Proportions of Polyunsaturated Fatty Acids in Umbilical Cord Blood at Birth Are Related to Atopic Eczema Development in the First Year of Life
Abstract
:1. Introduction
2. Materials and Methods
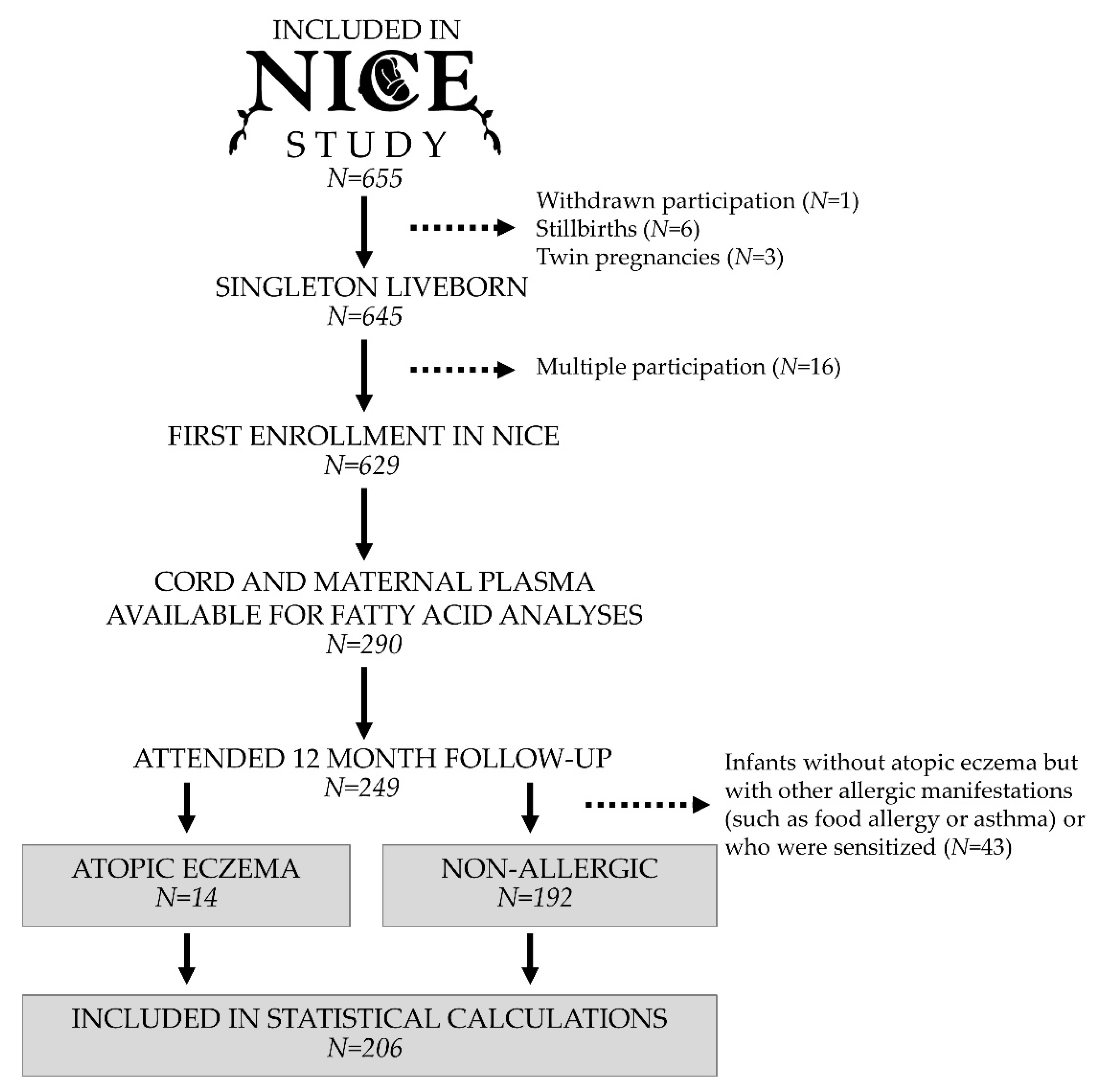
2.1. Study Population
2.2. Collection of Maternal and Infant Blood
2.3. Analysis of Fatty Acid Proportions in Plasma Phospholipids
2.4. Analysis of Fatty Acids by GC-MS
2.5. Dietary Assessments
2.6. Clinical Examination
2.7. Assessment of Filaggrin Gene (FLG) Mutations
2.8. Data Variables
2.9. Statistical Methods
3. Results
3.1. Prevalence of Eczema and Relationships between Eczema and Background Factors
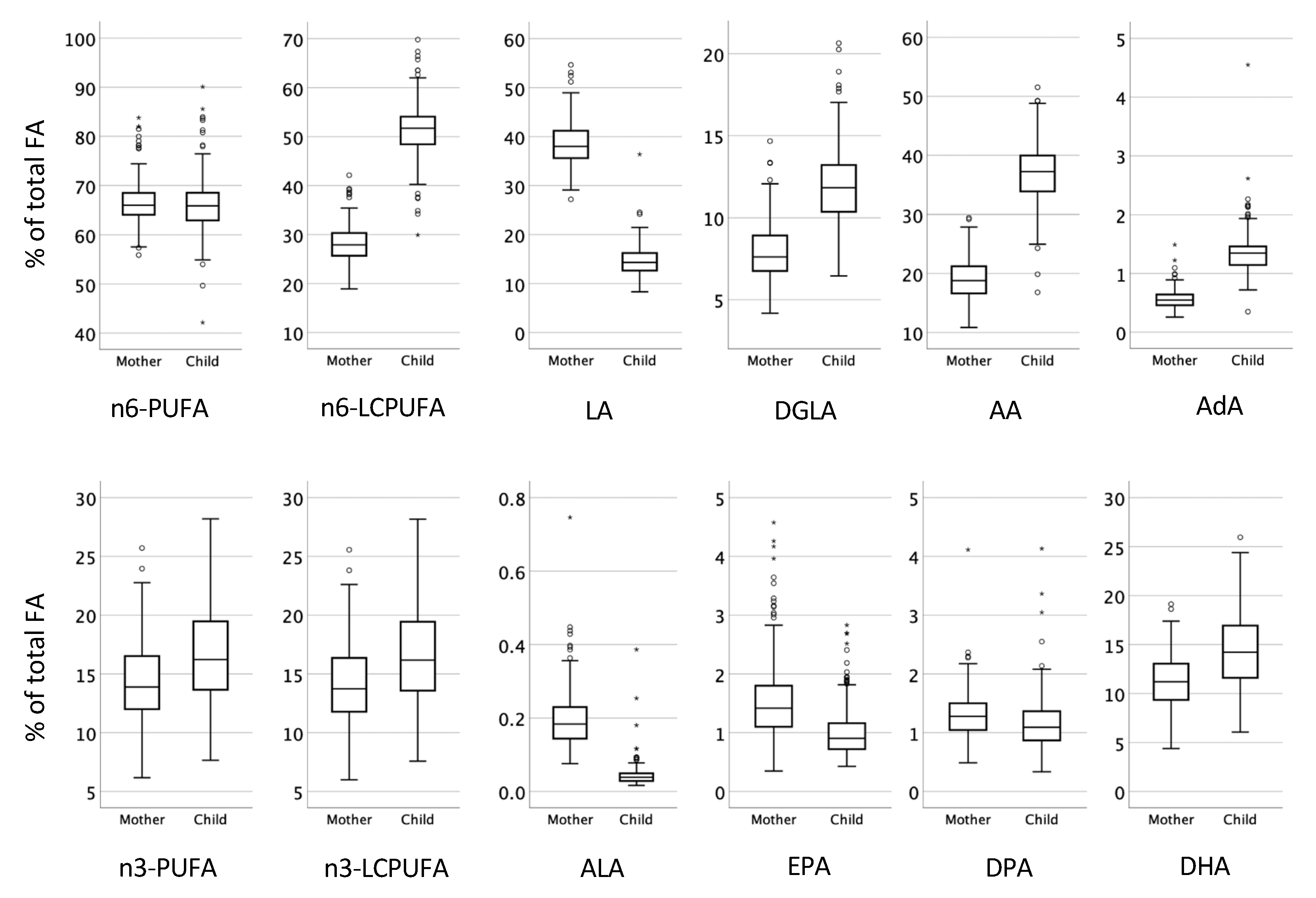
3.2. Proportions of Fatty Acids in Plasma Phospholipids at Birth in Relation to Presence of Atopic Eczema during the First Year of Life
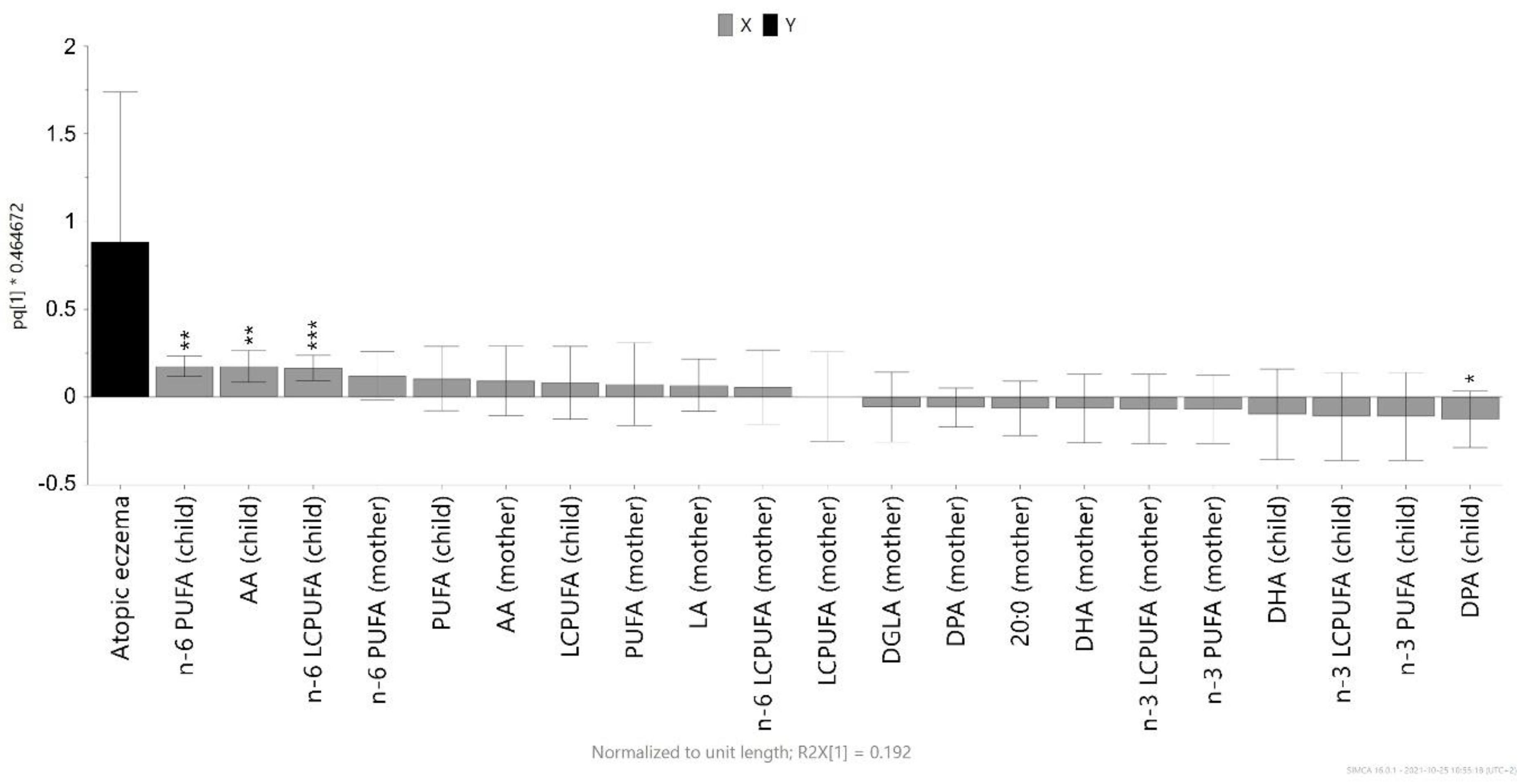
3.3. Magnitude of the Association between Infant Cord Blood Fatty Acids and Atopic Eczema
3.4. Associations between Fatty Acids in Maternal and Infant Cord Plasma and Maternal Food Intake
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66, 8–16. [Google Scholar] [CrossRef]
- Hill, D.A.; Spergel, J.M. The atopic march: Critical evidence and clinical relevance. Ann. Allergy Asthma Immunol. 2018, 120, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Werfel, T.; Allam, J.P.; Biedermann, T.; Eyerich, K.; Gilles, S.; Guttman-Yassky, E.; Hoetzenecker, W.; Knol, E.; Simon, H.U.; Wollenberg, A.; et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 336–349. [Google Scholar] [CrossRef] [Green Version]
- Black, P.N.; Sharpe, S. Dietary fat and asthma: Is there a connection? Eur. Respir. J. 1997, 10, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Sala-Vila, A.; Miles, E.A.; Calder, P.C. Fatty acid composition abnormalities in atopic disease: Evidence explored and role in the disease process examined. Clin. Exp. Allergy 2008, 38, 1432–1450. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Can early omega-3 fatty acid exposure reduce risk of childhood allergic disease? LID-784. Nutrients 2017, 9, 784. [Google Scholar] [CrossRef] [Green Version]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef]
- Burdge, G.C.; Wootton, S.A. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Dietary fatty acids and the immune system. Nutr. Rev. 1998, 56, S70–S83. [Google Scholar] [CrossRef]
- Calder, P.C. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 327–335. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Sundseth, S.S.; Jones, S.A.; Brown, P.J.; Wisely, G.B.; Koble, C.S.; Devchand, P.; Wahli, W.; Willson, T.M.; Lenhard, J.M.; et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. USA 1997, 94, 4318–4323. [Google Scholar] [CrossRef] [Green Version]
- Delerive, P.; Furman, C.; Teissier, E.; Fruchart, J.; Duriez, P.; Staels, B. Oxidized phospholipids activate PPARalpha in a phospholipase A2-dependent manner. FEBS Lett. 2000, 471, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Romieu, I.; Torrent, M.; Garcia-Esteban, R.; Ferrer, C.; Ribas-Fito, N.; Anto, J.M.; Sunyer, J. Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin. Exp. Allergy 2007, 37, 518–525. [Google Scholar] [CrossRef]
- Sausenthaler, S.; Koletzko, S.; Schaaf, B.; Lehmann, I.; Borte, M.; Herbarth, O.; von Berg, A.; Wichmann, H.E.; Heinrich, J.; Group, L.S. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am. J. Clin. Nutr. 2007, 85, 530–537. [Google Scholar]
- Barman, M.; Murray, F.; Bernardi, A.I.; Broberg, K.; Bolte, S.; Hesselmar, B.; Jacobsson, B.; Jonsson, K.; Kippler, M.; Rabe, H.; et al. Nutritional impact on Immunological maturation during Childhood in relation to the Environment (NICE): A prospective birth cohort in northern Sweden. BMJ Open 2018, 8, e022013. [Google Scholar] [CrossRef]
- Stravik, M.; Jonsson, K.; Hartvigsson, O.; Sandin, A.; Wold, A.E.; Sandberg, A.S.; Barman, M. Food and nutrient intake during pregnancy in relation to maternal characteristics: Results from the NICE Birth Cohort in Northern Sweden. Nutrients 2019, 11, 1680. [Google Scholar] [CrossRef] [Green Version]
- Stravik, M.; Barman, M.; Hesselmar, B.; Sandin, A.; Wold, A.E.; Sandberg, A.S. Maternal intake of cow’s milk during lactation is associated with lower prevalence of food allergy in offspring. Nutrients 2020, 12, 3680. [Google Scholar] [CrossRef]
- Swedish Food Agency. The Food Database. Available online: https://www.livsmedelsverket.se/en/food-and-content/naringsamnen/livsmedelsdatabasen (accessed on 22 September 2021).
- Williams, H.C.; Burney, P.G.; Hay, R.J.; Archer, C.B.; Shipley, M.J.; Hunter, J.J.; Bingham, E.A.; Finlay, A.Y.; Pembroke, A.C.; Graham-Brown, R.A.; et al. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br. J. Derm. 1994, 131, 383–396. [Google Scholar] [CrossRef]
- Williams, H.C.; Burney, P.G.; Pembroke, A.C.; Hay, R.J. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br. J. Derm. 1994, 131, 406–416. [Google Scholar] [CrossRef]
- Williams, H.C.; Burney, P.G.; Strachan, D.; Hay, R.J. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. II. Observer variation of clinical diagnosis and signs of atopic dermatitis. Br. J. Derm. 1994, 131, 397–405. [Google Scholar] [CrossRef]
- Wahlberg, K.; Liljedahl, E.R.; Alhamdow, A.; Lindh, C.; Lidén, C.; Albin, M.; Tinnerberg, H.; Broberg, K. Filaggrin variations are associated with PAH metabolites in urine and DNA alterations in blood. Envion. Res. 2019, 177, 108600. [Google Scholar] [CrossRef]
- Feingold, K.R.; Elias, P.M. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim. Biophys. Acta 2014, 1841, 280–294. [Google Scholar] [CrossRef]
- Khnykin, D.; Miner, J.H.; Jahnsen, F. Role of fatty acid transporters in epidermis: Implications for health and disease. Dermatoendocrinology 2011, 3, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Weidinger, S.; Illig, T.; Baurecht, H.; Irvine, A.D.; Rodriguez, E.; Diaz-Lacava, A.; Klopp, N.; Wagenpfeil, S.; Zhao, Y.; Liao, H.; et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J. Allergy Clin. Immunol. 2006, 118, 214–219. [Google Scholar] [CrossRef]
- Lack, G. Update on risk factors for food allergy. J. Allergy Clin. Immunol. 2012, 129, 1187–1197. [Google Scholar] [CrossRef]
- Weidinger, S.; O’Sullivan, M.; Illig, T.; Baurecht, H.; Depner, M.; Rodriguez, E.; Ruether, A.; Klopp, N.; Vogelberg, C.; Weiland, S.K.; et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J. Allergy Clin. Immunol. 2008, 121, 1203–1209.e1201. [Google Scholar] [CrossRef]
- Palmer, C.N.A.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Barman, M.; Rabe, H.; Hesselmar, B.; Johansen, S.; Sandberg, A.S.; Wold, A.E. Cord blood levels of EPA, a marker of fish intake, correlate with infants’ T- and B-lymphocyte phenotypes and risk for allergic disease. Nutrients 2020, 12, 3000. [Google Scholar] [CrossRef]
- Montes, R.; Chisaguano, A.M.; Castellote, A.I.; Morales, E.; Sunyer, J.; Lopez-Sabater, M.C. Fatty-acid composition of maternal and umbilical cord plasma and early childhood atopic eczema in a Spanish cohort. Eur. J. Clin. Nutr. 2013, 67, 658–663. [Google Scholar] [CrossRef]
- Newson, R.B.; Shaheen, S.O.; Henderson, A.J.; Emmett, P.M.; Sherriff, A.; Calder, P.C. Umbilical cord and maternal blood red cell fatty acids and early childhood wheezing and eczema. J. Allergy Clin. Immunol. 2004, 114, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Standl, M.; Demmelmair, H.; Koletzko, B.; Heinrich, J. Cord blood LC-PUFA composition and allergic diseases during the first 10 yr. Results from the LISAplus study. Pediatr. Allergy Immunol. 2014, 25, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Byberg, K.; Oymar, K.; Aksnes, L. Fatty acids in cord blood plasma, the relation to soluble CD23 and subsequent atopy. Prostaglandins Leukot. Essent. Fat. Acids 2008, 78, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, P.; McDermott, L. Long chain PUFA transport in human term placenta. J. Nutr. 2009, 139, 636–639. [Google Scholar] [CrossRef] [Green Version]
- Barman, M.; Jonsson, K.; Wold, A.E.; Sandberg, A.S. Exposure to a farm environment during pregnancy increases the proportion of arachidonic acid in the cord sera of offspring. Nutrients 2019, 11, 238. [Google Scholar] [CrossRef] [Green Version]
- Carnielli, V.P.; Wattimena, D.J.; Luijendijk, I.H.; Boerlage, A.; Degenhart, H.J.; Sauer, P.J. The very low birth weight premature infant is capable of synthesizing arachidonic and docosahexaenoic acids from linoleic and linolenic acids. Pediatr. Res. 1996, 40, 169–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gemming, L.; Ni Mhurchu, C. Dietary under-reporting: What foods and which meals are typically under-reported? Eur. J. Clin. Nutr. 2016, 70, 640–641. [Google Scholar] [CrossRef]
- Freedman, L.S.; Commins, J.M.; Moler, J.E.; Arab, L.; Baer, D.J.; Kipnis, V.; Midthune, D.; Moshfegh, A.J.; Neuhouser, M.L.; Prentice, R.L.; et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am. J. Epidemiol. 2014, 180, 172–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kipnis, V.; Midthune, D.; Freedman, L.; Bingham, S.; Day, N.E.; Riboli, E.; Ferrari, P.; Carroll, R.J. Bias in dietary-report instruments and its implications for nutritional epidemiology. Public Health Nutr. 2002, 5, 915–923. [Google Scholar] [CrossRef] [Green Version]
- Hjartaker, A.; Lund, E.; Bjerve, K.S. Serum phospholipid fatty acid composition and habitual intake of marine foods registered by a semi-quantitative food frequency questionnaire. Eur. J. Clin. Nutr. 1997, 51, 736–742. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, M.; Midthjell, K.; Bjerve, K.S. Long-term tracking of plasma phospholipid fatty acid concentrations and their correlation with the dietary intake of marine foods in newly diagnosed diabetic patients: Results from a follow-up of the HUNT Study, Norway. Br. J. Nutr. 2013, 109, 1123–1134. [Google Scholar] [CrossRef] [Green Version]
- Seah, J.Y.; Gay, G.M.; Su, J.; Tai, E.S.; Yuan, J.M.; Koh, W.P.; Ong, C.N.; van Dam, R.M. consumption of red meat, but not cooking oils high in polyunsaturated fat, is associated with higher arachidonic acid status in singapore chinese adults. Nutrients 2017, 9, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, A.J.; Johnson, L.; O’Dea, K.; Holman, R.T. Diets rich in lean beef increase arachidonic acid and long-chain omega 3 polyunsaturated fatty acid levels in plasma phospholipids. Lipids 1994, 29, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Willers, S.M.; Devereux, G.; Craig, L.C.; McNeill, G.; Wijga, A.H.; Abou El-Magd, W.; Turner, S.W.; Helms, P.J.; Seaton, A. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax 2007, 62, 773–779. [Google Scholar] [CrossRef] [Green Version]
- Jedrychowski, W.; Perera, F.; Maugeri, U.; Mrozek-Budzyn, D.; Miller, R.L.; Flak, E.; Mroz, E.; Jacek, R.; Spengler, J.D. Effects of prenatal and perinatal exposure to fine air pollutants and maternal fish consumption on the occurrence of infantile eczema. Int. Arch. Allergy Immunol. 2011, 155, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Baiz, N.; Just, J.; Chastang, J.; Forhan, A.; de Lauzon-Guillain, B.; Magnier, A.M.; Annesi-Maesano, I.; The EDEN Mother-Child Cohort Study Group. Maternal diet before and during pregnancy and risk of asthma and allergic rhinitis in children. Allergy Asthma Clin. Immunol. 2019, 15, 40. [Google Scholar] [CrossRef] [Green Version]
- Venter, C.; Brown, K.R.; Maslin, K.; Palmer, D.J. Maternal dietary intake in pregnancy and lactation and allergic disease outcomes in offspring. Pediatr. Allergy Immunol. 2017, 28, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Larsen, V.; Ierodiakonou, D.; Jarrold, K.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Ruparelia, A.; Devani, P.; Trivella, M.; et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: A systematic review and meta-analysis. PLoS Med. 2018, 15, e1002507. [Google Scholar] [CrossRef] [PubMed]
- Netting, M.J.; Middleton, P.F.; Makrides, M. Does maternal diet during pregnancy and lactation affect outcomes in offspring? A systematic review of food-based approaches. Nutrition 2014, 30, 1225–1241. [Google Scholar] [CrossRef] [Green Version]
- Kremmyda, L.S.; Vlachava, M.; Noakes, P.S.; Diaper, N.D.; Miles, E.A.; Calder, P.C. Atopy risk in infants and children in relation to early exposure to fish, oily fish, or long-chain omega-3 fatty acids: A systematic review. Clin. Rev. Allergy Immunol. 2011, 41, 36–66. [Google Scholar] [CrossRef] [PubMed]

| Non-Allergic (N = 192) | Atopic Eczema (N = 14) | p-Value | |||
|---|---|---|---|---|---|
| N | (%) | N | (%) | ||
| Male sex | 82 | (43) | 5 | (36) | 0.609 |
| Birth order | |||||
| First-born | 92 | (48) | 8 | (57) | 0.505 |
| Mother with ≥1 previous pregnancy | 100 | (52) | 6 | (43) | |
| Birthweight in grams | |||||
| <2500 | 1 | (<1) | 1 | (7) | 0.068 |
| 2500–4500 | 178 | (93) | 13 | (93) | |
| >4500 | 13 | (7) | 0 | (0) | |
| FLG null | |||||
| Heterozygous (FLG null) | 11 | (6) | 0 | (0) | |
| Homozygous Wild-Type (WT) | 159 | (94) | 11 | (100) | 1.000 |
| Breastfed | |||||
| Never | 6 | (3) | 0 | (0) | 0.467 |
| <4 months | 13 | (7) | 2 | (14) | |
| 4–5 months | 31 | (17) | 4 | (29) | |
| ≥6 months | 130 | (72) | 8 | (57) | |
| Missing | 12 | - | |||
| Pet ownership (first year of life) | |||||
| Dog | 62 | (32) | 4 | (29) | 1.000 |
| Cat | 47 | (25) | 3 | (21) | 1.000 |
| Other | 11 | (6) | 0 | (0) | 1.000 |
| Allergic heredity | |||||
| Maternal | 74 | (39) | 9 | (64) | 0.058 |
| Paternal | 80 | (42) | 9 | (64) | 0.099 |
| Sibling | 28 | (15) | 5 | (71) | 0.007 |
| Any | 125 | (65) | 13 | (93) | 0.033 |
| Maternal age at delivery (years) | |||||
| <25 | 19 | (10) | 2 | (14) | 0.815 |
| 26–30 | 89 | (46) | 5 | (36) | |
| 31–35 | 56 | (29) | 6 | (43) | |
| >35 | 28 | (15) | 1 | (7) | |
| Mother’s highest education level | |||||
| Elementary school (9 years) | 3 | (2) | 0 | (0) | 0.138 |
| Senior high school (12 years) | 51 | (27) | 7 | (50) | |
| University/other education (>12 years) | 138 | (72) | 7 | (50) | |
| Early pregnancy BMI, kg/m2 | |||||
| Underweight (<18.5) | 0 | (0) | 0 | (0) | 0.910 |
| Normal weight (18.5–24.9) | 82 | (56) | 7 | (54) | |
| Overweight (25–29.9) | 46 | (29) | 4 | (31) | |
| Obese (≥30) | 28 | (15) | 2 | (15) | |
| Missing | 6 | 1 | |||
| Residential address | |||||
| Town (central part) | 82 | (43) | 5 | (36) | 0.696 |
| Town (suburb) | 46 | (24) | 4 | (29) | |
| Countryside | 64 | (33) | 5 | (36) | |
| Maternal smoking before pregnancy | |||||
| Yes | 11 | (6) | 0 | (0) | 1.000 |
| No | 180 | (94) | 14 | (100) | |
| Missing | 1 | - | |||
| Gestational length | |||||
| Preterm | 4 | (2) | 1 | (7) | 0.365 |
| Term | 167 | (87) | 12 | (86) | |
| Post-term | 21 | (11) | 1 | (7) | |
| Birth mode | |||||
| Vaginal delivery | 180 | (94) | 12 | (86) | 0.244 |
| Cesarean section | 12 | (6) | 2 | (14) | |
| Association with Atopic Eczema During 1st Year of Life | |||||||
|---|---|---|---|---|---|---|---|
| Unadjusted Model | Adjusted Model 1 | Adjusted Model 2 | |||||
| Fatty Acid | % in Cord Blood Phospholipids Median (IQR) | OR 3 (95% CI) | p-Value | OR 3 (95% CI) | p-Value | OR 3 (95% CI) | p-Value |
| n-6 fatty acids | |||||||
| Linoleic acid, 18:2 n-6 | 14 (3.7) | 1.08 (0.66–1.79) | 0.754 | 1.01 (0.61–1.68) | 0.956 | 0.80 (0.36–1.76) | 0.578 |
| Arachidonic acid, 20:4 n-6 | 37 (6.1) | 2.75 (1.38–5.47) | 0.004 | 2.58 (1.32–5.04) | 0.005 | 2.61 (1.21–5.64) | 0.014 |
| n-6 LCPUFAs | 52 (5.7) | 2.11 (1.23–3.63) | 0.007 | 1.99 (1.17–3.39) | 0.012 | 1.91 (1.05–3.48) | 0.035 |
| n-6 PUFAs | 66 (5.6) | 1.79 (1.18–2.70) | 0.006 | 1.67 (1.11–2.51) | 0.015 | 1.52 (0.94–2.46) | 0.086 |
| n-3 fatty acids | |||||||
| α-linolenic acid, 18:3 n-3 | 0.039 (0.021) | 0.33 (0.12–0.93) | 0.036 | 0.34 (0.12–0.94) | 0.038 | 0.39 (0.13–1.19) | 0.099 |
| EPA, 20:5 n-3 | 0.92 (0.48) | 0.80 (0.41–1.53) | 0.494 | 0.74 (0.38–1.44) | 0.371 | 0.81 (0.39–1.70) | 0.583 |
| DPA, 22:5 n-3 | 1.1 (0.52) | 0.41 (0.17–1.01) | 0.052 | 0.40 (0.16–1.00) | 0.049 | 0.56 (0.22–1.40) | 0.215 |
| DHA, 22:6 n-3 | 14 (5.5) | 0.53 (0.22–1.29) | 0.160 | 0.53 (0.22–1.30) | 0.165 | 0.83 (0.32–2.16) | 0.699 |
| n-3 LCPUFAs | 16 (5.9) | 0.49 (0.20–1.19) | 0.114 | 0.48 (0.20–1.18) | 0.109 | 0.76 (0.30–1.95) | 0.571 |
| n-3 PUFAs | 16 (5.9) | 0.49 (0.20–1.18) | 0.111 | 0.48 (0.20–1.17) | 0.107 | 0.76 (0.29–1.95) | 0.563 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barman, M.; Stråvik, M.; Broberg, K.; Sandin, A.; Wold, A.E.; Sandberg, A.-S. Proportions of Polyunsaturated Fatty Acids in Umbilical Cord Blood at Birth Are Related to Atopic Eczema Development in the First Year of Life. Nutrients 2021, 13, 3779. https://doi.org/10.3390/nu13113779
Barman M, Stråvik M, Broberg K, Sandin A, Wold AE, Sandberg A-S. Proportions of Polyunsaturated Fatty Acids in Umbilical Cord Blood at Birth Are Related to Atopic Eczema Development in the First Year of Life. Nutrients. 2021; 13(11):3779. https://doi.org/10.3390/nu13113779
Chicago/Turabian StyleBarman, Malin, Mia Stråvik, Karin Broberg, Anna Sandin, Agnes E. Wold, and Ann-Sofie Sandberg. 2021. "Proportions of Polyunsaturated Fatty Acids in Umbilical Cord Blood at Birth Are Related to Atopic Eczema Development in the First Year of Life" Nutrients 13, no. 11: 3779. https://doi.org/10.3390/nu13113779
APA StyleBarman, M., Stråvik, M., Broberg, K., Sandin, A., Wold, A. E., & Sandberg, A.-S. (2021). Proportions of Polyunsaturated Fatty Acids in Umbilical Cord Blood at Birth Are Related to Atopic Eczema Development in the First Year of Life. Nutrients, 13(11), 3779. https://doi.org/10.3390/nu13113779







