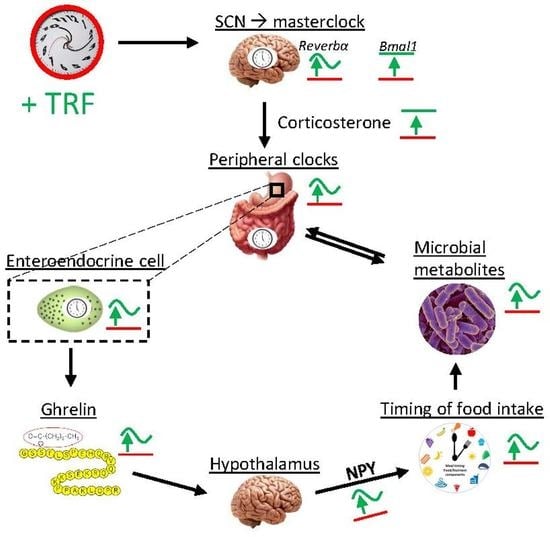
Time-Restricted Feeding in Mice Prevents the Disruption of the Peripheral Circadian Clocks and Its Metabolic Impact during Chronic Jetlag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Experimental Design
2.2.1. Chronic Jetlag Model
2.2.2. Time-Restricted Feeding Model
2.3. Analysis of Fecal SCFA Concentrations
2.4. Quantitative Real-Time PCR
2.5. Plasma Hormone Measurements
2.6. Statistical Analysis
3. Results
3.1. TRF Selectively Restored the Disruption of the Central Circadian Clock during Chronic Jetlag
3.2. TRF Prevented the Increase in Body Mass Induced by Chronic Jetlag
3.3. TRF Partially Prevented the Changes in Rhythmicity of Plasma Ghrelin and Blood Glucose Levels Caused by Chronic Jetlag
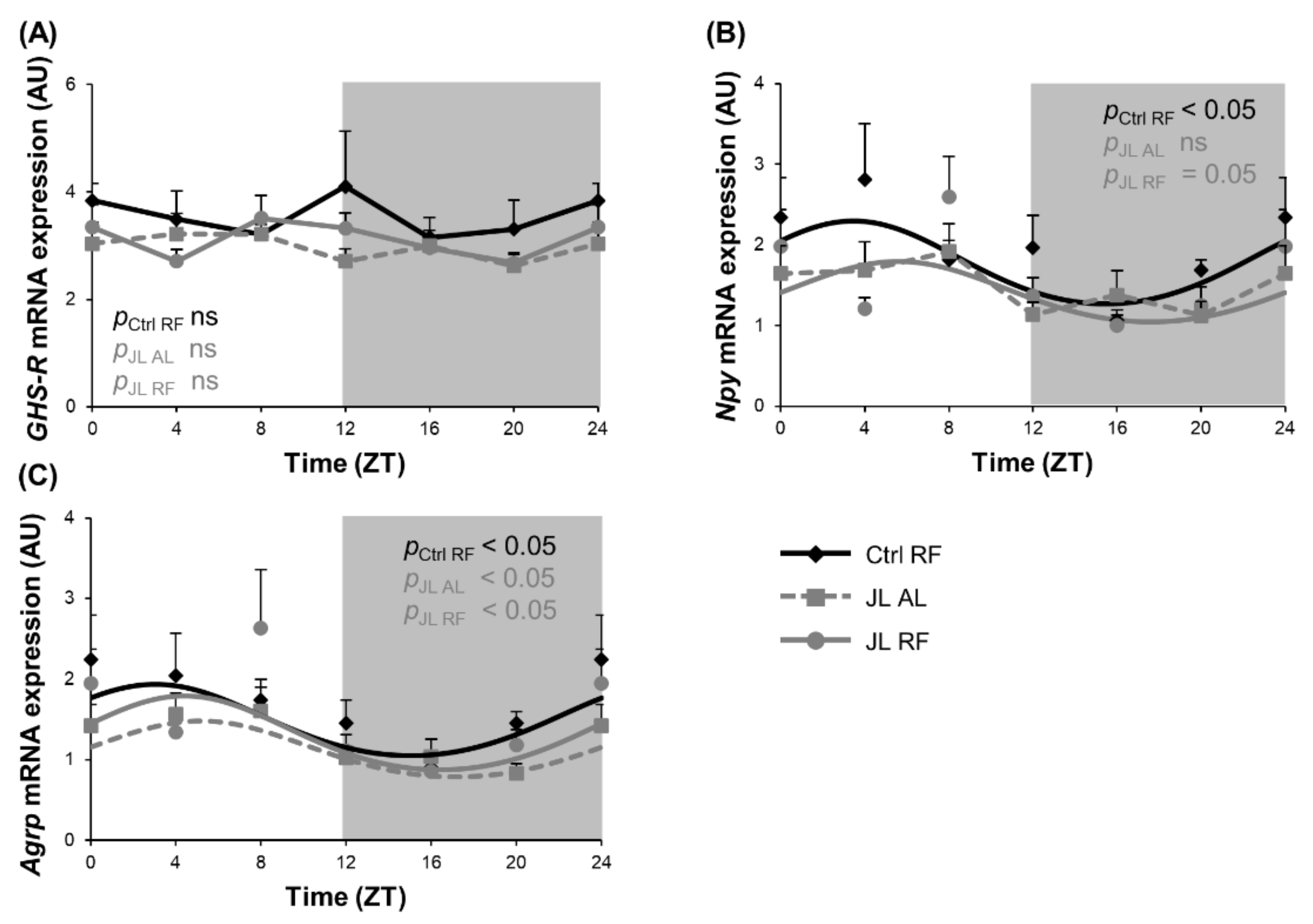
3.4. TRF Prevented the Rhythmicity in Npy mRNA Expression in the Hypothalamus That Was Abolished by Chronic Jetlag
3.5. TRF Prevented Alterations in Diurnal Fluctuations in Fecal SCFA Concentrations Caused by Chronic Jetlag
3.6. TRF Partially Prevented Changes in Clock Gene Expression of Colonic Mucosa Caused by Chronic Jetlag
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segers, A.; Depoortere, I. Circadian clocks in the digestive system. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balsalobre, A. Brown Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 2000, 289, 2344–2347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiola, F.; Le Minh, N. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaix, A.; Lin, T. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 2019, 29, 303–319. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [Green Version]
- Erren, T.C.; Reiter, R.J. Defining chronodisruption. J. Pineal. Res. 2009, 46, 245–247. [Google Scholar] [CrossRef]
- Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Laermans, J.; Depoortere, I. Chronobesity: Role of the circadian system in the obesity epidemic. Obes. Rev. 2016, 17, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Laermans, J.; Broers, C. Shifting the circadian rhythm of feeding in mice induces gastrointestinal, metabolic and immune alterations which are influenced by ghrelin and the core clock gene Bmal1. PLoS ONE 2014, 9, e110176. [Google Scholar] [CrossRef]
- Bae, S.A.; Fang, M.Z. At the Interface of Lifestyle, Behavior, and Circadian Rhythms: Metabolic Implications. Front. Nutr. 2019, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, A.; Kobiita, A. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, E6691–E6698. [Google Scholar] [CrossRef] [Green Version]
- Knutsson, A.; Boggild, H. Gastrointestinal disorders among shift workers. Scand. J. Work Environ. Health 2010, 36, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaiss, C.A.; Zeevi, D. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [Green Version]
- Kettner, N.M.; Mayo, S.A. Circadian Dysfunction Induces Leptin Resistance in Mice. Cell Metab. 2015, 22, 448–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmet, L.; Thijs, T. Chronodisruption by chronic jetlag impacts metabolic and gastrointestinal homeostasis in male mice. Acta Physiol. 2021, e13703. [Google Scholar] [CrossRef]
- Logan, R.W.; McClung, C.A. Rhythms of life: Circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Crowther, M.E.; Ferguson, S.A. Non-Pharmacological Interventions to Improve Chronic Disease Risk Factors and Sleep in Shift Workers: A Systematic Review and Meta-Analysis. Clocks Sleep 2021, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, J.; Navas-Carrillo, D. Alterations of circadian rhythms and their impact on obesity, metabolic syndrome and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2020, 60, 1038–1047. [Google Scholar] [CrossRef]
- Morgan, M.N.; Dvuchbabny, S. The Cancer Clock Is (Not) Ticking: Links between Circadian Rhythms and Cancer. Clocks Sleep 2019, 1, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homolak, J.; Mudrovčić, M. Circadian Rhythm and Alzheimer’s Disease. Med. Sci. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamshed, H.; Beyl, R.A. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatori, M.; Vollmers, C. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cienfuegos, S.; Gabel, K. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Beyl, R. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221. [Google Scholar] [CrossRef] [Green Version]
- Chaix, A.; Zarrinpar, A. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [Green Version]
- Mistlberger, R.E. Neurobiology of food anticipatory circadian rhythms. Physiol. Behav. 2011, 104, 535–545. [Google Scholar] [CrossRef]
- Landry, G.J.; Yamakawa, G.R. The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J. Biol. Rhythm. 2007, 22, 467–478. [Google Scholar] [CrossRef]
- Power, S.C.; Mistlberger, R.E. Food anticipatory circadian rhythms in mice entrained to long or short day photoperiods. Physiol. Behav. 2020, 222, 112939. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol. Metab. 2014, 3, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Trumbauer, M.E. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 2004, 145, 2607–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laermans, J.; Vancleef, L. Role of the clock gene Bmal1 and the gastric ghrelin-secreting cell in the circadian regulation of the ghrelin-GOAT system. Sci. Rep. 2015, 5, 16748. [Google Scholar] [CrossRef] [Green Version]
- Iijima, M.; Takemi, S. The suppressive effect of REVERBs on ghrelin and GOAT transcription in gastric ghrelin-producing cells. Neuropeptides 2021, 90, 102187. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Knight, Z.A. Ablation of AgRP neurons impairs adaption to restricted feeding. Mol. Metab. 2014, 3, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, X. Timing of Calorie Restriction in Mice Impacts Host Metabolic Phenotype with Correlative Changes in Gut Microbiota. Msystems 2019, 4, e00348-19. [Google Scholar] [CrossRef] [Green Version]
- Zarrinpar, A.; Chaix, A. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef] [Green Version]
- Segers, A.; Desmet, L. The circadian clock regulates the diurnal levels of microbial short-chain fatty acids and their rhythmic effects on colon contractility in mice. Acta Physiol. 2019, 225, e13193. [Google Scholar] [CrossRef]
- Govindarajan, K.; MacSharry, J. Unconjugated Bile Acids Influence Expression of Circadian Genes: A Potential Mechanism for Microbe-Host Crosstalk. PLoS ONE 2016, 11, e0167319. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [Green Version]
- Janssen, S.; Laermans, J. Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl. Acad. Sci. USA 2011, 108, 2094–2099. [Google Scholar] [CrossRef] [Green Version]
- Nelson, W.; Tong, Y.L. Methods for cosinor-rhythmometry. Chronobiologia 1979, 6, 305–323. [Google Scholar] [PubMed]
- Tahara, Y.; Yamazaki, M. Gut Microbiota-Derived Short Chain Fatty Acids Induce Circadian Clock Entrainment in Mouse Peripheral Tissue. Sci. Rep. 2018, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Ono, D.; Honma, S. Dissociation of Per1 and Bmal1 circadian rhythms in the suprachiasmatic nucleus in parallel with behavioral outputs. Proc. Natl. Acad. Sci. USA 2017, 114, E3699. [Google Scholar] [CrossRef] [Green Version]
- Kiessling, S.; Eichele, G. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J. Clin. Investig. 2010, 120, 2600–2609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.; Lee, E.J. Cooperative roles of the suprachiasmatic nucleus central clock and the adrenal clock in controlling circadian glucocorticoid rhythm. Sci. Rep. 2017, 7, 46404. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; la Fleur, S.E. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 2003, 464, 36–48. [Google Scholar] [CrossRef]
- Ren, B.; Ma, C. Impact of Time-Restricted Feeding to Late Night on Adaptation to a 6 h Phase Advance of the Light-Dark Cycle in Mice. Front. Physiol. 2021, 12, 634187. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, M.P.; Allison, D.B. Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA 2014, 111, 16647–16653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, J.A.; Richardson, J.D. The causal role of breakfast in energy balance and health: A randomized controlled trial in lean adults. Am. J. Clin. Nutr. 2014, 100, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Coomans, C.P.; van den Berg, S.A. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes 2013, 62, 1102–1108. [Google Scholar] [CrossRef] [Green Version]
- Stoynev, A.G.; Ikonomov, O.C. Feeding pattern and light-dark variations in water intake and renal excretion after suprachiasmatic nuclei lesions in rats. Physiol. Behav. 1982, 29, 35–40. [Google Scholar] [CrossRef]
- LeSauter, J.; Hoque, N. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc. Natl. Acad. Sci. USA 2009, 106, 13582–13587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yannielli, P.C.; Molyneux, P.C. Ghrelin effects on the circadian system of mice. J. Neurosci. 2007, 27, 2890–2895. [Google Scholar] [CrossRef] [Green Version]
- Yi, C.X.; Challet, E. A circulating ghrelin mimetic attenuates light-induced phase delay of mice and light-induced Fos expression in the suprachiasmatic nucleus of rats. Eur. J. Neurosci. 2008, 27, 1965–1972. [Google Scholar] [CrossRef] [PubMed]
- Zigman, J.M.; Jones, J.E. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 2006, 494, 528–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Campos, M.; Aguilera, C.M.A. Ghrelin: A hormone regulating food intake and energy homeostasis. Br. J. Nutr. 2006, 96, 201–226. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.R.; Chen, H. Ghrelin receptors mediate ghrelin-induced excitation of agouti-related protein/neuropeptide Y but not pro-opiomelanocortin neurons. J. Neurochem. 2017, 142, 512–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazato, M.; Murakami, N. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Kamegai, J.; Tamura, H. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes 2001, 50, 2438–2443. [Google Scholar] [CrossRef] [Green Version]
- Clemenzi, M.N.; Martchenko, A. Analysis of Western diet, palmitate and BMAL1 regulation of neuropeptide Y expression in the murine hypothalamus and BMAL1 knockout cell models. Mol. Cell. Endocrinol. 2020, 507, 110773. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, N.; Salehi, A. Bisphenol A Alters Bmal1, Per2, and Rev-Erba mRNA and Requires Bmal1 to Increase Neuropeptide Y Expression in Hypothalamic Neurons. Endocrinology 2019, 160, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cedernaes, J.; Huang, W. Transcriptional Basis for Rhythmic Control of Hunger and Metabolism within the AgRP Neuron. Cell Metab. 2019, 29, 1078–1091. [Google Scholar] [CrossRef]
- Versteeg, R.I.; Serlie, M.J. Serotonin, a possible intermediate between disturbed circadian rhythms and metabolic disease. Neuroscience 2015, 301, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Sasaki, H. The Timing Effects of Soy Protein Intake on Mice Gut Microbiota. Nutrients 2020, 12, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segers, A.; Desmet, L. Night-time feeding of Bmal1-/- mice restores SCFA rhythms and their effect on ghrelin. J. Endocrinol. 2020, 245, 155–164. [Google Scholar] [CrossRef]
- Ye, Y.; Xu, H. Time-Restricted Feeding Reduces the Detrimental Effects of a High-Fat Diet, Possibly by Modulating the Circadian Rhythm of Hepatic Lipid Metabolism and Gut Microbiota. Front. Nutr. 2020, 7, 596285. [Google Scholar] [CrossRef]
- Zeb, F.; Wu, X. Time-restricted feeding is associated with changes in human gut microbiota related to nutrient intake. Nutrition 2020, 78, 110797. [Google Scholar] [CrossRef]
- Beli, E.; Yan, Y. Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice. Diabetes 2018, 67, 1867–1879. [Google Scholar] [CrossRef] [Green Version]
- Zeb, F.; Wu, X. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br. J. Nutr. 2020, 123, 1216–1226. [Google Scholar] [CrossRef]
- De Goede, P.; Sen, S. Differential effects of diet composition and timing of feeding behavior on rat brown adipose tissue and skeletal muscle peripheral clocks. Neurobiol. Sleep Circadian Rhythm. 2018, 4, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Segall, L.A.; Verwey, M. Timed restricted feeding restores the rhythms of expression of the clock protein, Period2, in the oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in adrenalectomized rats. Neuroscience 2008, 157, 52–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Patel, M.S. The molecular clock mediates leptin-regulated bone formation. Cell 2005, 122, 803–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Dang, F. Glucagon-CREB/CRTC2 signaling cascade regulates hepatic BMAL1 protein. J. Biol. Chem. 2015, 290, 2189–2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Yin, Y. Ghrelin Restores the Disruption of the Circadian Clock in Steatotic Liver. Int. J. Mol. Sci. 2018, 19, 3134. [Google Scholar] [CrossRef] [Green Version]






| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| β-actin | CCTGTGCTGCTCACCGAGGC | GACCCCGTCTCTCCGGAGTCCATC |
| Cycloph | GGAGATGGCACAGGAGGAAA | CCCGTAGTGCTTCAGCTTGAA |
| Hmbs | CTGAAGGATGTGCCTACCATAC | AAGGTTTCCAGGGTCTTTCC |
| Tbp | AGGATGCTCTAGGGAAGAT | TGAATAGGCTGTGGAGTAAGT |
| Npy | CCGCTCTGCGACACTACAT | TGTCTCAGGGCTGGATCTCT |
| Agrp | GCGGAGGTGCTAGATCCA | AGGACTCGTGCAGCCTTA |
| GHS-R | TCAGGGACCAGAACCACAAA | CCAGCAGAGGATGAAAGCAA |
| Arntl | CGTTTCTCGACACGCAATAGAT | TCCTGTGGTAGATACGCCAAAA |
| Reverbα | CCCTGGACTCCAATAACAACACA | GCCATTGGAGCTGTCACTGTAG |
| Condition | p Value Cosinor | Acrophase (h) (±SD) | Amplitude | Mesor (±SD) | |||
|---|---|---|---|---|---|---|---|
| Hypothalamus | Circadian Clock | Arntl | Ctrl RF | <0.001 | 23h38 (±0h46) | 0.38 | 1.44 (±0.05) |
| JL AL | ns | NA | NA | NA | |||
| JL RF | ns | NA | NA | NA | |||
| Reverbα | Ctrl RF | <0.001 | 11h47 (±0h56) | 0.39 | 1.52 (±0.07) | ||
| JL AL | <0.05 | 16h07 (±1h11) ** | 0.25 | 1.39 (±0.06) | |||
| JL RF | <0.05 | 11h50 (±1h23) # | 0.25 | 1.49 (±0.06) | |||
| GHS-R | Ctrl RF | NA | NA | NA | NA | ||
| JL AL | NA | NA | NA | NA | |||
| JL RF | NA | NA | NA | NA | |||
| Npy | Ctrl RF | <0.05 | 3h29 (±1h19) | 0.52 | 1.71 (±0.13) | ||
| JL AL | ns | NA | NA | NA | |||
| JL RF | =0.05 | 5h37 (±1h22) | 0.38 | 1.37 (±0.10) * | |||
| Agrp | Ctrl RF | <0.05 | 3h02 (±1h24) | 0.44 | 1.43 (±0.12) | ||
| JL AL | <0.05 | 5h10 (±1h16) | 0.35 | 1.08 (±0.08) * | |||
| JL RF | <0.05 | 4h24 (±1h07) | 0.46 | 1.25 (±0.10) | |||
| Plasma | Corticosterone | Ctrl RF | <0.05 | 14h46 (±1h16) | 27996.33 | 34348.46 (±5488.43) | |
| JL AL | ns | NA | NA | NA | |||
| JL RF | ns | NA | NA | NA | |||
| Total ghrelin | Ctrl RF | <0.001 | 8h03 (±0h29) | 4073.69 | 9306.82 (±351.71) | ||
| JL AL | ns | NA | NA | NA | |||
| JL RF | <0.001 | 8h04 (±0h37) | 2765.73 * | 7359.93 (±312.97) *** | |||
| Octanoylated ghrelin | Ctrl RF | <0.001 | 7h36 (±0h46) | 16.99 | 43.89 (±2.42) | ||
| JL AL | ns | NA | NA | NA | |||
| JL RF | <0.05 | 9h49 (±1h16) | 9.73 | 43.07 (±2.27) | |||
| Glucose | Ctrl RF | <0.05 | 4h42 (±1h18) | 18.88 | 226.65 (±4.92) | ||
| JL AL | ns | NA | NA | NA | |||
| JL RF | <0.05 | 6h37 (±1h03) | 22.88 | 207.22 (±4.45) ** | |||
| Fecal SCFA | Acetate | Ctrl RF | <0.01 | 4h28 (±0h57) | 3.84 | 24.62 (±0.67) | |
| JL AL | ns | NA | NA | NA | |||
| JL RF | <0.05 | 6h39 (±1h14) | 3.08 | 25.64 (±0.71) | |||
| Butyrate | Ctrl RF | <0.001 | 3h00 (±0h32) | 1.78 | 2.66 (±0.16) | ||
| JL AL | ns | NA | NA | NA | |||
| JL RF | <0.001 | 3h13 (±0h41) | 1.23 | 2.47 (±0.15) | |||
| Propionate | Ctrl RF | <0.001 | 5h44 (±0h41) | 0.68 | 2.11 (±0.86) | ||
| JL AL | <0.05 | 12h48 (±1h24) *** | 0.44 | 2.89 (±0.12) *** | |||
| JL RF | <0.05 | 5h35 (±1h12) ### | 0.49 | 2.58 (±0.11) *** | |||
| Total SCFA | Ctrl RF | <0.001 | 4h14 (±0h45) | 2.92 | 29.68 (±0.88) | ||
| JL AL | ns | NA | NA | NA | |||
| JL RF | <0.05 | 5h51 (±1h04) | 4.70 | 30.99 (±0.93) | |||
| Distal Colonic Mucosa | Circadian Clock | Arntl | Ctrl RF | <0.001 | 1h07 (±0h11) | 0.57 | 0.50 (±0.02) |
| JL AL | <0.001 | 7h02 (±0h15) *** | 0.44 ** | 0.52 (±0.02) | |||
| JL RF | <0.001 | 3h05 (±0h12) *** ### | 0.54 # | 0.49 (±0.02) | |||
| Reverbα | Ctrl RF | <0.001 | 9h54 (±0h11) | 7.60 | 1.97 (±0.14) | ||
| JL AL | <0.001 | 16h23 (±0h14) *** | 5.03 * | 2.10 (±0.15) | |||
| JL RF | <0.001 | 12h29 (±0h12) *** ### | 6.03 | 2.01 (±0.15) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desmet, L.; Thijs, T.; Mas, R.; Verbeke, K.; Depoortere, I. Time-Restricted Feeding in Mice Prevents the Disruption of the Peripheral Circadian Clocks and Its Metabolic Impact during Chronic Jetlag. Nutrients 2021, 13, 3846. https://doi.org/10.3390/nu13113846
Desmet L, Thijs T, Mas R, Verbeke K, Depoortere I. Time-Restricted Feeding in Mice Prevents the Disruption of the Peripheral Circadian Clocks and Its Metabolic Impact during Chronic Jetlag. Nutrients. 2021; 13(11):3846. https://doi.org/10.3390/nu13113846
Chicago/Turabian StyleDesmet, Louis, Theo Thijs, Rosalie Mas, Kristin Verbeke, and Inge Depoortere. 2021. "Time-Restricted Feeding in Mice Prevents the Disruption of the Peripheral Circadian Clocks and Its Metabolic Impact during Chronic Jetlag" Nutrients 13, no. 11: 3846. https://doi.org/10.3390/nu13113846
APA StyleDesmet, L., Thijs, T., Mas, R., Verbeke, K., & Depoortere, I. (2021). Time-Restricted Feeding in Mice Prevents the Disruption of the Peripheral Circadian Clocks and Its Metabolic Impact during Chronic Jetlag. Nutrients, 13(11), 3846. https://doi.org/10.3390/nu13113846