Effect of Nutritional Habits on the Glycemic Response to Different Carbohydrate Diet in Children with Type 1 Diabetes Mellitus
Abstract
:1. Introduction
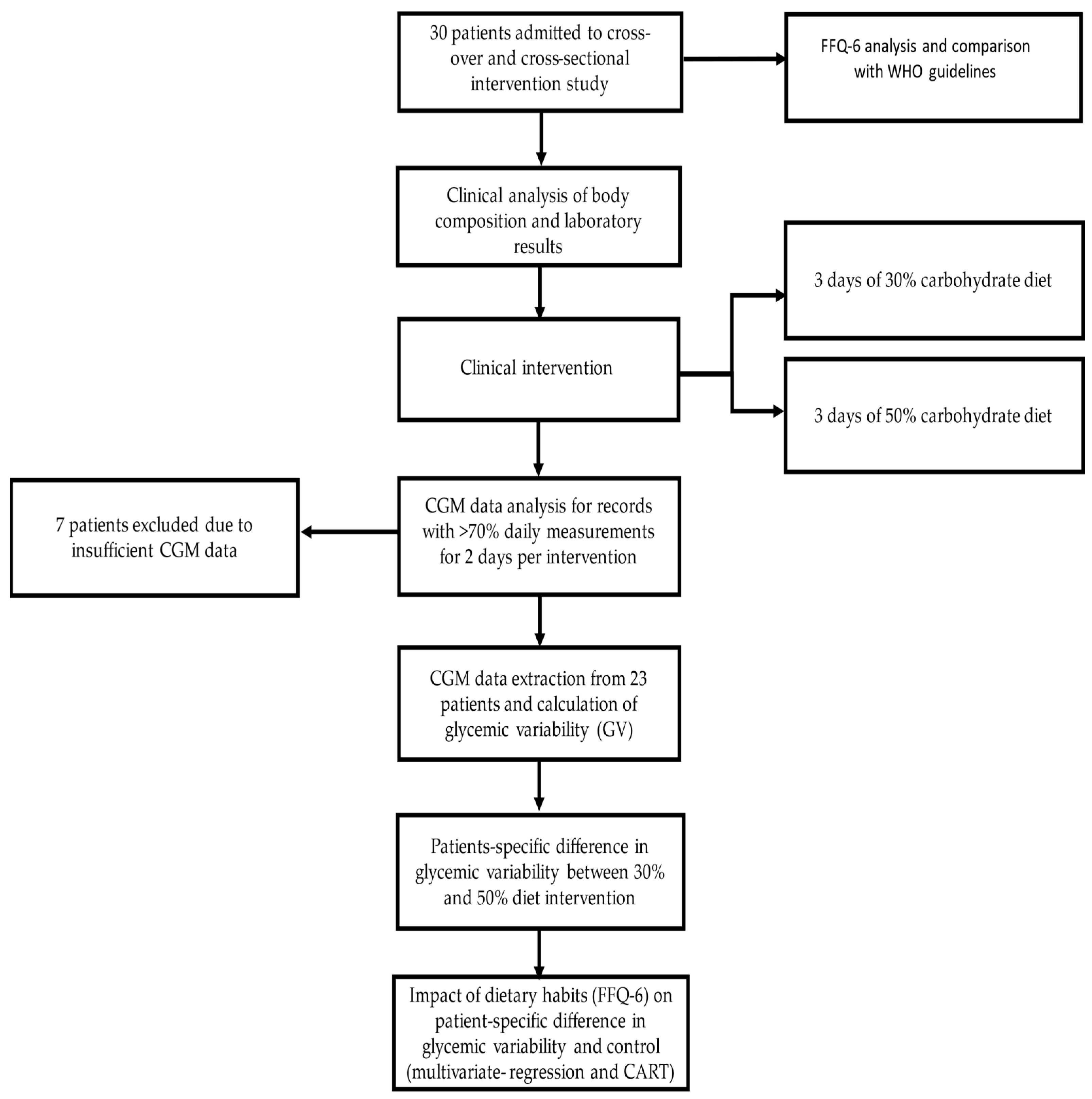
2. Materials and Methods
2.1. Participants, Recruitment, and Study Design
2.2. Ethics Statement
2.3. Collecting Clinical and Food Preferences Data
2.4. Collecting Continuous Glucose Monitoring Data
2.5. Statistical Analysis
3. Results
3.1. Study Group Characteristics
3.2. FFQ-6 Results and Interpretation
3.3. Glycaemic Variability Analysis Based on Used Diet
3.4. Analysis of Glycaemic Variability Indices between Diets in the Context of Nutritional Habits
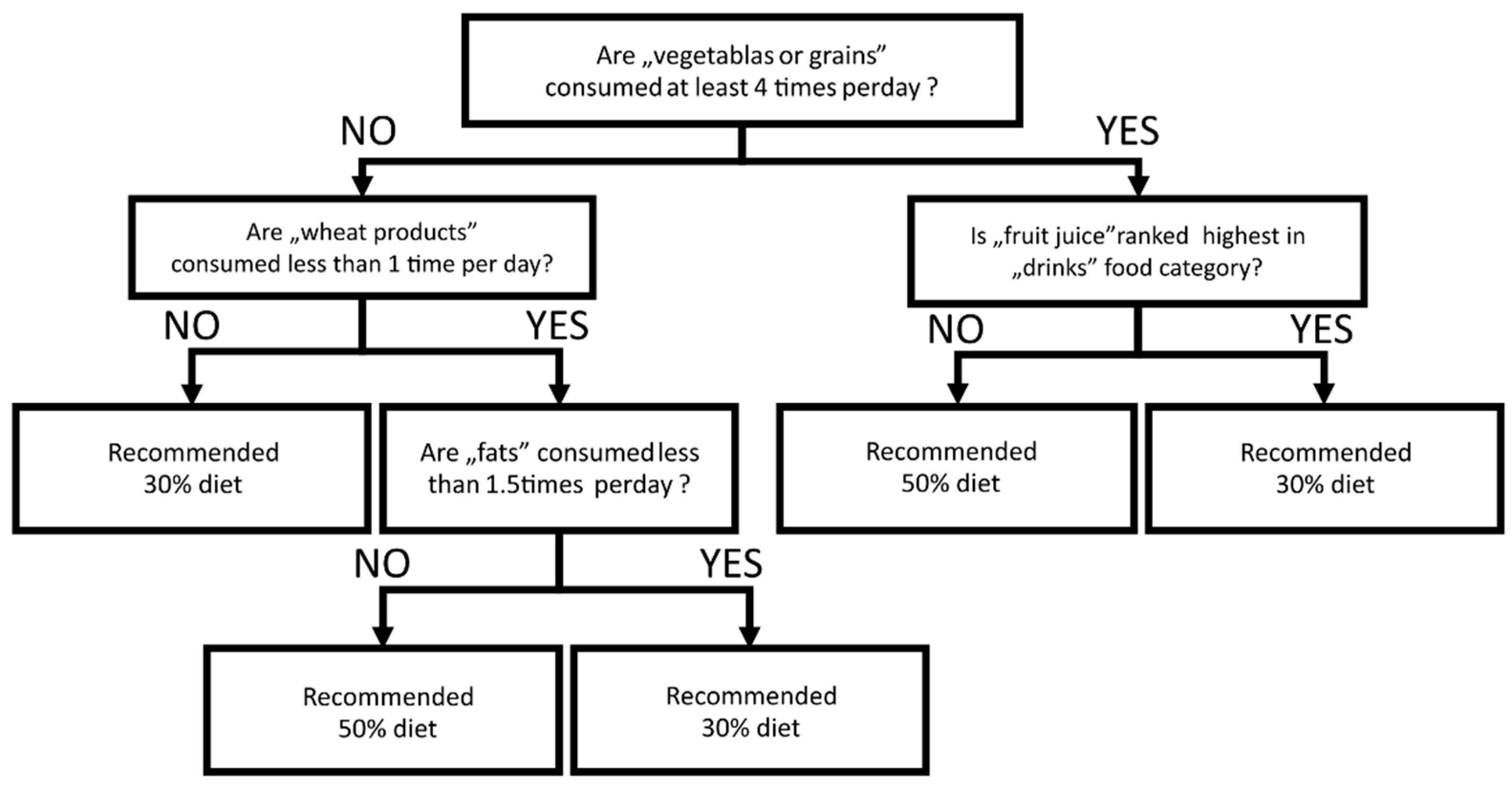
3.5. Individual Response to Low Carbohydrate Diet Predicted by FFQ-6 Questionnaires
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Standards of Medical Care in Diabetes—2021 Abridged for Primary Care Providers. Clin. Diabetes 2021, 39, 14–43. [CrossRef]
- Pathak, V.; Pathak, N.M.; O’Neill, C.L.; Guduric-Fuchs, J.; Medina, R.J. Therapies for Type 1 Diabetes: Current Scenario and Future Perspectives. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 1179551419844521. [Google Scholar] [CrossRef]
- Szalecki, M.; Wysocka-Mincewicz, M.; Ramotowska, A.; Mazur, A.; Lisowicz, L.; Beń-Skowronek, I.; Sieniawska, J.; Klonowska, B.; Charemska, D.; Nawrotek, J.; et al. Epidemiology of type 1 diabetes in Polish children: A multicentre cohort study. Diabetes/Metab. Res. Rev. 2018, 34, e2962. [Google Scholar] [CrossRef]
- Grabia, M.; Markiewicz-Żukowska, R.; Socha, K. Prevalence of Metabolic Syndrome in Children and Adolescents with Type 1 Diabetes Mellitus and Possibilities of Prevention and Treatment: A Systematic Review. Nutrients 2021, 13, 1782. [Google Scholar] [CrossRef] [PubMed]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Borys, S.; Budzyński, A.; Cyganek, K.; Cypryk, K.; Czech, A.; Czupryniak, L.; Drzewoski, J.; Dzida, G.; et al. 2021 Guidelines on the management of patients with diabetes. A position of Diabetes Poland. Clin. Diabetol. 2021, 10, 1–113. [Google Scholar] [CrossRef]
- Danne, T.; Nimri, R.; Battelino, T.; Bergenstal, R.M.; Close, K.L.; DeVries, J.H.; Garg, S.; Heinemann, L.; Hirsch, I.; Amiel, S.A.; et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017, 40, 1631–1640. [Google Scholar] [CrossRef] [Green Version]
- Puhr, S.; Derdzinski, M.; Welsh, J.B.; Parker, A.S.; Walker, T.; Price, D.A. Real-World Hypoglycemia Avoidance with a Con-tinuous Glucose Monitoring System’s Predictive Low Glucose Alert. Diabetes Technol. Ther. 2019, 21, 155–158. [Google Scholar] [CrossRef]
- Patton, S.R. Adherence to diet in youth with type 1 diabetes. J. Am. Diet Assoc. 2011, 111, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Nansel, T.R.; Lipsky, L.M.; Liu, A. Greater diet quality is associated with more optimal glycemic control in a longitudinal study of youth with type 1 diabetes 1,2. Am. J. Clin. Nutr. 2016, 104, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Meissner, T.; Wolf, J.; Kersting, M.; Frohlich-Reiterer, E.; Flechtner-Mors, M.; Salgin, B.; Stahl-Pehe, A.; Holl, R.W. Carbohydrate intake in relation toBMI, HbA1c and lipid profile in children and adolescents with type 1diabetes. Clin. Nutr. 2014, 33, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Clinical Recommendations for the Management of Patients with Diabetes 2021. Statement of the Polish Diabetes Society. Available online: www.journals.viamedica.pl (accessed on 11 October 2021).
- Krebs, J.D.; Parry Strong, A.; Cresswell, P.; Reynolds, A.N.; Hanna, A.; Haeusler, S. A randomised trial of the feasibility of a low carbohydrate diet vs standard carbohydrate counting in adults with type 1 diabetes taking body weight into account. Asia Pac. J. Clin. Nutr. 2016, 25, 78–84. [Google Scholar]
- Seckold, R.; Fisher, E.; de Bock, M.; King, B.R.; Smart, C.E. The ups and downs of low-carbohydrate diets in the management of Type 1 diabetes: A review of clinical outcomes. Diabet. Med. 2019, 36, 326–334. [Google Scholar] [CrossRef]
- World Health Organization. Reducing Childhood Obesity in Poland by Effective Policies; World Health Organization: Copenhagen, Denmark, 2017. [Google Scholar]
- Szczyrska, J.; Jankowska, A.; Brzeziński, M.; Jankowski, M.; Metelska, P.; Szlagatys-Sidorkiewicz, A. Prevalence of Overweight and Obesity in 6–7-Year-Old Children—A Result of 9-Year Analysis of Big City Population in Poland. Int. J. Environ. Res. Public Health 2020, 17, 3480. [Google Scholar] [CrossRef]
- Bandurska, E.; Brzeziński, M.; Metelska, P.; Zarzeczna-Baran, M. Cost-Effectiveness of an Obesity Management Program for 6- to 15-Year-Old Children in Poland: Data from Over Three Thousand Participants. Obes. Facts 2020, 13, 487–498. [Google Scholar] [CrossRef]
- Głąbska, D.; Skolmowska, D.; Guzek, D. Population-Based Study of the Changes in the Food Choice Determinants of Secondary School Students: Polish Adolescents’ COVID-19 Experience (PLACE-19) Study. Nutrients 2020, 12, 2640. [Google Scholar] [CrossRef]
- Łuszczki, E.; Bartosiewicz, A.; Pezdan-Śliż, I.; Kuchciak, M.; Jagielski, P.; Oleksy, Ł.; Stolarczyk, A.; Dereń, K. Children’s Eating Habits, Physical Activity, Sleep, and Media Usage before and during COVID-19 Pandemic in Poland. Nutrients 2021, 13, 2447. [Google Scholar] [CrossRef]
- Craig, M.E.; Hattersley, A.; Donaghue, K.C. Definition, epidemiology and classification of diabetes in children and adoles-cents. Pediatr. Diabetes 2009, 10 (Suppl. S12), 3–12. [Google Scholar] [CrossRef]
- Aliant Diet Calculator. Available online: www.aliant.com.pl (accessed on 11 October 2021).
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Nutrition Standards for the Population of Poland and Their Application; PZH: Warsaw, Poland, 2020. [Google Scholar]
- Niedzwiedzka, E.; Wadolowska, L. Accuracy analysis of the food intake variety questionnaire (FIVeQ). Reproducibility as-sessment among older people. Pak. J. Nutr. 2008, 7, 426–435. [Google Scholar] [CrossRef] [Green Version]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [Green Version]
- Gaabay, M.A.L.; Rodacki, M.; Callairi, L.E. Time in range: A new parameter to evaluate blood glucose control in patients with diabetes. Diabetol. Metab. Syndr. 2020, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Campbell, F.M.; Murphy, N.P.; Stewart, C. Outcomes of using flash glucose monitoring technology by children and young people with type 1 diabetes in a single arm study. Pediatr. Diabetes 2018, 19, 1294–1301. [Google Scholar] [CrossRef] [Green Version]
- Order of the President of the National Health Fund No. 67/2011//DSOZ of October 18, 2011 on Defining the Conditions for Concluding and Implementing Contracts Such as Health Services Contracted Separately. Available online: www.eur-lex.europa.eu (accessed on 11 October 2021).
- De Souza Bosco Paiva, C.; Lima, M.H.M. Introducing a very low carbohydrate diet for a child with type 1 diabetes. Br. J. Nurs. 2019, 28, 1015–1019. [Google Scholar] [CrossRef]
- Lennerz, B.S.; Barton, A.; Bernstein, R.K.; Dikeman, R.D.; Diulus, C.; Hallberg, S.; Rhodes, E.T.; Ebbeling, C.B.; Westman, E.C.; Yancy, W.S.; et al. Management of Type 1 Diabetes with a Very Low–Carbohydrate Diet. Pediatrics 2018, 141, e20173349. [Google Scholar] [CrossRef] [Green Version]
- Scott, S.N.; Anderson, L.; Morton, J.P.; Wagenmakers, A.J.; Riddell, M.C. Riddell Carbohydrate Restriction in Type 1 Diabetes: A Realistic Therapy for Improved Glycaemic Control and Athletic Performance? Nutrients 2019, 11, 1022. [Google Scholar] [CrossRef] [Green Version]
- Campbell, M.D.; Kime, N.; McKenna, J. Exercise and physical activity in patients with type 1 diabetes. Lancet Diabetes Endocrinol. 2017, 5, 493. [Google Scholar] [CrossRef] [Green Version]
- Myśliwiec, A.; Skalska, M.; Michalak, A.; Chrzanowski, J.; Szmigiero-Kawko, M.; Lejk, A.; Jastrzębska, J.; Radzimiński, Ł.; López-Sánchez, G.F.; Gawrecki, A.; et al. Responses to Low- and High-Intensity Exercise in Adolescents with Type 1 Diabetes in Relation to Their Level of VO2 Max. Int. J. Environ. Res. Public Health 2021, 18, 692. [Google Scholar] [CrossRef]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Bally, L.; Zueger, T.; Buehler, T.; Dokumaci, A.S.; Speck, C.; Pasi, N.; Ciller, C.; Paganini, D.; Feller, K.; Loher, H.; et al. Metabolic and hormonal response to intermittent high-intensity and continuous moderate intensity exercise in individuals with type 1 diabetes: A randomised crossover study. Diabetologia 2016, 59, 776–784. [Google Scholar] [CrossRef] [Green Version]
- De Bock, M.; Lobley, K.; Anderson, D.; Davis, E.; Donaghue, K.; Pappas, M.; Siafarikas, A.; Cho, Y.H.; Jones, T.; Smart, C. Endocrine and metabolic consequences due to restrictive carbohydrate diets in children with type 1 diabetes: An illustrative case series. Pediatr. Diabetes 2018, 19, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Mansoor, N.; Vinknes, K.J.; Veierød, M.B.; Retterstøl, K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2016, 115, 466–479. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Isaksson, S.S.; Bacos, M.B.; Eliasson, B.; Adolfsson, E.T.; Rawshani, A.; Lindblad, U.; Jendle, J.; Berglund, A.; Lind, M.; Axelsen, M. Effects of nutrition education using a food-based approach, carbohydrate counting or routine care in type 1 diabetes: 12 months prospective randomized trial. BMJ Open Diabetes Res. Care 2021, 9, e001971. [Google Scholar] [CrossRef]
- Bell, K.J.; Smart, C.E.; Steil, G.M.; Brand-Miller, J.C.; King, B.; Wolpert, H.A. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: Implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care 2015, 38, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Adolfsson, P.; Riddell, M.C.; Taplin, C.E.; Davis, E.A.; Fournier, P.A.; Annan, F.; Scaramuzza, A.E.; Hasnani, D.; Hofer, S.E. ISPAD Clinical Practice Consensus Guidelines 2018: Exercise in children and adolescents with diabetes. Pediatr. Diabetes 2018, 19 (Suppl. S27), 205–226. [Google Scholar] [CrossRef]

| Category (n = 30) | Median (25–75%) | Min–Max |
|---|---|---|
| Age [years] | 16.00 (13.00–17.00) | 10–17 |
| Disease time [years] | 6.00 (3.00–8.00) | 1.00–15.00 |
| BMI centile | 78.21 (55.62–89.94) | 8.15–97.68 |
| Time using pump [years] | 3.00 (1.00–7.00) | 1.00–14.00 |
| Initial HbA1c [%] | 7.25 (6.90–7.70) | 5.40–8.10 |
| Initial HbA1c [mmol/mol] | 55.738 (51.913–60.656) | 35.519–65.027 |
| Mean daily insulin requirement [u/day/kg of weight] | 0.75 (0.59–0.90) | 0.20–1.40 |
| Body fat % [Tanita] | 20.30 (14.50–29.20) | 12.70–39.00 |
| AST [U/L] | 17.00 (15.00–19.00) | 12.00–40.00 |
| ALAT [U/L] | 13.00 (10.00–15.00) | 5.00–26.00 |
| TC | 169.0 (149.0–188.0) | 119.0–257.0 |
| LDL | 94.50 (75.0–107.0) | 44.0–175.0 |
| HDL | 61.50 (52.0–70.0) | 30.0–92.0 |
| TG | 71.0 (62.0–90.0) | 28.0–181.0 |
| Vitamin D ng/mL | 21.65 (18.00–27.80) | 6.00–40.70 |
| FFQ-6 Category | Median (25–75 Cent.) Portions/d | Min–Max Portions/d | European Recommendations Portions/d | N (%) below the EU Recommendations | N (%) of Patients that Achieved EU Recommendations | N (%) above the EU Recommendations |
|---|---|---|---|---|---|---|
| Meat and fish | 1.6 (0.7–2.2) | 0.1–3.1 | 1–2 | 4 (13.33%) | 21 (70.00%) | 5 (16.67%) |
| Fats | 2.2 (1.7–2.7) | 0.4–5.2 | 2 | 5 (16.67%) | 12 (40.00%) | 13 (43.33%) |
| Fruits | 2.6 (1.7–3.5) | 0.1–8.3 | ~1–2 | 3 (10.00%) | 11 (36.67%) | 16 (53.33%) |
| Fruits and vegetables | 5.4 (3.6–6.8) | 0.1–15.6 | ~5–6 | 7 (23.33%) | 11 (36.67%) | 12 (40.00%) |
| Dairy products and eggs | 2.3 (1.4–2.8) | 0.8–5.7 | 3–4 | 18 (60.00%) | 9 (30.00%) | 3 (10.00%) |
| Sweets and snacks | 0.9 (0.6–1.7) | 0.2–3.5 | 0 | - | 5 (16.67%) | 25 (83.33%) |
| Vegetables | 2.5 (1.5–3.3) | 0.0–10.0 | ~4–5 | 23 (76.67%) | 4 (13.33%) | 3 (10.00%) |
| Bread, grains, potatoes | 3.1 (2.3–3.6) | 2.0–5.1 | ~5–6 | 28 (93.33%) | 2 (6.67%) | - |
| GV | Diet 30% Mean ± SD (n = 23) | Diet 50% Mean ± SD (n = 23) | Change for Diet 30% Mean ± SD (n = 111) | p |
|---|---|---|---|---|
| Mean glucose (mg/dL) | 133.16 ± 23.94 | 125.95 ± 22.88 | +5.77 ± 22.70 | 0.0620 |
| Median glucose (mg/dL) | 129.78 ± 23.46 | 120.95 ± 25.49 | +6.17 ± 21.53 | 0.0470 |
| 25th centile glucose (mg/dL) | 105.14 ± 20.24 | 96.00 ± 21.49 | +5.23 ± 16.81 | 0.0060 |
| 75th centile glucose (mg/dL) | 156.75 ± 29.09 | 149.02 ± 26.64 | +5.69 ± 31.72 | 0.1450 |
| 5th centile glucose (mg/dL) | 83.40 ± 17.11 | 72.69 ± 16.92 | +6.69 ± 16.30 | 0.0001 |
| 95th centile glucose (mg/dL) | 197.52 ± 39.39 | 201.43 ± 36.91 | +9.37 ± 57.84 | 0.6280 |
| SD (mg/dL) | 35.4 ± 9.653 | 39.39 ± 10.12 | +0.16 ± 16.38 | 0.0750 |
| CV (%) | 26.50 ± 5.43 | 31.68 ± 7.57 | −2.45 ± 8.12 | 0.0005 |
| Time below target range <54 mg/dL (<3 mmol/L) (%) | 0.43 ± 0.91 | 1.98 ± 3.91 | +0.07 ±3.51 | 0.0950 |
| Time below target range <70 mg/dL (<3.9 mmol/L) (%) | 2.49 ± 2.76 | 6.49 ± 6.56 | −1.01 ± 4.80 | 0.0003 |
| Time in target range 70–180 mg/dL (%) | 82.45 ± 13.83 | 77.89 ± 13.76 | −1.65 ± 13.42 | 0.1440 |
| Time in target range 180–250 mg/dl (>10 mmol/l) (%) | 12.74 ± 11.93 | 11.88 ± 11.63 | +1.58 ± 11.66 | 0.7460 |
| Time above target range >250 mg/dl (>13.9 mmol/l) (%) | 1.90 ± 3.22 | 1.77 ± 2.66 | +1.02 ± 5.30 | 0.8270 |
| ΔGV (50–30% Diet) | R2 | Observed FFQ6 and Clinical Data Influence on ΔGV |
|---|---|---|
| Mean | 0.5084 | −0.40 × (white bread most common (0/1)) 0.57 × (frequency of meat/fish meals consumed per day) |
| CV% | 0.4401 | 0.60 × (frequency of grains meals consumed per day) −0.42 × (frequency of drinks consumed per day) |
| TBR < 70 mg/dL | 0.2453 | 0.53 × (frequency of grains consumed per day) |
| TIR 70–180 mg/dL | 0.6398 | 0.42 × (white bread most common (0/1)) 0.35 × (potatoes most common (0/1)) −0.54 × (frequency of meat/fish meals consumed per day) |
| TAR > 180 mg/dL | 0.6180 | 0.57 × (frequency of meat/fish meals consumed per day) 0.39 × (frequency of sweets and snacks consumed per day) 0.37 × (level of 25OHD3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lejk, A.; Chrzanowski, J.; Cieślak, A.; Fendler, W.; Myśliwiec, M. Effect of Nutritional Habits on the Glycemic Response to Different Carbohydrate Diet in Children with Type 1 Diabetes Mellitus. Nutrients 2021, 13, 3815. https://doi.org/10.3390/nu13113815
Lejk A, Chrzanowski J, Cieślak A, Fendler W, Myśliwiec M. Effect of Nutritional Habits on the Glycemic Response to Different Carbohydrate Diet in Children with Type 1 Diabetes Mellitus. Nutrients. 2021; 13(11):3815. https://doi.org/10.3390/nu13113815
Chicago/Turabian StyleLejk, Agnieszka, Jędrzej Chrzanowski, Adrianna Cieślak, Wojciech Fendler, and Małgorzata Myśliwiec. 2021. "Effect of Nutritional Habits on the Glycemic Response to Different Carbohydrate Diet in Children with Type 1 Diabetes Mellitus" Nutrients 13, no. 11: 3815. https://doi.org/10.3390/nu13113815
APA StyleLejk, A., Chrzanowski, J., Cieślak, A., Fendler, W., & Myśliwiec, M. (2021). Effect of Nutritional Habits on the Glycemic Response to Different Carbohydrate Diet in Children with Type 1 Diabetes Mellitus. Nutrients, 13(11), 3815. https://doi.org/10.3390/nu13113815






