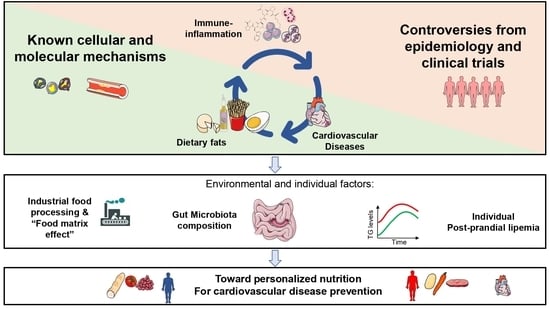
Molecular Immune-Inflammatory Connections between Dietary Fats and Atherosclerotic Cardiovascular Disease: Which Translation into Clinics?
Abstract
:1. Introduction
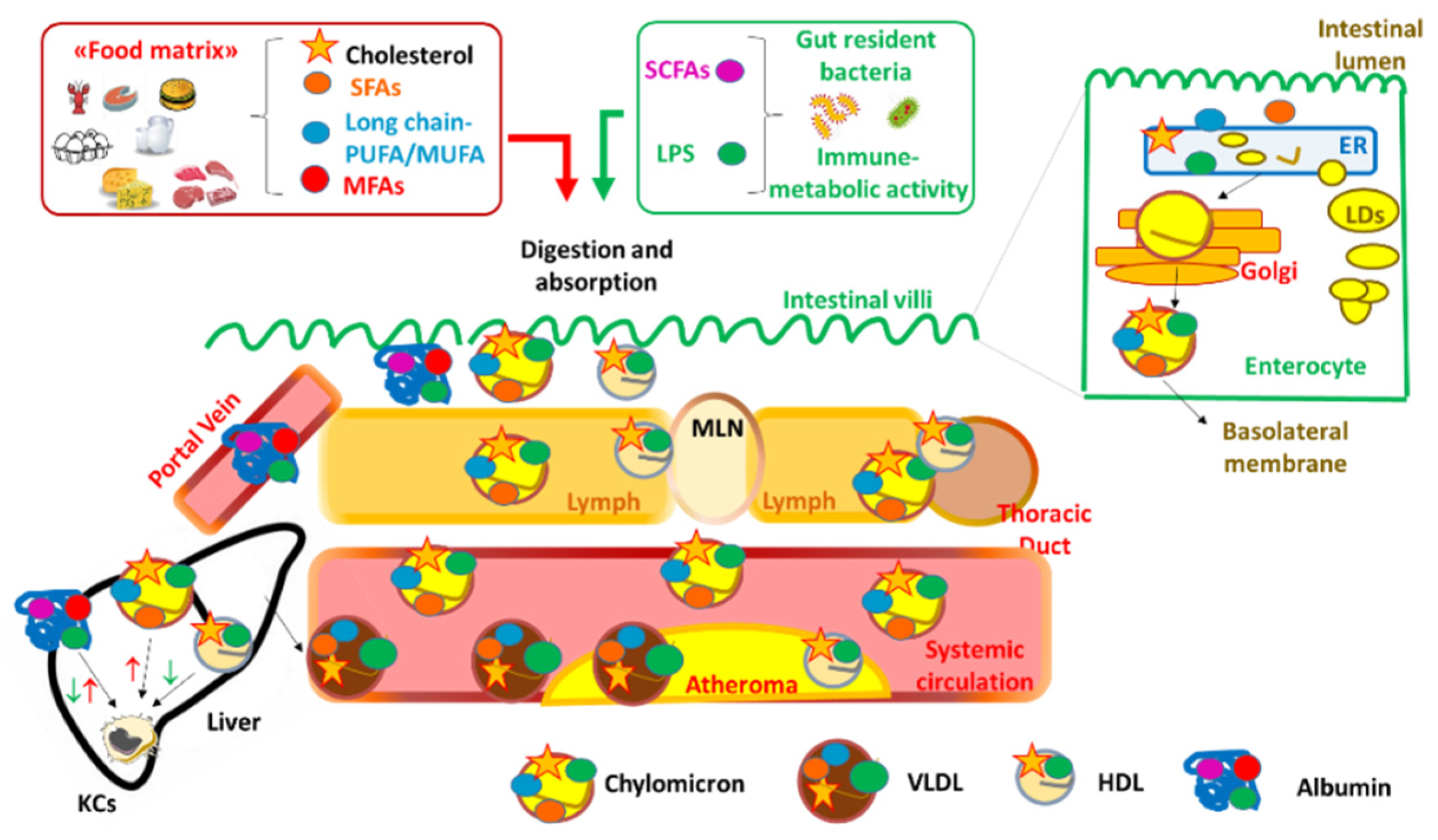
2. Dietary Fats, Inflammation and Atherosclerosis
2.1. Short Chain Fatty Acids
2.2. Medium and Long Chain Fatty Acids
2.3. The Contribution of Dietary Cholesterol as Compared to Serum Cholesterol
3. Data Linking Intake of Dietary Fats, Markers of Inflammation, and Risk of CVD
3.1. Epidemiological Studies
3.2. Interventional Clinical Trials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aiello, L.C.; Wheeler, P. The Expensive-Tissue Hypothesis: The Brain and the Digestive System in Human and Primate Evolution. Curr. Anthropol. 1995, 36, 199–221. [Google Scholar] [CrossRef]
- Holmes, E.; Li, J.V.; Marchesi, J.R.; Nicholson, J.K. Gut Microbiota Composition and Activity in Relation to Host Metabolic Phenotype and Disease Risk. Cell Metab. 2012, 16, 559–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Meier, T.; Gräfe, K.; Senn, F.; Sur, P.; Stangl, G.I.; Dawczynski, C.; März, W.; Kleber, M.E.; Lorkowski, S. Cardiovascular mortality attributable to dietary risk factors in 51 countries in the WHO European Region from 1990 to 2016: A systematic analysis of the Global Burden of Disease Study. Eur. J. Epidemiol. 2019, 34, 37–55. [Google Scholar] [CrossRef] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Yu, E.; Willett, W.C.; Hu, F.B. Understanding Nutritional Epidemiology and Its Role in Policy. Adv. Nutr. 2015, 6, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Maki, K.C.; Slavin, J.L.; Rains, T.M.; Kris-Etherton, P.M. Limitations of Observational Evidence: Implications for Evidence-Based Dietary Recommendations. Adv. Nutr. 2014, 5, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.B.; Satija, A.; Rimm, E.B.; Spiegelman, D.; Sampson, L.; Rosner, B.; Camargo, C.A.; Stampfer, M.; Willett, W.C. Diet Assessment Methods in the Nurses’ Health Studies and Contribution to Evidence-Based Nutritional Policies and Guidelines. Am. J. Public Health 2016, 106, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; de Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Kim, J.E.; Campbell, W.W. Total red meat intake of ≥0.5 servings/d does not negatively influence cardiovascular disease risk factors: A systemically searched meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 105, 57–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, V.W.; Van Horn, L.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Allen, N.B. Associations of Processed Meat, Unprocessed Red Meat, Poultry, or Fish Intake With Incident Cardiovascular Disease and All-Cause Mortality. JAMA Intern. Med. 2020, 180, 503. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Torres-Acosta, N.; O’Keefe, E.L.; Saeed, I.M.; Lavie, C.J.; Smith, S.E.; Ros, E. A Pesco-Mediterranean Diet With Intermittent Fasting. J. Am. Coll. Cardiol. 2020, 76, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Patsch, J.R.; Miesenböck, G.; Hopferwieser, T.; Mühlberger, V.; Knapp, E.; Dunn, J.K.; Gotto, A.M.; Patsch, W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler. Thromb. J. Vasc. Biol. 1992, 12, 1336–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjærg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. J. Am. Med. Assoc. 2007, 298, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norata, G.D.; Grigore, L.; Raselli, S.; Redaelli, L.; Hamsten, A.; Maggi, F.; Eriksson, P.; Catapano, A.L. Post-prandial endothelial dysfunction in hypertriglyceridemic subjects: Molecular mechanisms and gene expression studies. Atherosclerosis 2007, 193, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Bernelot Moens, S.J.; Verweij, S.L.; Schnitzler, J.G.; Stiekema, L.C.A.; Bos, M.; Langsted, A.; Kuijk, C.; Bekkering, S.; Voermans, C.; Verberne, H.J.; et al. Remnant Cholesterol Elicits Arterial Wall Inflammation and a Multilevel Cellular Immune Response in Humans. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 969–975. [Google Scholar] [CrossRef] [Green Version]
- Norata, G.D.; Grigore, L.; Raselli, S.; Seccomandi, P.M.; Hamsten, A.; Maggi, F.M.; Eriksson, P.; Catapano, A.L. Triglyceride-rich lipoproteins from hypertriglyceridemic subjects induce a pro-inflammatory response in the endothelium: Molecular mechanisms and gene expression studies. J. Mol. Cell. Cardiol. 2006, 40, 484–494. [Google Scholar] [CrossRef]
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J.; Capdevila, J.; Hadjigeorgiou, G.; Davies, R.; Al Khatib, H.; et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020, 26, 964–973. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, G.; Manwani, D.; Mortha, A.; Xu, C.; Faith, J.J.; Burk, R.D.; Kunisaki, Y.; Jang, J.-E.; Scheiermann, C.; et al. Neutrophil ageing is regulated by the microbiome. Nature 2015, 525, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Uehara, K.; Takahashi, T.; Fujii, H.; Shimizu, H.; Omori, E.; Matsumi, M.; Yokoyama, M.; Morita, K.; Akagi, R.; Sassa, S. The lower intestinal tract-specific induction of heme oxygenase-1 by glutamine protects against endotoxemic intestinal injury. Crit. Care Med. 2005, 33, 381–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, W.-K.; Yin, J.; Zhu, X.-P.; Liu, G.; Li, N.-Z.; Peng, Y.-Y.; Yin, Y.-Y. Glutamine on Intestinal Inflammation: A Mechanistic Perspective. Eur. J. Inflamm. 2013, 11, 315–326. [Google Scholar] [CrossRef]
- Ren, W.; Yin, J.; Wu, M.; Liu, G.; Yang, G.; Xion, Y.; Su, D.; Wu, L.; Li, T.; Chen, S.; et al. Serum Amino Acids Profile and the Beneficial Effects of L-Arginine or L-Glutamine Supplementation in Dextran Sulfate Sodium Colitis. PLoS ONE 2014, 9, e88335. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Lin, G.; Hu, S.; Wang, B.; Dai, Z.; Wu, G. Glycine Stimulates Protein Synthesis and Inhibits Oxidative Stress in Pig Small Intestinal Epithelial Cells. J. Nutr. 2014, 144, 1540–1548. [Google Scholar] [CrossRef] [Green Version]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.Y.; Liu, M.; Portincasa, P.; Wang, D.Q.-H. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur. J. Clin. Investig. 2013, 43, 1203–1233. [Google Scholar] [CrossRef] [Green Version]
- Beilstein, F.; Carrière, V.; Leturque, A.; Demignot, S. Characteristics and functions of lipid droplets and associated proteins in enterocytes. Exp. Cell Res. 2016, 340, 172–179. [Google Scholar] [CrossRef]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleophas, M.C.P.; Ratter, J.M.; Bekkering, S.; Quintin, J.; Schraa, K.; Stroes, E.S.; Netea, M.G.; Joosten, L.A.B. Effects of oral butyrate supplementation on inflammatory potential of circulating peripheral blood mononuclear cells in healthy and obese males. Sci. Rep. 2019, 9, 775. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, X.; Su, C.; Xi, M.; Zhang, X.; Jiang, Z.; Wang, L.; Hong, B. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Br. J. Pharmacol. 2020, 177, 1754–1772. [Google Scholar] [CrossRef] [PubMed]
- Baragetti, A.; Severgnini, M.; Olmastroni, E.; Dioguardi, C.C.; Mattavelli, E.; Angius, A.; Rotta, L.; Cibella, J.; Caredda, G.; Consolandi, C.; et al. Gut microbiota functional dysbiosis relates to individual diet in subclinical carotid atherosclerosis. Nutrients 2021, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Markó, L.; Höges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G.; et al. Short-Chain Fatty Acid Propionate Protects from Hypertensive Cardiovascular Damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.T. Gut commensals make choline too. Nat. Microbiol. 2019, 4, 4–5. [Google Scholar] [CrossRef]
- Koeth, R.A.; Lam-Galvez, B.R.; Kirsop, J.; Wang, Z.; Levison, B.S.; Gu, X.; Copeland, M.F.; Bartlett, D.; Cody, D.B.; Dai, H.J.; et al. L-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Investig. 2019, 129, 373–387. [Google Scholar] [CrossRef]
- Caesar, R.; Reigstad, C.S.; Bäckhed, H.K.; Reinhardt, C.; Ketonen, M.; Lundén, G.Ö.; Cani, P.D.; Bäckhed, F. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut 2012, 61, 1701–1707. [Google Scholar] [CrossRef] [Green Version]
- d’Hennezel, E.; Abubucker, S.; Murphy, L.O.; Cullen, T.W. Total Lipopolysaccharide from the Human Gut Microbiome Silences Toll-Like Receptor Signaling. mSystems 2017, 2, e00046-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnevale, R.; Sciarretta, S.; Valenti, V.; di Nonno, F.; Calvieri, C.; Nocella, C.; Frati, G.; Forte, M.; d’Amati, G.; Pignataro, M.G.; et al. Low-grade endotoxaemia enhances artery thrombus growth via toll-like receptor 4: Implication for myocardial infarction. Eur. Heart J. 2020, 41, 3156–3165. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [Green Version]
- Randolph, G.J.; Miller, N.E. Lymphatic transport of high-density lipoproteins and chylomicrons. J. Clin. Investig. 2014, 124, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Bonnardel, J.; T’Jonck, W.; Gaublomme, D.; Browaeys, R.; Scott, C.L.; Martens, L.; Vanneste, B.; De Prijck, S.; Nedospasov, S.A.; Kremer, A.; et al. Stellate Cells, Hepatocytes, and Endothelial Cells Imprint the Kupffer Cell Identity on Monocytes Colonizing the Liver Macrophage Niche. Immunity 2019, 51, 638–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.-H.; Onufer, E.J.; Huang, L.-H.; Sprung, R.W.; Davidson, W.S.; Czepielewski, R.S.; Wohltmann, M.; Sorci-Thomas, M.G.; Warner, B.W.; Randolph, G.J. Enterically derived high-density lipoprotein restrains liver injury through the portal vein. Science 2021, 373, eabe6729. [Google Scholar] [CrossRef]
- Norata, G.D.; Catapano, A.L. Molecular mechanisms responsible for the antiinflammatory and protective effect of HDL on the endothelium. Vasc. Health Risk Manag. 2005, 1, 119–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Garrett, W.S. Immune recognition of microbial metabolites. Nat. Rev. Immunol. 2020, 20, 91–92. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [Green Version]
- Björkman, L.; Mårtensson, J.; Winther, M.; Gabl, M.; Holdfeldt, A.; Uhrbom, M.; Bylund, J.; Højgaard Hansen, A.; Pandey, S.K.; Ulven, T.; et al. The Neutrophil Response Induced by an Agonist for Free Fatty Acid Receptor 2 (GPR43) Is Primed by Tumor Necrosis Factor Alpha and by Receptor Uncoupling from the Cytoskeleton but Attenuated by Tissue Recruitment. Mol. Cell. Biol. 2016, 36, 2583–2595. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, N.E.; Kotarsky, K.; Owman, C.; Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003, 303, 1047–1052. [Google Scholar] [CrossRef]
- Ohira, H.; Fujioka, Y.; Katagiri, C.; Mamoto, R.; Aoyama-Ishikawa, M.; Amako, K.; Izumi, Y.; Nishiumi, S.; Yoshida, M.; Usami, M.; et al. Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J. Atheroscler. Thromb. 2013, 20, 425–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; Rudensky, A.Y.; Donald, B.; et al. Metabolites produced by commensal bacteria promote peripheral regolatory T cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Masui, R.; Sasaki, M.; Funaki, Y.; Ogasawara, N.; Mizuno, M.; Iida, A.; Izawa, S.; Kondo, Y.; Ito, Y.; Tamura, Y.; et al. G protein-coupled receptor 43 moderates gut inflammation through cytokine regulation from mononuclear cells. Inflamm. Bowel Dis. 2013, 19, 2848–2856. [Google Scholar] [CrossRef]
- Fachi, J.L.; Sécca, C.; Rodrigues, P.B.; de Mato, F.C.P.; Di Luccia, B.; de Souza Felipe, J.; Pral, L.P.; Rungue, M.; de Melo Rocha, V.; Sato, F.T.; et al. Acetate coordinates neutrophil and ILC3 responses against C. difficile through FFAR2. J. Exp. Med. 2020, 217, e20190489. [Google Scholar] [CrossRef] [PubMed]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Baragetti, A.; Catapano, A.L.; Magni, P. Multifactorial activation of nlrp3 inflammasome: Relevance for a precision approach to atherosclerotic cardiovascular risk and disease. Int. J. Mol. Sci. 2020, 21, 4459. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.C.A.M.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Bäckhed, F.; Lusis, A.J.; et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef]
- Bultman, S.J. Bacterial butyrate prevents atherosclerosis. Nat. Microbiol. 2018, 3, 1332–1333. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.Y.; Kargi, A.Y.; Omer, M.; Chan, C.K.; Wabitsch, M.; O’Brien, K.D.; Wight, T.N.; Chait, A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: Dissociation of adipocyte hypertrophy from inflammation. Diabetes 2010, 59, 386–396. [Google Scholar] [CrossRef] [Green Version]
- Suganami, T.; Nishida, J.; Ogawa, Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: Role of free fatty acids and tumor necrosis factor α. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2062–2068. [Google Scholar] [CrossRef] [Green Version]
- Suganami, T.; Tanimoto-Koyama, K.; Nishida, J.; Itoh, M.; Yuan, X.; Mizuarai, S.; Kotani, H.; Yamaoka, S.; Miyake, K.; Aoe, S.; et al. Role of the Toll-like receptor 4/NF-κB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.J.; Kim, K.H.; Yoon, J.M.; Kim, J.B. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem. Biophys. Res. Commun. 2006, 346, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Ajuwon, K.M.; Spurlock, M.E. Palmitate Activates the NF-κB Transcription Factor and Induces IL-6 and TNFα Expression in 3T3-L1 Adipocytes. J. Nutr. 2005, 135, 1841–1846. [Google Scholar] [CrossRef]
- Lee, J.Y.; Zhao, L.; Youn, H.S.; Weatherill, A.R.; Tapping, R.; Feng, L.; Lee, W.H.; Fitzgerald, K.A.; Hwang, D.H. Saturated Fatty Acid Activates but Polyunsaturated Fatty Acid Inhibits Toll-like Receptor 2 Dimerized with Toll-like Receptor 6 or 1. J. Biol. Chem. 2004, 279, 16971–16979. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Plakidas, A.; Lee, W.H.; Heikkinen, A.; Chanmugam, P.; Bray, G.; Hwang, D.H. Differential modulation of Toll-like receptors by fatty acids: Preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 2003, 44, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Schaeffler, A.; Gross, P.; Buettner, R.; Bollheimer, C.; Buechler, C.; Neumeier, M.; Kopp, A.; Schoelmerich, J.; Falk, W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-κB pathway in adipocytes links nutritional signalling with innate immunity. Immunology 2009, 126, 233–245. [Google Scholar] [CrossRef]
- Zanoni, I.; Ostuni, R.; Marker, L.R.; Barresi, S.; Barbalat, R.; Barton, G.M.; Granucci, F.; Kagan, J.C. CD14 controls the LPS-induced endocytosis of Tool-like Receptor 4. Cell 2011, 147, 868–880. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.H.; Brickey, W.J.; Ting, J.P.-Y. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Talbot, N.A.; Wheeler-Jones, C.P.; Cleasby, M.E. Palmitoleic acid prevents palmitic acid-induced macrophage activation and consequent p38 MAPK-mediated skeletal muscle insulin resistance. Mol. Cell. Endocrinol. 2014, 393, 129–142. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Finucane, O.M.; Lyons, C.L.; Murphy, A.M.; Reynolds, C.M.; Klinger, R.; Healy, N.P.; Cooke, A.A.; Coll, R.C.; Mcallan, L.; Nilaweera, K.N.; et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1β secretion and insulin resistance despite obesity. Diabetes 2015, 64, 2116–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiyama, J.; Taguchi, R.; Akasaka, Y.; Shibata, S.; Ito, M.; Nagasawa, M.; Murakami, K. Unsaturated FAs prevent palmitate-induced LOX-1 induction via inhibition of ER stress in macrophages. J. Lipid Res. 2011, 52, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Ma, M.; Li, Z.; Hao, Y.; Li, H.; Fu, P.; Jin, S. Posttreatment of Maresin1 Inhibits NLRP3 inflammasome activation via promotion of NLRP3 ubiquitination. FASEB J. 2020, 34, 11944–11956. [Google Scholar] [CrossRef]
- Viola, J.R.; Lemnitzer, P.; Jansen, Y.; Csaba, G.; Winter, C.; Neideck, C.; Silvestre-Roig, C.; Dittmar, G.; Döring, Y.; Drechsler, M.; et al. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circ. Res. 2016, 119, 1030–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, A.-C.; Lie, K.K.; Moren, M.; Skjærven, K.H. High dietary arachidonic acid levels induce changes in complex lipids and immune-related eicosanoids and increase levels of oxidised metabolites in zebrafish (Danio rerio). Br. J. Nutr. 2017, 117, 1075–1085. [Google Scholar] [CrossRef] [Green Version]
- Holladay, C.S.; Wright, R.M.; Spangelo, B.L. Arachidonic acid stimulates interleukin-6 release from rat peritoneal macrophages in vitro: Evidence for a prostacyclin-dependent mechanism. Prostaglandins Leukot. Essent. Fat. Acids 1993, 49, 915–922. [Google Scholar] [CrossRef]
- Shen, Z.; Ma, Y.; Ji, Z.; Hao, Y.; Yan, X.; Zhong, Y.; Tang, X.; Ren, W. Arachidonic acid induces macrophage cell cycle arrest through the JNK signaling pathway. Lipids Health Dis. 2018, 17, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellenstein, M.D.; de Visser, K.E. Fatty Acids Corrupt Neutrophils in Cancer. Cancer Cell 2019, 35, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Tyurin, V.A.; Blasi, M.; De Leo, A.; Kossenkov, A.V.; Donthireddy, L.; To, T.K.J.; Schug, Z.; Basu, S.; Wang, F.; et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 2019, 569, 73–78. [Google Scholar] [CrossRef]
- Guillot, E.; Vaugelade, P.; Lemarchali, P.; Re Rat, A. Intestinal absorption and liver uptake of medium-chain fatty acids in non-anaesthetized pigs. Br. J. Nutr. 1993, 69, 431–442. [Google Scholar] [CrossRef]
- Cerovic, V.; Bain, C.C.; Mowat, A.M.; Milling, S.W.F. Intestinal macrophages and dendritic cells: What’s the difference? Trends Immunol. 2014, 35, 270–277. [Google Scholar] [CrossRef]
- Bogunovic, M.; Ginhoux, F.; Helft, J.; Shang, L.; Greter, M.; Liu, K.; Jakubzick, C.; Ingersoll, M.A.; Leboeuf, M.; Stanley, E.R.; et al. Origin of the Lamina Propria Dendritic Cell Network. Immunity 2009, 31, 513–525. [Google Scholar] [CrossRef] [Green Version]
- Bernier-Latmani, J.; Petrova, T.V. Intestinal lymphatic vasculature: Structure, mechanisms and functions. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.; Harrison, O.J.; Schmitt, V.; Pelletier, M.; Spencer, S.P.; Urban, J.F.; Ploch, M.; Ramalingam, T.R.; Siegel, R.M.; Belkaid, Y. Critical role of fatty acid metabolism in ILC2-mediated barrier protection during malnutrition and helminth infection. J. Exp. Med. 2016, 213, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Bsat, M.; Chapuy, L.; Rubio, M.; Wassef, R.; Richard, C.; Schwenter, F.; Loungnarath, R.; Soucy, G.; Mehta, H.; Sarfati, M. Differential Pathogenic Th17 Profile in Mesenteric Lymph Nodes of Crohn’s Disease and Ulcerative Colitis Patients. Front. Immunol. 2019, 10, 1177. [Google Scholar] [CrossRef] [Green Version]
- Farnsworth, R.H.; Karnezis, T.; Maciburko, S.J.; Mueller, S.N.; Stacker, S.A. The Interplay Between Lymphatic Vessels and Chemokines. Front. Immunol. 2019, 10, 518. [Google Scholar] [CrossRef] [Green Version]
- Ghoshal, S.; Witta, J.; Zhong, J.; de Villiers, W.; Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009, 50, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Mjösberg, J.M.; Trifari, S.; Crellin, N.K.; Peters, C.P.; van Drunen, C.M.; Piet, B.; Fokkens, W.J.; Cupedo, T.; Spits, H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 2011, 12, 1055–1062. [Google Scholar] [CrossRef]
- Xue, L.; Salimi, M.; Panse, I.; Mjösberg, J.M.; McKenzie, A.N.J.; Spits, H.; Klenerman, P.; Ogg, G. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J. Allergy Clin. Immunol. 2014, 133, 1184–1194.e7. [Google Scholar] [CrossRef] [Green Version]
- Pelly, V.S.; Kannan, Y.; Coomes, S.M.; Entwistle, L.J.; Rückerl, D.; Seddon, B.; MacDonald, A.S.; McKenzie, A.; Wilson, M.S. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol. 2016, 9, 1407–1417. [Google Scholar] [CrossRef] [Green Version]
- Alsina-Sanchis, E.; Mülfarth, R.; Moll, I.; Mogler, C.; Rodriguez-Vita, J.; Fischer, A. Intraperitoneal oil application causes local inflammation with depletion of resident peritoneal macrophages. Mol. Cancer Res. 2021, 19, 288–300. [Google Scholar] [CrossRef]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. Postprandial lipemia as a cardiometabolic risk factor. Curr. Med. Res. Opin. 2014, 30, 1489–1503. [Google Scholar] [CrossRef]
- Gower, R.M.; Wu, H.; Foster, G.A.; Devaraj, S.; Jialal, I.; Ballantyne, C.M.; Knowlton, A.A.; Simon, S.I. CD11c/CD18 Expression Is Upregulated on Blood Monocytes During Hypertriglyceridemia and Enhances Adhesion to Vascular Cell Adhesion Molecule-1. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Lipopolysaccharide (LPS)-Binding Protein Mediates LPS Detoxification by Chylomicrons. Available online: https://www.jimmunol.org/content/170/3/1399 (accessed on 2 August 2021). [CrossRef] [Green Version]
- Shepherd, J. The role of the exogenous pathway in hypercholesterolaemia. Eur. Heart J. Suppl. 2001, 3, E2–E5. [Google Scholar] [CrossRef]
- Kapourchali, F.R.; Surendiran, G.; Goulet, A.; Moghadasian, M.H. The Role of Dietary Cholesterol in Lipoprotein Metabolism and Related Metabolic Abnormalities: A Mini-review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2408–2415. [Google Scholar] [CrossRef]
- Lopez-Miranda, J.; Williams, C.; Lairon, D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br. J. Nutr. 2007, 98, 458–473. [Google Scholar] [CrossRef] [Green Version]
- Sharrett, A.R.; Heiss, G.; Chambless, L.E.; Boerwinkle, E.; Coady, S.A.; Folsom, A.R.; Patsch, W. Metabolic and lifestyle determinants of postprandial lipemia differ from those of fasting triglycerides the Atherosclerosis Risk in Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 275–281. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Agriculture. FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 2 August 2021).
- European Institute of Oncology Food Composition Database for Epidemiological Studies in Italy. Available online: http://www.bda-ieo.it/wordpress/en/ (accessed on 2 August 2021).
- Berger, S.; Raman, G.; Vishwanathan, R.; Jacques, P.F.; Johnson, E.J. Dietary cholesterol and cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2015, 102, 276–294. [Google Scholar] [CrossRef] [Green Version]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Miller, N.H.; Hubbard, V.S.; Lee, I.-M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk. Circulation 2014, 129, S76–S99. [Google Scholar] [CrossRef] [Green Version]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Sheedy, F.J.; Grebe, A.; Rayner, K.J.; Kalantari, P.; Ramkhelawon, B.; Carpenter, S.B.; Becker, C.E.; Ediriweera, H.N.; Mullick, A.E.; Golenbock, D.T.; et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013, 14, 812–820. [Google Scholar] [CrossRef] [Green Version]
- Yvan-Charvet, L.; Pagler, T.; Gautier, E.L.; Avagyan, S.; Siry, R.L.; Han, S.; Welch, C.L.; Wang, N.; Randolph, G.J.; Snoeck, H.W.; et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 2010, 328, 1689–1693. [Google Scholar] [CrossRef] [Green Version]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [Green Version]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nũez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef] [Green Version]
- Rajamäki, K.; Lappalainen, J.; Öörni, K.; Välimäki, E.; Matikainen, S.; Kovanen, P.T.; Kari, E.K. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS ONE 2010, 5, e11765. [Google Scholar] [CrossRef] [Green Version]
- Bekkering, S.; Quintin, J.; Joosten, L.A.B.; van der Meer, J.W.M.; Netea, M.G.; Riksen, N.P. Oxidized Low-Density Lipoprotein Induces Long-Term Proinflammatory Cytokine Production and Foam Cell Formation via Epigenetic Reprogramming of Monocytes. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef]
- Christ, A.; Günther, P.; Lauterbach, M.A.R.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 2018, 172, 162–175. [Google Scholar] [CrossRef] [Green Version]
- Baragetti, A.; Bonacina, F.; Catapano, A.L.; Norata, G.D. Effect of Lipids and Lipoproteins on Hematopoietic Cell Metabolism and Commitment in Atherosclerosis. Immunometabolism 2021, 3, e210014. [Google Scholar] [CrossRef]
- Murphy, A.J.; Akhtari, M.; Tolani, S.; Pagler, T.; Bijl, N.; Kuo, C.-L.; Wang, M.; Sanson, M.; Abramowicz, S.; Welch, C.; et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Investig. 2011, 121, 4138–4149. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, M.S.; Julien, P.; Pérusse, L.; Vohl, M.C.; Rudkowska, I. Natural Rumen-Derived trans Fatty Acids Are Associated with Metabolic Markers of Cardiac Health. Lipids 2015, 50, 873–882. [Google Scholar] [CrossRef]
- Chandra, A.; Lyngbakken, M.N.; Eide, I.A.; Røsjø, H.; Vigen, T.; Ihle-Hansen, H.; Orstad, E.B.; Rønning, O.M.; Berge, T.; Schmidt, E.B.; et al. Plasma trans fatty acid levels, cardiovascular risk factors and lifestyle: Results from the Akershus cardiac examination 1950 study. Nutrients 2020, 12, 1419. [Google Scholar] [CrossRef]
- Lopez-Garcia, E.; Schulze, M.B.; Manson, J.A.E.; Meigs, J.B.; Albert, C.M.; Rifai, N.; Willett, W.C.; Hu, F.B. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J. Nutr. 2005, 135, 562–566. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Manson, J.A.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern. Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef]
- Livingstone, K.M.; Givens, D.I.; Cockcroft, J.R.; Pickering, J.E.; Lovegrove, J.A. Is fatty acid intake a predictor of arterial stiffness and blood pressure in men? Evidence from the Caerphilly Prospective Study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1079–1085. [Google Scholar] [CrossRef]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated Fats Compared with Unsaturated Fats and Sources of Carbohydrates in Relation to Risk of Coronary Heart Disease A Prospective Cohort Study. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, B.M.; Nielsen, M.M.; Jakobsen, M.U.; Nielsen, C.J.; Holst, C.; Larsen, T.M.; Bendsen, N.T.; Bysted, A.; Leth, T.; Hougaard, D.M.; et al. A cross-sectional study on trans-fatty acids and risk markers of CHD among middle-aged men representing a broad range of BMI. Br. J. Nutr. 2011, 106, 1245–1252. [Google Scholar] [CrossRef] [Green Version]
- Oh, K.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Willett, W.C. Dietary fat intake and risk of coronary heart disease in women: 20 Years of follow-up of the nurses’ health study. Am. J. Epidemiol. 2005, 161, 672–679. [Google Scholar] [CrossRef] [Green Version]
- Sohn, C.; Kim, J.; Bae, W. The framingham risk score, diet, and inflammatory markers in Korean men with metabolic syndrome. Nutr. Res. Pract. 2012, 6, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Khayyatzadeh, S.S.; Kazemi-Bajestani, S.M.R.; Bagherniya, M.; Mehramiz, M.; Tayefi, M.; Ebrahimi, M.; Ferns, G.A.; Safarian, M.; Ghayour-Mobarhan, M. Serum high C reactive protein concentrations are related to the intake of dietary macronutrients and fiber: Findings from a large representative Persian population sample. Clin. Biochem. 2017, 50, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Garcia-Arellano, A.; Estruch, R.; Marquez-Sandoval, F.; Corella, D.; Fiol, M.; Gómez-Gracia, E.; Viñoles, E.; Arós, F.; Herrera, C.; et al. Components of the mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur. J. Clin. Nutr. 2008, 62, 651–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guasch-Ferré, M.; Liu, G.; Li, Y.; Sampson, L.; Manson, J.A.E.; Salas-Salvadó, J.; Martínez-González, M.A.; Stampfer, M.J.; Willett, W.C.; Sun, Q.; et al. Olive Oil Consumption and Cardiovascular Risk in U.S. Adults. J. Am. Coll. Cardiol. 2020, 75, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Zong, G.; Willett, W.C.; Zock, P.L.; Wanders, A.J.; Hu, F.B.; Sun, Q. Associations of Monounsaturated Fatty Acids From Plant and Animal Sources With Total and Cause-Specific Mortality in Two US Prospective Cohort Studies. Circ. Res. 2019, 124, 1266–1275. [Google Scholar] [CrossRef]
- Imran, T.F.; Kim, E.; Buring, J.E.; Lee, I.M.; Gaziano, J.M.; Djousse, L. Nut consumption, risk of cardiovascular mortality, and potential mediating mechanisms: The Women’s Health Study. J. Clin. Lipidol. 2021, 15, 266–274. [Google Scholar] [CrossRef]
- Garcia-Arellano, A.; Ramallal, R.; Ruiz-Canela, M.; Salas-Salvadó, J.; Corella, D.; Shivappa, N.; Schröder, H.; Hébert, J.R.; Ros, E.; Gómez-Garcia, E.; et al. Dietary inflammatory index and incidence of cardiovascular disease in the PREDIMED study. Nutrients 2015, 7, 4124–4138. [Google Scholar] [CrossRef] [Green Version]
- De Souza, R.J.; Dehghan, M.; Mente, A.; Bangdiwala, S.I.; Ahmed, S.H.; Alhabib, K.F.; Altuntas, Y.; Basiak-Rasała, A.; Dagenais, G.R.; Diaz, R.; et al. Association of nut intake with risk factors, cardiovascular disease, and mortality in 16 countries from 5 continents: Analysis from the Prospective Urban and Rural Epidemiology (PURE) study. Am. J. Clin. Nutr. 2020, 112, 208–219. [Google Scholar] [CrossRef]
- Lopez-Garcia, E.; Schulze, M.B.; Manson, J.A.E.; Meigs, J.B.; Albert, C.M.; Rifai, N.; Willett, W.C.; Hu, F.B. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J. Nutr. 2004, 134, 1806–1811. [Google Scholar] [CrossRef] [Green Version]
- Pischon, T.; Hankinson, S.E.; Hotamisligil, G.S.; Rifai, N.; Willett, W.C.; Rimm, E.B. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation 2003, 108, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Ninomiya, T.; Nagata, M.; Hata, J.; Hirakawa, Y.; Ozawa, M.; Yoshida, D.; Ohara, T.; Kishimoto, H.; Mukai, N.; Fukuhara, M.; et al. Association between ratio of serum eicosapentaenoic acid to arachidonic acid and risk of cardiovascular disease: The Hisayama Study. Atherosclerosis 2013, 231, 261–267. [Google Scholar] [CrossRef]
- Madsen, T.; Skou, H.A.; Hansen, V.E.; Fog, L.; Christensen, J.H.; Toft, E.; Schmidt, E.B. C-Reactive Protein, Dietary n-3 Fatty Acid, and the Extent of Coronary Artery Disease. Am. J. Cardiol. 2001, 88, 1139–1142. [Google Scholar] [CrossRef]
- Manger, M.S.; Strand, E.; Ebbing, M.; Seifert, R.; Refsum, H.; Nordrehaug, J.E.; Nilsen, D.W.; Drevon, C.A.; Tell, G.S.; Bleie, Ø.; et al. Dietary intake of n-3 long-chain polyunsaturated fatty acids and coronary events in Norwegian patients with coronary artery disease. Am. J. Clin. Nutr. 2010, 92, 244–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffarian, D.; Ascherio, A.; Hu, F.B.; Stampfer, M.J.; Willett, W.C.; Siscovick, D.S.; Rimm, E.B. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation 2005, 111, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Bergendal, E. Dietary Fat and Cholesterol and Risk of Cardiovascular Disease in Older Adults: The Health ABC Study. Bone 2008, 23, 1–7. [Google Scholar] [CrossRef]
- Bagherniya, M.; Khayyatzadeh, S.S.; Bakavoli, A.R.H.; Ferns, G.A.; Ebrahimi, M.; Safarian, M.; Nematy, M.; Ghayour-Mobarhan, M. Serum high-sensitive C-reactive protein is associated with dietary intakes in diabetic patients with and without hypertension: A cross-sectional study. Ann. Clin. Biochem. 2018, 55, 422–429. [Google Scholar] [CrossRef]
- Virtanen, J.K.; Mursu, J.; Tuomainen, T.P.; Virtanen, H.E.K.; Voutilainen, S. Egg consumption and risk of incident type 2 diabetes in men: The kuopio ischaemic heart disease risk factor study. Am. J. Clin. Nutr. 2015, 101, 1088–1096. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, A.M.; Virtanen, H.E.K.; Voutilainen, S.; Kurl, S.; Tuomainen, T.P.; Salonen, J.T.; Virtanen, J.K. Egg consumption, cholesterol intake, and risk of incident stroke in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2019, 110, 169–176. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Rimm, E.B.; Manson, J.A.E.; Ascherio, A.; Colditz, G.A.; Rosner, B.A.; Spiegelman, D.; Speizer, F.E.; Sacks, F.M.; et al. A prospective study of egg consumption and risk of cardiovascular disease in men and women. J. Am. Med. Assoc. 1999, 281, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Pai, J.K.; Pischon, T.; Ma, J.; Manson, J.E.; Hankinson, S.E.; Joshipura, K.; Curhan, G.C.; Rifai, N.; Cannuscio, C.C.; Stampfer, M.J.; et al. Inflammatory Markers and the Risk of Coronary Heart Disease in Men and Women. N. Engl. J. Med. 2004, 351, 2599–2610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalogeropoulos, A.; Georgiopoulou, V.; Psaty, B.M.; Rodondi, N.; Smith, A.L.; Harrison, D.G.; Liu, Y.; Hoffmann, U.; Bauer, D.C.; Newman, A.B.; et al. Inflammatory Markers and Incident Heart Failure Risk in Older Adults. The Health ABC (Health, Aging, and Body Composition) Study. J. Am. Coll. Cardiol. 2010, 55, 2129–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodondi, N.; Marques-Vidal, P.; Butler, J.; Sutton-Tyrrell, K.; Cornuz, J.; Satterfield, S.; Harris, T.; Bauer, D.C.; Ferrucci, L.; Vittinghoff, E.; et al. Markers of atherosclerosis and inflammation for prediction of coronary heart disease in older adults. Am. J. Epidemiol. 2010, 171, 540–549. [Google Scholar] [CrossRef] [Green Version]
- Corley, J.; Shivappa, N.; Hébert, J.R.; Starr, J.M.; Deary, I.J. Associations between Dietary Inflammatory Index Scores and Inflammatory Biomarkers among Older Adults in the Lothian Birth Cohort 1936 Study. J. Nutr. Health Aging 2019, 23, 628–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramallal, R.; Toledo, E.; Martínez-González, M.A.; Hernández-Hernández, A.; García-Arellano, A.; Shivappa, N.; Hébert, J.R.; Ruiz-Canela, M. Dietary inflammatory index and incidence of cardiovascular disease in the SUN cohort. PLoS ONE 2015, 10, e0135221. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef] [Green Version]
- Kouvari, M.; Panagiotakos, D.B.; Chrysohoou, C.; Georgousopoulou, E.N.; Yannakoulia, M.; Tousoulis, D.; Pitsavos, C.; Skoumas, Y.; Katinioti, N.; Papadimitriou, L.; et al. Dairy products, surrogate markers, and cardiovascular disease; a sex-specific analysis from the ATTICA prospective study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2194–2206. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Shipley, M.; Armitage, J.; Collins, R.; Harris, W. Plasma phospholipid fatty acids and CHD in older men: Whitehall study of London civil servants. Br. J. Nutr. 2009, 102, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Lahoz, C.; Castillo, E.; Mostaza, J.M.; de Dios, O.; Salinero-Fort, M.A.; González-Alegre, T.; García-Iglesias, F.; Estirado, E.; Laguna, F.; Sanchez, V.; et al. Relationship of the adherence to a mediterranean diet and its main components with CRP levels in the Spanish population. Nutrients 2018, 10, 379. [Google Scholar] [CrossRef] [Green Version]
- Soliman, G.A. Dietary cholesterol and the lack of evidence in cardiovascular disease. Nutrients 2018, 10, 780. [Google Scholar] [CrossRef] [Green Version]
- Bechthold, A.; Boeing, H.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Iqbal, K.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; Schlesinger, S.; et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 1071–1090. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Baer, D.J.; Judd, J.T.; Clevidence, B.A.; Tracy, R.P. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: A randomized crossover study. Am. J. Clin. Nutr. 2004, 79, 969–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebauer, S.K.; Dionisi, F.; Krauss, R.M.; Baer, D.J. Vaccenic acid and trans fatty acid isomers from partially hydrogenated oil both adversely affect LDL cholesterol: A double-blind, randomized. Am. J. Clin. Nutr. 2015, 102, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Kien, C.L.; Bunn, J.Y.; Fukagawa, N.K.; Anathy, V.; Matthews, D.E.; Crain, K.I.; Ebenstein, D.B.; Tarleton, E.K.; Pratley, R.E.; Poynter, M.E. Lipidomic evidence that lowering the typical dietary palmitate to oleate ratio in humans decreases the leukocyte production of proinflammatory cytokines and muscle expression of redox-sensitive genes. J. Nutr. Biochem. 2015, 26, 1599–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholls, S.J.; Lundman, P.; Harmer, J.A.; Ons, B.S.C.H.; Cutri, B.; Ons, B.M.E.D.S.C.H.; Griffiths, K.A.; Rye, K.; Barter, P.J.; Celermajer, D.S. Consumption of Saturated Fat Impairs the Anti-Inflammatory Properties of High-Density Lipoproteins and Endothelial Function. J. Am. Coll. Cardiol. 2006, 48, 715–720. [Google Scholar] [CrossRef]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; West, S.G.; Gillies, P.J.; Kris-Etherton, P.M. Dietary α-Linolenic Acid Reduces Inflammatory and Lipid Cardiovascular Risk Factors in Hypercholesterolemic Men and Women. J. Nutr. 2004, 134, 2991–2997. [Google Scholar] [CrossRef] [PubMed]
- Brassard, D.; Tessier-Grenier, M.; Allaire, J.; Rajendiran, E.; She, Y.; Ramprasath, V.; Gigleux, I.; Talbot, D.; Levy, E.; Tremblay, A.; et al. Comparison of the impact of SFAs from cheese and butter on cardiometabolic risk factors: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 800–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, K.; Faun, L.; Ratna, S.; Nesaretnam, K.; Sanders, T.A.B. Effects of exchanging carbohydrate or monounsaturated fat with saturated fat on in fl ammatory and thrombogenic responses in subjects with abdominal obesity: A randomized controlled trial. Clin. Nutr. 2017, 36, 1250–1258. [Google Scholar] [CrossRef] [Green Version]
- Vafeiadou, K.; Weech, M.; Altowaijri, H.; Todd, S.; Yaqoob, P.; Jackson, K.G.; Lovegrove, J.A. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: Results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS). Am. J. Clin. Nutr. 2015, 102, 40–48. [Google Scholar] [CrossRef]
- Voon, P.T.; Ng, T.K.W.; Lee, V.K.M.; Nesaretnam, K. Diets high in palmitic acid (16:0), lauric and myristic acids (12:0 + 14:0), or oleic acid (18:1) do not alter postprandial or fasting plasma homocysteine and inflammatory markers in healthy Malaysian adults. Am. J. Clin. Nutr. 2012, 95, 780. [Google Scholar] [CrossRef] [Green Version]
- Faintuch, J.; Horie, L.M.; Barbeiro, H.V.; Barbeiro, D.F.; Soriano, F.G.; Ishida, R.K.; Cecconello, I. Systemic inflammation in morbidly obese subjects: Response to oral supplementation with alpha-linolenic acid. Obes. Surg. 2007, 17, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.O.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Rallidis, L.S.; Paschos, G.; Liakos, G.K.; Velissaridou, A.H.; Anastasiadis, G.; Zampelas, A. Dietary α-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis 2003, 167, 237–242. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Mitjavila, M.T.; Fandos, M.; Salas-Salvadó, J.; Covas, M.-I.; Borrego, S.; Estruch, R.; Lamuela-Raventós, R.; Corella, D.; Martínez-Gonzalez, M.Á.; Sánchez, J.M.; et al. The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. Clin. Nutr. 2013, 32, 172–178. [Google Scholar] [CrossRef]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-Style Diet on Endothelial Dysfunction and Markers of Vascular Inflammation in the Metabolic Syndrome. JAMA 2004, 292, 1440. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.R.; Hodgson, J.M.; Woodman, R.; Bryan, J.; Wilson, C.; Murphy, K.J. A Mediterranean diet lowers blood pressure and improves endothelial function: Results from the MedLey randomized intervention trial. Am. J. Clin. Nutr. 2017, 105, 1305–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberg, R.L.; Brook, R.D.; Rubenfire, M.; Eagle, K.A. Cardiovascular Impact of Nutritional Supplementation With Omega-3 Fatty Acids: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, C.E.; Casillas, R.; Bulló, M.; Castañer, O.; Ros, E.; Sáez, G.T.; Toledo, E.; Estruch, R.; Ruiz-Gutiérrez, V.; Fitó, M.; et al. A Mediterranean diet supplemented with extra virgin olive oil or nuts improves endothelial markers involved in blood pressure control in hypertensive women. Eur. J. Nutr. 2017, 56, 89–97. [Google Scholar] [CrossRef]
- Lambert, C.; Cubedo, J.; Padró, T.; Sánchez-Hernández, J.; Antonijoan, R.; Perez, A.; Badimon, L. Phytosterols and Omega 3 Supplementation Exert Novel Regulatory Effects on Metabolic and Inflammatory Pathways: A Proteomic Study. Nutrients 2017, 9, 599. [Google Scholar] [CrossRef] [Green Version]
- Kirkhus, B.; Lamglait, A.; Eilertsen, K.; Falch, E.; Haider, T.; Vik, H.; Hoem, N.; Hagve, T.; Basu, S.; Olsen, E.; et al. Effects of similar intakes of marine n-3 fatty acids from enriched food products and fish oil on cardiovascular risk markers in healthy human subjects. Br. J. Nutr. 2012, 107, 1339–1349. [Google Scholar] [CrossRef] [Green Version]
- Krantz, M.J.; Havranek, E.P.; Pereira, R.I.; Beaty, B.; Mehler, P.S.; Long, C.S. Effects of omega-3 fatty acids on arterial stiffness in patients with hypertension: A randomized pilot study. J. Negat. Results Biomed. 2017, 14, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volk, B.; Volek, J.S.; Fernandez, M.L. Effects of carbohydrate restriction and dietary cholesterol provided by eggs on clinical risk factors in metabolic syndrome. J. Clin. Lipidol. 2013, 7, 463–471. [Google Scholar] [CrossRef]
- Morgantini, C.; Trifirò, S.; Tricò, D.; Meriwether, D.; Baldi, S.; Mengozzi, A.; Reddy, S.T.; Natali, A. A short-term increase in dietary cholesterol and fat intake affects high-density lipoprotein composition in healthy subjects. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Tricò, D.; Trifirò, S.; Mengozzi, A.; Morgantini, C.; Baldi, S.; Mari, A.; Natali, A. Reducing cholesterol and fat intake improves glucose tolerance by enhancing B cell function in nondiabetic subjects. J. Clin. Endocrinol. Metab. 2018, 103, 622–631. [Google Scholar] [CrossRef]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volek, J.S.; Luz, M. Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk-free egg substitute in individuals with metabolic syndrome. Metabolism 2013, 62, 400–410. [Google Scholar] [CrossRef]
- Neelakantan, N.; Seah, J.Y.H.; van Dam, R.M. The Effect of Coconut Oil Consumption on Cardiovascular Risk Factors. Circulation 2020, 141, 803–814. [Google Scholar] [CrossRef]
- Desroches, S.; Archer, W.R.; Paradis, M.; Dériaz, O.; Couture, P.; Bergeron, J.; Bergeron, N.; Lamarche, B. Baseline Plasma C-Reactive Protein Concentrations Influence Lipid and Lipoprotein Responses to Low-Fat and High Monounsaturated Fatty Acid Diets in Healthy Men. J. Nutr. 2006, 136, 1005–1011. [Google Scholar] [CrossRef] [Green Version]
- Manson, J.E.; Cook, N.R.; Lee, I.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine Omega-3 Fatty Acids and Prevention of Vascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Allaire, J.; Couture, P.; Leclerc, M.; Charest, A.; Marin, J.; Marie-claude, L.; Talbot, D.; Tchernof, A. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: The Comparing EPA to DHA (ComparED). Am. J. Clin. Nutr. 2016, 3, 280–287. [Google Scholar] [CrossRef]
- Swann, J.R.; Rajilic-Stojanovic, M.; Salonen, A.; Sakwinska, O.; Gill, C.; Meynier, A.; Fança-Berthon, P.; Schelkle, B.; Segata, N.; Shortt, C.; et al. Considerations for the design and conduct of human gut microbiota intervention studies relating to foods. Eur. J. Nutr. 2020, 59, 3347–3368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [Green Version]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [Green Version]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Khatib, H.A.; et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef] [PubMed]


| EPIDEMIOLOGICAL STUDIES | ||
|---|---|---|
| Dietary fats | Prevalent Effects on Inflammatory Markers | Effects on CVD Risk/Risk Factors |
| Trans fats | ↑ [116] [117] [118] ↓ ↔ [119,120] [121] [122] | ↑ [116,119,120,121] [117] [118,123] ↓ ↔ [122] |
| Saturated fats | ↔ [119,120] [121] [124,125] | ↑ [119] ↔ [120] [121] [123,124] |
| Monounsaturated fats | ↓ [119] [126,127] | ↓ [119] [126,127] ↔ [121] [128] [123] |
| Polyunsaturated fats | ↔ [119] [121] [126] [129] | ↓ [119] [121] [123] [126] [130,131] [129] |
| n-3 and derivates | ↓ [129] [132,133,134,135] ↔ [136] | ↓ [132,137] [134] ↔ [136] |
| n-6 and derivates | ↔ [119] | ↓ [119] |
| Cholesterol | ↑ [125] ↔ [138,139,140,141] | ↑ [138] ↔ [139,140,141,142] |
| INTERVENTIONAL TRIALS | ||
|---|---|---|
| Dietary Lipids | Prevalent Effects on Inflammatory Markers | Effects on CVD Risk/Risk Factors |
| Trans fats | ↑ [156] ↔ [157] | ↑ [155] [156] |
| Saturated fats | ↑ [158] [159] [160] ↔ [156] [161,162] [163] | ↑ [156] [158,161] [159,160,163] ↔ [162] |
| Monounsaturated fats | ↓ [156] [158] ↔ [161,162] [163] [164] [165] | ↓ [156] [158,161,162] [163] [164] |
| Polyunsaturated fats | ↓ [159] ↔ [161] [163] | ↓ [161] [159,163] [166] |
| Ω-3 and derivates | ↓ [167,168,169,170,171] [165] ↔ [160] [172] [173,174] [175,176] | ↓ [160] [168,169,170,171,173] ↔ [167,172] [165,174,175,176] |
| Ω-6 and derivates | ↔ [160] [167] | ↔ [160] [167] |
| Cholesterol | ↓ [177] ↑ [178] ↔ [179] | ↓ [180] ↑ [179] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattavelli, E.; Catapano, A.L.; Baragetti, A. Molecular Immune-Inflammatory Connections between Dietary Fats and Atherosclerotic Cardiovascular Disease: Which Translation into Clinics? Nutrients 2021, 13, 3768. https://doi.org/10.3390/nu13113768
Mattavelli E, Catapano AL, Baragetti A. Molecular Immune-Inflammatory Connections between Dietary Fats and Atherosclerotic Cardiovascular Disease: Which Translation into Clinics? Nutrients. 2021; 13(11):3768. https://doi.org/10.3390/nu13113768
Chicago/Turabian StyleMattavelli, Elisa, Alberico Luigi Catapano, and Andrea Baragetti. 2021. "Molecular Immune-Inflammatory Connections between Dietary Fats and Atherosclerotic Cardiovascular Disease: Which Translation into Clinics?" Nutrients 13, no. 11: 3768. https://doi.org/10.3390/nu13113768