008, 283, 16514–16524. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Ghoshal, S.; Porter, T.D. Phosphorylation of hepatic AMP-activated protein kinase and liver kinase B1 is increased after a single oral dose of green tea extract to mice. Nutr. Res. 2012, 32, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Fisher, F.F.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohl, E.E.; Rupprecht, A.; Macher, G.; Hilse, K.E. Important trends in UCP3 investigation. Front. Physiol. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Hilse, K.E.; Kalinovich, A.V.; Rupprecht, A.; Smorodchenko, A.; Zeitz, U.; Staniek, K.; Erben, R.G.; Pohl, E.E. The expression of UCP3 directly correlates to UCP1 abundance in brown adipose tissue. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Toime, L.J.; Brand, M.D. Uncoupling protein-3 lowers reactive oxygen species production in isolated mitochondria. Free Radic. Biol. Med. 2010, 49, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uldry, M.; Yang, W.; St-Pierre, J.; Lin, J.; Seale, P.; Spiegelman, B.M. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006, 3, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiner, S.; Mepani, R.J.; Laznik, D.; Ye, L.; Jurczak, M.J.; Jornayvaz, F.R.; Estall, J.L.; Bhowmick, D.C.; Shulman, G.I.; Spiegelman, B.M. Development of insulin resistance in mice lacking PGC-1α in adipose tissues. Proc. Natl. Acad. Sci. USA 2012, 109, 9635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldiss, P.; Betts, J.; Sale, C.; Pope, M.; Budge, H.; Symonds, M.E. Exercise-induced ‘browning’ of adipose tissues. Metabolism 2018, 81, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Stanford, K.I.; Middelbeek, R.J.W.; Townsend, K.L.; Lee, M.Y.; Takahashi, H.; So, K.; Hitchcox, K.M.; Markan, K.R.; Hellbach, K.; Hirshman, M.F.; et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 2015, 64, 2002–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanford, K.I.; Middelbeek, R.J.W.; Goodyear, L.J. Exercise effects on white adipose tissue: Beiging and metabolic adaptations. Diabetes 2015, 64, 2361–2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Kazak, L.; Rahbani, J.F.; Samborska, B.; Lu, G.Z.; Jedrychowski, M.P.; Lajoie, M.; Zhang, S.; Ramsay, L.A.; Dou, F.Y.; Tenen, D.; et al. Ablation of adipocyte creatine transport impairs thermogenesis and causes diet-induced obesity. Nat. Metab. 2019, 1, 360–370. [Google Scholar] [CrossRef]
- Kazak, L.; Chouchani, E.T.; Lu, G.Z.; Jedrychowski, M.P.; Bare, C.J.; Mina, A.I.; Kumari, M.; Zhang, S.; Vuckovic, I.; Laznik-Bogoslavski, D.; et al. Genetic Depletion of Adipocyte Creatine Metabolism Inhibits Diet-Induced Thermogenesis and Drives Obesity. Cell Metab. 2017, 26, 660–671. [Google Scholar] [CrossRef] [Green Version]
- Kazak, L.; Chouchani, E.T.; Jedrychowski, M.P.; Erickson, B.K.; Shinoda, K.; Cohen, P.; Vetrivelan, R.; Lu, G.Z.; Laznik-Bogoslavski, D.; Hasenfuss, S.C.; et al. A Creatine-Driven Substrate Cycle Enhances Energy Expenditure and Thermogenesis in Beige Fat. Cell 2015, 163, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Bertholet, A.M.; Kazak, L.; Chouchani, E.T.; Bogaczyńska, M.G.; Paranjpe, I.; Wainwright, G.L.; Bétourné, A.; Kajimura, S.; Spiegelman, B.M.; Kirichok, Y. Mitochondrial Patch Clamp of Beige Adipocytes Reveals UCP1-Positive and UCP1-Negative Cells Both Exhibiting Futile Creatine Cycling. Cell Metab. 2017, 25, 811–822. [Google Scholar] [CrossRef] [Green Version]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- De Matteis, R.; Lucertini, F.; Guescini, M.; Polidori, E.; Zeppa, S.; Stocchi, V.; Cinti, S.; Cuppini, R. Exercise as a new physiological stimulus for brown adipose tissue activity. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 582–590. [Google Scholar] [CrossRef]
- Wu, M.V.; Bikopoulos, G.; Hung, S.; Ceddia, R.B. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: Impact on whole-body energy expenditure. J. Biol. Chem. 2014, 289, 34129–34140. [Google Scholar] [CrossRef] [Green Version]
- Fromme, T.; Klingenspor, M. Uncoupling protein 1 expression and high-fat diets. Am. J. Physiol. 2011, 300, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dewal, R.S.; Stanford, K.I. Effects of exercise on brown and beige adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Barneda, D.; Planas-Iglesias, J.; Gaspar, M.L.; Mohammadyani, D.; Prasannan, S.; Dormann, D.; Han, G.-S.; Jesch, S.A.; Carman, G.M.; Kagan, V.; et al. The brown adipocyte protein CIDEA promotes lipid droplet fusion via a phosphatidic acid-binding amphipathic helix. eLife 2015, 4, e07485. [Google Scholar] [CrossRef] [Green Version]
- Qiao, L.; Yoo, H.S.; Bosco, C.; Lee, B.; Feng, G.S.; Schaack, J.; Chi, N.W.; Shao, J. Adiponectin reduces thermogenesis by inhibiting brown adipose tissue activation in mice. Diabetologia 2014, 57, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Masand, R.; Paulo, E.; Wu, D.; Wang, Y.; Swaney, D.L.; Jimenez-Morales, D.; Krogan, N.J.; Wang, B. Proteome Imbalance of Mitochondrial Electron Transport Chain in Brown Adipocytes Leads to Metabolic Benefits. Cell Metab. 2018, 27, 616–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Liang, X.; Sun, X.; Zhang, L.; Fu, X.; Rogers, C.J.; Berim, A.; Zhang, S.; Wang, S.; Wang, B.; et al. AMPK/α-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell Metab. 2016, 24, 542–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, A.K.; Ruderman, N.B. Malonyl-CoA and AMP-activated protein kinase: An expanding partnership. Mol. Cell. Biochem. 2003, 253, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, E.M.; Steinberg, G.R. Emerging Role of AMPK in Brown and Beige Adipose Tissue (BAT): Implications for Obesity, Insulin Resistance, and Type 2 Diabetes. Curr. Diab. Rep. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Root-Mccaig, J.; Castellani, L.; Kemp, B.E.; Steinberg, G.R.; Wright, D.C. Evidence for the role of AMPK in regulating PGC-1 alpha expression and mitochondrial proteins in mouse epididymal adipose tissue. Obesity 2014, 22, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kaushik, V.K.; Constant, S.; Prentki, M.; Przybytkowski, E.; Ruderman, N.; Saha, A.K. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J. Biol. Chem. 2002, 277, 32571–32577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfram, S.; Wang, Y.; Thielecke, F. Anti-obesity effects of green tea: From bedside to bench. Mol. Nutr. Food Res. 2006, 50, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Wang, L.; Wang, L.; Tanaka, Y.; Zhang, T.; Ashida, H. Oolong, black and pu-erh tea suppresses adiposity in mice via activation of AMP-activated protein kinase. Food Funct. 2014, 5, 2420–2429. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Y.; Xie, Z.; Zhou, Y.; Zhang, Y.; Wan, X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur. J. Clin. Nutr. 2014, 68, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Seydoux, J.; Girardier, L. Potentiation of the thermogenic antiobesity effects of ephedrine by dietary methylxanthines: Adenosine antagonism or phosphodiesterase inhibition? Metabolism 1992, 41, 1233–1241. [Google Scholar] [CrossRef]
- Leijten, P.A.A.; Van Breemen, C. The Effects of Caffeine on the Noradrenaline-Sensitive Calcium Store in Rabbit Aorta. J. Physiol. 1984, 357, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Xuong, N.H.; Taylor, S.S. Crystal structure of a complex between the catalytic and regulatory (RIα) subunits of PKA. Science 2005, 307, 690–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Meng, J.; Shen, Q.; Zhang, Y.; Pan, S.; Chen, Z.; Zhu, L.Q.; Lu, Y.; Huang, Y.; Zhang, G. Caffeine inhibits hypothalamic A 1 R to excite oxytocin neuron and ameliorate dietary obesity in mice. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.M.; Irwin, C.; Seay, R.F.; Clarke, H.E.; Allegro, D.; Desbrow, B. Caffeine, coffee, and appetite control: A review. Int. J. Food Sci. Nutr. 2017, 68, 901–912. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, P.; Levack, R.; Watters, J.; Xu, Z.; Yang, Y. Caffeine increases food intake while reducing anxiety-related behaviors. Appetite 2016, 101, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.; SanMiguel, N.; López-Cruz, L.; Carratalá-Ros, C.; Olivares-García, R.; Salamone, J.D. Caffeine modulates food intake depending on the context that gives access to food: Comparison with dopamine depletion. Front. Psychiatry 2018, 9, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licholai, J.A.; Nguyen, K.P.; Fobbs, W.C.; Schuster, C.J.; Ali, M.A.; Kravitz, A.V. Why Do Mice Overeat High-Fat Diets? How High-Fat Diet Alters the Regulation of Daily Caloric Intake in Mice. Obesity 2018, 26, 1026–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, M.R.; Butryn, M.L. Hedonic hunger: A new dimension of appetite? Physiol. Behav. 2007, 91, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Van De Giessen, E.; La Fleur, S.E.; De Bruin, K.; Van Den Brink, W.; Booij, J. Free-choice and no-choice high-fat diets affect striatal dopamine D 2/3 receptor availability, caloric intake, and adiposity. Obesity 2012, 20, 1738–1740. [Google Scholar] [CrossRef] [PubMed]
- Melhorn, S.J.; Krause, E.G.; Scott, K.A.; Mooney, M.R.; Johnson, J.D.; Woods, S.C.; Sakai, R.R. Acute exposure to a high-fat diet alters meal patterns and body composition. Physiol. Behav. 2010, 99, 33. [Google Scholar] [CrossRef] [Green Version]
- Tschöp, M.H.; Speakman, J.R.; Arch, J.R.S.; Auwerx, J.; Brüning, J.C.; Chan, L.; Eckel, R.H.; Farese, R.V.; Galgani, J.E.; Hambly, C.; et al. A guide to analysis of mouse energy metabolism. Nat. Methods 2012, 9, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellacott, K.L.J.; Morton, G.J.; Woods, S.C.; Tso, P.; Schwartz, M.W. Assessment of feeding behavior in laboratory mice. Cell Metab. 2010, 12, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A.; Kravitz, A.V. Challenges in quantifying food intake in rodents. Brain Res. 2018, 1693, 188–191. [Google Scholar] [CrossRef]
- Santamarina, A.B.; Oliveira, J.L.; Silva, F.P.; Carnier, J.; Mennitti, L.V.; Santana, A.A.; de Souza, G.H.I.; Ribeiro, E.B.; Oller do Nascimento, C.M.; Lira, F.S.; et al. Green Tea Extract Rich in Epigallocatechin-3-Gallate Prevents Fatty Liver by AMPK Activation via LKB1 in Mice Fed a High-Fat Diet. PLoS ONE 2015, 10, e0141227. [Google Scholar] [CrossRef] [PubMed]
- Shivaprasad, H.N.; Gopalakrishna, S.; Mariyanna, B.; Thekkoot, M.; Reddy, R.; Tippeswamy, B.S. Effect of Coleus forskohliiextract on cafeteria diet-induced obesity in rats. Pharmacogn. Res. 2014, 6, 42–45. [Google Scholar] [CrossRef] [Green Version]
- Harada, K.; Shen, W.-J.; Patel, S.; Natu, V.; Wang, J.; Osuga, J.; Ishibashi, S.; Kraemer, F.B. Resistance to high-fat diet-induced obesity and altered expression of adipose-specific genes in HSL-deficient mice Downloaded from. Am. J. Physiol. Endocrinol. Metab. 2003, 285, 1182–1195. [Google Scholar] [CrossRef] [Green Version]
- Serra, D.; Mera, P.; Malandrino, M.I.; Mir, J.F.; Herrero, L. Mitochondrial fatty acid oxidation in obesity. Antioxid. Redox Signal. 2013, 19, 269–284. [Google Scholar] [CrossRef] [Green Version]
- Trayhurn, P.; Wood, I.S. Signalling role of adipose tissue: Adipokines and inflammation in obesity. Biochem. Soc. Trans. 2005, 33, 1078–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Greevenbroek, M.M.J.; Schalkwijk, C.G.; Stehouwer, C.D.A. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: Causes and consequences. Neth. J. Med. 2013, 71, 174–187. [Google Scholar] [PubMed]
- Senn, J.J.; Klover, P.J.; Nowak, I.A.; Mooney, R.A. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 2002, 51, 3391–3399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.; Song, S.J.; Choi, M.S.; Yu, R.; Park, T. IL-7 receptor deletion ameliorates diet-induced obesity and insulin resistance in mice. Diabetologia 2015, 58, 2361–2370. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.S.; Park, H.S.; Kawada, T.; Kim, J.H.; Lim, D.; Hubbard, N.E.; Kwon, B.S.; Erickson, K.L.; Yu, R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int. J. Obes. 2006, 30, 1347–1355. [Google Scholar] [CrossRef] [Green Version]
- Carmona, M.C.; Lefebvre, P.; Lefebvre, B.; Galinier, A.; Benani, A.; Jeanson, Y.; Louche, K.; Flajollet, S.; Ktorza, A.; Dacquet, C.; et al. Coadministration of coenzyme Q prevents Rosiglitazone-induced adipogenesis in ob/ob mice. Int. J. Obes. 2009, 33, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Lira, F.S.; Rosa, J.C.; Cunha, C.A.; Ribeiro, E.B.; Oller Do Nascimento, C.; Oyama, L.M.; Mota, J.F. Supplementing alpha-tocopherol (vitamin E) and vitamin D3 in high fat diet decrease IL-6 production in murine epididymal adipose tissue and 3T3-L1 adipocytes following LPS stimulation. Lipids Health Dis. 2011, 10, 6–10. [Google Scholar] [CrossRef] [Green Version]
- You, T.; Arsenis, N.C.; Disanzo, B.L.; Lamonte, M.J. Effects of exercise training on chronic inflammation in obesity: Current evidence and potential mechanisms. Sport. Med. 2013, 43, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Mizokami, T.; Yano, H.; Suzuki, K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med. Sci. Sports Exerc. 2013, 45, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- De Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Leduc-Gaudet, J.P.; Reynaud, O.; Chabot, F.; Mercier, J.; Andrich, D.E.; St-Pierre, D.H.; Gouspillou, G. The impact of a short-term high-fat diet on mitochondrial respiration, reactive oxygen species production, and dynamics in oxidative and glycolytic skeletal muscles of young rats. Physiol. Rep. 2018, 6. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.H.; Weber, T.M.; Cathey, B.L.; Brophy, P.M.; Gilliam, L.A.A.; Kane, C.L.; Maples, J.M.; Gavin, T.P.; Houmard, J.A.; Neufer, P.D. Mitochondrial respiratory capacity and content are normal in young insulin-resistant obese humans. Diabetes 2014, 63, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Boushel, R.; Gnaiger, E.; Schjerling, P.; Skovbro, M.; Kraunsøe, R.; Dela, F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 2007, 50, 790–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancock, C.R.; Han, D.H.; Chen, M.; Terada, S.; Yasuda, T.; Wright, D.C.; Holloszy, J.O. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc. Natl. Acad. Sci. USA 2008, 105, 7815–7820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cormier, R.P.J.; Champigny, C.M.; Simard, C.J.; St-Coeur, P.D.; Pichaud, N. Dynamic mitochondrial responses to a high-fat diet in Drosophila melanogaster. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paglialunga, S.; Ludzki, A.; Root-McCaig, J.; Holloway, G.P. In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice. Diabetologia 2015, 58, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Politis-Barber, V.; Brunetta, H.S.; Paglialunga, S.; Petrick, H.L.; Holloway, G.P. Long-term high-fat feeding exacerbates short-term increases in adipose mitochondrial reactive oxygen species, without impairing mitochondrial respiration. Am. J. Physiol. Metab. 2020, 319, E376–E387. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.T.M.; Sverdlov, A.L.; Karki, S.; Macartney-Coxson, D.; Stubbs, R.S.; Farb, M.G.; Carmine, B.; Hess, D.T.; Colucci, W.S.; Gokce, N. Oxidative modifications of mitochondrial complex II are associated with insulin resistance of visceral fat in obesity. Am. J. Physiol. Metab. 2019, 316, E168–E177. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nederveen, J.P.; Manta, K.; Bujak, A.L.; Simone, A.C.; Fuda, M.R.; Nilsson, M.I.; Hettinga, B.P.; Hughes, M.C.; Perry, C.G.R.; Tarnopolsky, M.A. A Novel Multi-Ingredient Supplement Activates a Browning Program in White Adipose Tissue and Mitigates Weight Gain in High-Fat Diet-Fed Mice. Nutrients 2021, 13, 3726. https://doi.org/10.3390/nu13113726
Nederveen JP, Manta K, Bujak AL, Simone AC, Fuda MR, Nilsson MI, Hettinga BP, Hughes MC, Perry CGR, Tarnopolsky MA. A Novel Multi-Ingredient Supplement Activates a Browning Program in White Adipose Tissue and Mitigates Weight Gain in High-Fat Diet-Fed Mice. Nutrients. 2021; 13(11):3726. https://doi.org/10.3390/nu13113726
Chicago/Turabian StyleNederveen, Joshua P., Katherine Manta, Adam L. Bujak, Alexander C. Simone, Matthew R. Fuda, Mats I. Nilsson, Bart P. Hettinga, Meghan C. Hughes, Christopher G. R. Perry, and Mark A. Tarnopolsky. 2021. "A Novel Multi-Ingredient Supplement Activates a Browning Program in White Adipose Tissue and Mitigates Weight Gain in High-Fat Diet-Fed Mice" Nutrients 13, no. 11: 3726. https://doi.org/10.3390/nu13113726
APA StyleNederveen, J. P., Manta, K., Bujak, A. L., Simone, A. C., Fuda, M. R., Nilsson, M. I., Hettinga, B. P., Hughes, M. C., Perry, C. G. R., & Tarnopolsky, M. A. (2021). A Novel Multi-Ingredient Supplement Activates a Browning Program in White Adipose Tissue and Mitigates Weight Gain in High-Fat Diet-Fed Mice. Nutrients, 13(11), 3726. https://doi.org/10.3390/nu13113726







