Efficacy of Creatine Supplementation Combined with Resistance Training on Muscle Strength and Muscle Mass in Older Females: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy, Study Selection, and Data Extraction
2.2. Eligibility Criteria
2.3. Assessment of Methodological Quality of Included Studies
2.4. Data Analysis
2.5. Analysis of the Level of Evidence
3. Results
4. Discussion
4.1. Quality of Evidence
4.2. Adverse Events
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ganapathy, A.; Nieves, J.W. Nutrition and Sarcopenia-What Do We Know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, Dynapenia, And the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javed, A.A.; Mayhew, A.J.; Shea, A.K.; Raina, P. Association Between Hormone Therapy and Muscle Mass in Postmenopausal Women: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1910154. [Google Scholar] [CrossRef]
- Iannuzzi-Sucich, M.; Prestwood, K.M.; Kenny, A.M. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M772–M777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Wang, X.; Xie, H.; Zheng, S.; Wu, X.; Zhu, X.; Zhang, X.; Xue, S.; Li, H.; Hong, W.; et al. Sex differences in the prevalence and adverse outcomes of sarcopenia and sarcopenic obesity in community dwelling elderly in East China using the AWGS criteria. BMC Endocr. Disord. 2019, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchengast, S.; Huber, J. Gender and age differences in lean soft tissue mass and sarcopenia among healthy elderly. Anthropol. Anz. 2009, 67, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef] [Green Version]
- Bermon, S.; Venembre, P.; Sachet, C.; Valour, S.; Dolisi, C. Effects of creatine monohydrate ingestion in sedentary and weight-trained older adults. Acta Physiol. Scand. 1998, 164, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.L.; Botelho, P.B.; Carneiro, J.A.; Mota, J.F. Impact of creatine supplementation in combination with resistance training on lean mass in the elderly. J. Cachexia Sarcopenia Muscle 2016, 7, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Brose, A.; Parise, G.; Tarnopolsky, M.A. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2003, 58, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candow, D.G.; Vogt, E.; Johannsmeyer, S.; Forbes, S.C.; Farthing, J.P. Strategic creatine supplementation and resistance training in healthy older adults. Appl. Physiol. Nutr. Metab. 2015, 40, 689–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.C.; Tu, Y.K.; Wang, T.G.; Huang, Y.T.; Chien, K.L. Effects of resistance training, endurance training and whole-body vibration on lean body mass, muscle strength and physical performance in older people: A systematic review and network meta-analysis. Age Ageing 2018, 47, 367–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, S.; Granic, A.; Sayer, A.A. Nutrition and Muscle Strength, As the Key Component of Sarcopenia: An Overview of Current Evidence. Nutrients 2019, 11, 2942. [Google Scholar] [CrossRef] [Green Version]
- Candow, D.G.; Chilibeck, P.D.; Forbes, S.C. Creatine supplementation and aging musculoskeletal health. Endocrine 2014, 45, 354–361. [Google Scholar] [CrossRef]
- Forbes, S.C.; Candow, D.G.; Ostojic, S.M.; Roberts, M.D.; Chilibeck, P.D. Meta-Analysis Examining the Importance of Creatine Ingestion Strategies on Lean Tissue Mass and Strength in Older Adults. Nutrients 2021, 13, 1912. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Kaviani, M.; Candow, D.G.; Zello, G.A. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: A meta-analysis. Open Access J. Sports Med. 2017, 8, 213–226. [Google Scholar] [CrossRef] [Green Version]
- Devries, M.C.; Phillips, S.M. Creatine supplementation during resistance training in older adults-a meta-analysis. Med. Sci. Sports Exerc. 2014, 46, 1194–1203. [Google Scholar] [CrossRef]
- Smith-Ryan, A.E.; Cabre, H.E.; Eckerson, J.M.; Candow, D.G. Creatine Supplementation in Women’s Health: A Lifespan Perspective. Nutrients 2021, 13, 877. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef] [Green Version]
- de Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Johannsmeyer, S.; Candow, D.G.; Brahms, C.M.; Michel, D.; Zello, G.A. Effect of creatine supplementation and drop-set resistance training in untrained aging adults. Exp. Gerontol. 2016, 83, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar, A.F.; Januário, R.S.; Junior, R.P.; Gerage, A.M.; Pina, F.L.; do Nascimento, M.A.; Padovani, C.R.; Cyrino, E.S. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur. J. Appl. Physiol. 2013, 113, 987–996. [Google Scholar] [CrossRef]
- Alves, C.R.; Merege Filho, C.A.; Benatti, F.B.; Brucki, S.; Pereira, R.M.; de Sá Pinto, A.L.; Lima, F.R.; Roschel, H.; Gualano, B. Creatine supplementation associated or not with strength training upon emotional and cognitive measures in older women: A randomized double-blind study. PLoS ONE 2013, 8, e76301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilibeck, P.D.; Candow, D.G.; Landeryou, T.; Kaviani, M.; Paus-Jenssen, L. Effects of Creatine and Resistance Training on Bone Health in Postmenopausal Women. Med. Sci. Sports Exerc. 2015, 47, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Macedo, A.R.; Alves, C.R.; Roschel, H.; Benatti, F.B.; Takayama, L.; de Sá Pinto, A.L.; Lima, F.R.; Pereira, R.M. Creatine supplementation and resistance training in vulnerable older women: A randomized double-blind placebo-controlled clinical trial. Exp. Gerontol. 2014, 53, 7–15. [Google Scholar] [CrossRef]
- Neves, M., Jr.; Gualano, B.; Roschel, H.; Fuller, R.; Benatti, F.B.; Pinto, A.L.; Lima, F.R.; Pereira, R.M.; Lancha, A.H., Jr.; Bonfá, E. Beneficial effect of creatine supplementation in knee osteoarthritis. Med. Sci. Sports Exerc. 2011, 43, 1538–1543. [Google Scholar] [CrossRef]
- Nawrocka, A.; Polechoński, J.; Garbaciak, W.; Mynarski, W. Functional Fitness and Quality of Life among Women over 60 Years of Age Depending on Their Level of Objectively Measured Physical Activity. Int. J. Environ. Res. Public Health 2019, 16, 972. [Google Scholar] [CrossRef] [Green Version]
- Candow, D.G.; Chilibeck, P.D. Differences in Size, Strength, and Power of Upper and Lower Body Muscle Groups in Young and Older Men. J. Gerontol. Ser. A 2005, 60, 148–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rava, A.; Pihlak, A.; Ereline, J.; Gapeyeva, H.; Kums, T.; Purge, P.; Jürimäe, J.; Pääsuke, M. Body Composition, Neuromuscular Performance, and Mobility: Comparison Between Regularly Exercising and Inactive Older Women. J. Aging Phys. Act. 2017, 25, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Seguin, R.; Nelson, M.E. The benefits of strength training for older adults. Am. J. Prev. Med. 2003, 25, 141–149. [Google Scholar] [CrossRef]
- da Silva, R.B.; Costa-Paiva, L.; Morais, S.S.; Mezzalira, R.; Ferreira Nde, O.; Pinto-Neto, A.M. Predictors of falls in women with and without osteoporosis. J. Orthop. Sports Phys. Ther. 2010, 40, 582–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinaki, M.; Brey, R.H.; Hughes, C.A.; Larson, D.R.; Kaufman, K.R. Balance disorder and increased risk of falls in osteoporosis and kyphosis: Significance of kyphotic posture and muscle strength. Osteoporos. Int. 2005, 16, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Bazzucchi, I.; Felici, F.; Sacchetti, M. Effect of short-term creatine supplementation on neuromuscular function. Med. Sci. Sports Exerc. 2009, 41, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Candow, D.G.; Forbes, S.C.; Kirk, B.; Duque, G. Current Evidence and Possible Future Applications of Creatine Supplementation for Older Adults. Nutrients 2021, 13, 745. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- de Guingand, D.L.; Palmer, K.R.; Snow, R.J.; Davies-Tuck, M.L.; Ellery, S.J. Risk of Adverse Outcomes in Females Taking Oral Creatine Monohydrate: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1780. [Google Scholar] [CrossRef] [PubMed]



| Author (Year) | Population | n = Per Group | Age (year) | Intervention | Outcome Measure (Italics Indicate Effect Significant Cr) | Duration | |
|---|---|---|---|---|---|---|---|
| Supplementation | Training | ||||||
| Aguiar et al. (2013) [27] | healthy women > 60 years | 18 Cr = 9 Pl = 9 | Cr: 64 ± 4 Pl: 65 ± 6 | Cr or Pl: 5 g/day | RT 3 x/week | MS: 1RM (bench press, knee extension and biceps curl) BC: DEXA (muscle mass) | 12 weeks |
| Alves et al. (2013) [28] | healthy older women > 60 years | 22 Cr = 12 Pl = 10 | Cr: 66.4 ± 5.6 Pl: 63.9 ± 3.8 | Cr or Pl: 20 g/day for 5 days; followed by 5 g/day | RT 2 x/week | MS: 1RM (chest press and leg press) | 24 weeks |
| Bermon et al. (1998) [11] | healthy older adults | 8 Cr = 4 Pl = 4 | Cr: 71.0 ± 1.9 Pl: 69.3 ± 0.4 | Cr: 20 g Cr + 8 g glucose/day for 5 days; followed by 3 g Cr + 2 g glucose/day Pl: 28 g glucose/day for 5 days; followed by 5 g glucose/day | RT 3 x/week | MS: 1RM (leg press, chest press and leg extension) | 52 days |
| Brose et al. * (2003) [13] | healthy older adults (women were postmenopausal) | 13 Cr = 6 Pl = 7 | Cr: 70.8 ± 6.1 Pl: 69.9 ± 5.6 | Cr: 5 g Cr/day + 2 g dextrose/day Pl: 7 g dextrose/day | RT 3 x/week | MS: 1RM (leg press, chest press, arm flexion and Knee extension) BC: DEXA (muscle mass) | 14 weeks |
| Candow et al. * (2015) [14] | healthy ≥ 50 years older adults (women were postmenopausal) | 22 Cr = 13 Pl = 9 | 56 ± 5 | Cr: 0.1 g Cr/kg/day Pl: 0.1 g malt/kg/day | RT 3 x/week | MS: 1RM (Cr before and after—leg press and chest press) BC: DEXA (Cr after—muscle mass) | 32 weeks |
| Chilibeck et al. (2015) [29] | postmenopausal women | 33 Cr = 15 Pl = 18 | Cr: 57 ± 4 Pl: 57 ± 7 | Cr: 0.1 g Cr/kg/day Pl: 0.1 g malt/kg/day | RT 3 x/week | MS: 1RM (bench press and hack squat) BC: DEXA (muscle mass) | 52 weeks |
| Gualano et al. (2014) [30] | postmenopausal women with osteopenia or osteoporosis | 30 Cr = 15 Pl = 15 | Cr: 67.1 ± 5.6 Pl: 63.6 ± 3.6 | Cr or Pl: 20 g/day for 5 days; followed by 5 g/day | RT 2 x/week | MS: 1RM (bench press and leg press) BC: DEXA (muscle mass) | 24 weeks |
| Johannsmeyer et al. * (2016) [25] | Older adults (women were postmenopausal) | 14 Cr = 7 Pl = 7 | Cr: 59 ± 3 Pl: 58 ± 6 | Cr: 0.1 g Cr + 0.1 g dextrose/kg/day Pl: 0.2 g dextrose/kg/day | RT 3 x/week | MS: 1RM (leg press, chest press, hack Squat and lateral pull-down) BC: DEXA (muscle mass) | 12 weeks |
| Neves et al. (2011) [31] | postmenopausal women with knee osteoarthritis | 24 Cr = 3 Pl = 11 | Cr: 58 ± 3 Pl: 56 ± 3 | Cr or Pl: 20 g/day for 7 days; followed by 5 g/day | RT 3 x/week | MS: 1RM (leg press) BC: DEXA (muscle mass, lower limb muscle mass) | 12 weeks |
| Pinto et al. (2016) [12] | healthy older adults | 27 Cr = 13 Pl = 14 | Cr: 67.4 ± 4.7 Pl: 67.1 ± 6.3 | Cr: 5 g Cr/day Pl: 5 g malt/day | RT 3 x/week | MS: 10RM (bench press and leg press) BC: DEXA (muscle mass) | 12 weeks |
| Study | Random Allocation | Concealed Allocation | Groups Similar at Baseline | Participant Blinding | Therapist Blinding | Examiner Blinding | <15% Dropouts | Intention to Treat Analysis | Between Group Difference Reported | Point Estimate and Variability Reported | Total (0–10) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aguiar et al. (2013) [27] | Y | N | Y | Y | Y | Y | Y | N | Y | Y | 8 |
| Alves et al. (2013) [28] | Y | N | Y | Y | Y | Y | N | N | Y | Y | 7 |
| Bermon et al. (1998) [11] | Y | N | Y | Y | N | N | Y | N | Y | Y | 6 |
| Brose et al. (2003) [13] | Y | N | Y | Y | Y | N | Y | N | Y | Y | 7 |
| Candow et al. (2015) [14] | Y | Y | Y | Y | Y | Y | N | N | Y | Y | 8 |
| Chilibeck et al. (2015) [29] | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | 9 |
| Gualano et al. (2014) [30] | Y | N | Y | Y | Y | Y | N | N | Y | Y | 7 |
| Johannsmeyer et al. (2016) [25] | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Neves et al. (2011) [31] | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | 9 |
| Pinto et al. (2016) [12] | Y | N | Y | Y | Y | Y | N | N | Y | Y | 7 |
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Outcome | Number of Subjects with Cr | Number of Subjects with Pl | Absolute Effect(95% CI) | Quality of Evidence (GRADE) | Importance |
|---|---|---|---|---|---|---|---|---|---|---|---|
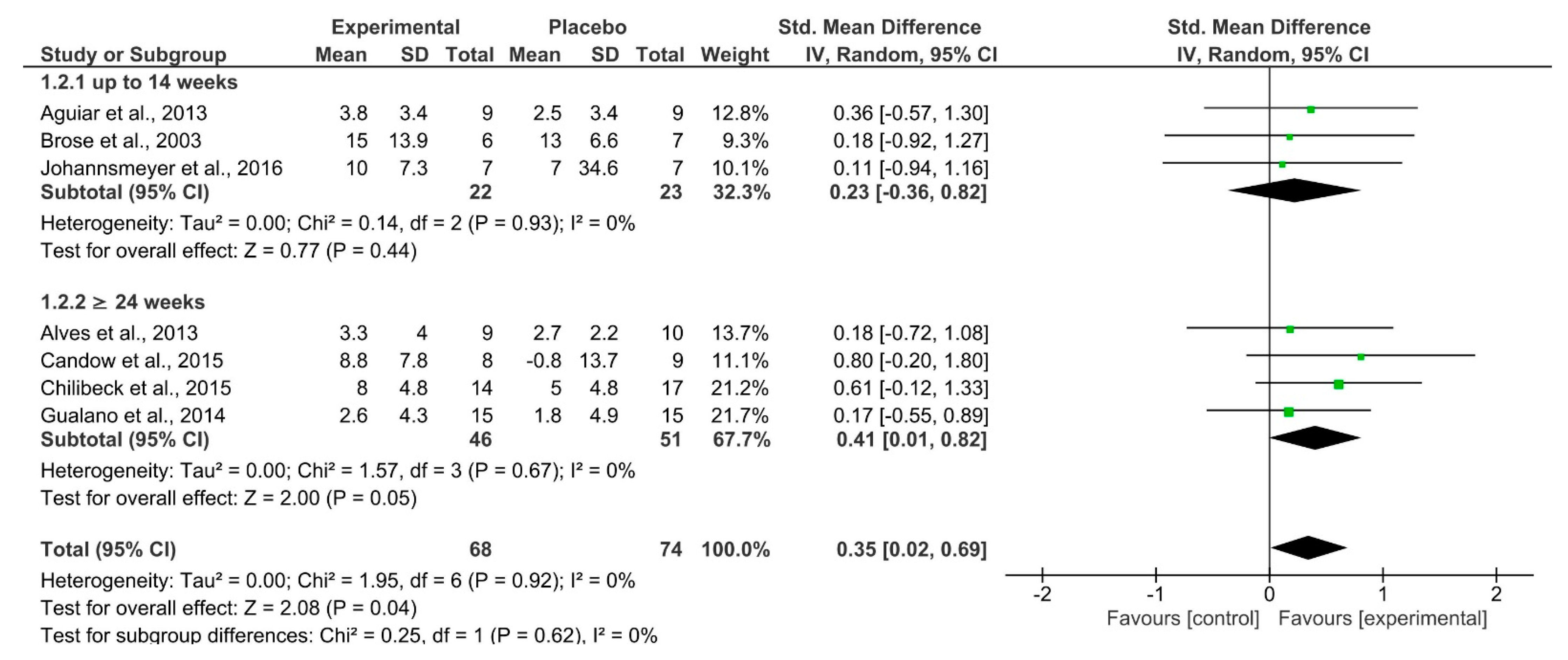
| 7 | RCT | Not serious | Not serious | Not serious | Extremely serious a | Upper-body Strength | 68 | 74 | SMD 0.35 (0.02 –0.69) | ⨁⨁◯◯ LOW | Critical |
| 7 | RCT | Not serious | Not serious | Not serious | Extremely serious a | Lower-body Strength | 75 | 76 | SMD 0.22 (−0.10–0.55) | ⨁⨁◯◯ LOW | Critical |
| 6 | RCT | Not serious | Not serious | Not serious | Extremely serious a | Muscle Mass | 70 | 66 | SMD 0.24 (−0.10–0.59) | ⨁⨁◯◯ LOW | Important |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, E.E.P.; de Araújo, R.C.; Candow, D.G.; Forbes, S.C.; Guijo, J.A.; de Almeida Santana, C.C.; Prado, W.L.d.; Botero, J.P. Efficacy of Creatine Supplementation Combined with Resistance Training on Muscle Strength and Muscle Mass in Older Females: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3757. https://doi.org/10.3390/nu13113757
dos Santos EEP, de Araújo RC, Candow DG, Forbes SC, Guijo JA, de Almeida Santana CC, Prado WLd, Botero JP. Efficacy of Creatine Supplementation Combined with Resistance Training on Muscle Strength and Muscle Mass in Older Females: A Systematic Review and Meta-Analysis. Nutrients. 2021; 13(11):3757. https://doi.org/10.3390/nu13113757
Chicago/Turabian Styledos Santos, Ellem Eduarda Pinheiro, Rodrigo Cappato de Araújo, Darren G. Candow, Scott C. Forbes, Jaddy Antunes Guijo, Carla Caroliny de Almeida Santana, Wagner Luiz do Prado, and João Paulo Botero. 2021. "Efficacy of Creatine Supplementation Combined with Resistance Training on Muscle Strength and Muscle Mass in Older Females: A Systematic Review and Meta-Analysis" Nutrients 13, no. 11: 3757. https://doi.org/10.3390/nu13113757
APA Styledos Santos, E. E. P., de Araújo, R. C., Candow, D. G., Forbes, S. C., Guijo, J. A., de Almeida Santana, C. C., Prado, W. L. d., & Botero, J. P. (2021). Efficacy of Creatine Supplementation Combined with Resistance Training on Muscle Strength and Muscle Mass in Older Females: A Systematic Review and Meta-Analysis. Nutrients, 13(11), 3757. https://doi.org/10.3390/nu13113757









