KDP, a Lactobacilli Product from Kimchi, Enhances Mucosal Immunity by Increasing Secretory IgA in Mice and Exhibits Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Lactic Acid Bacteria KDP
2.3. Chemicals
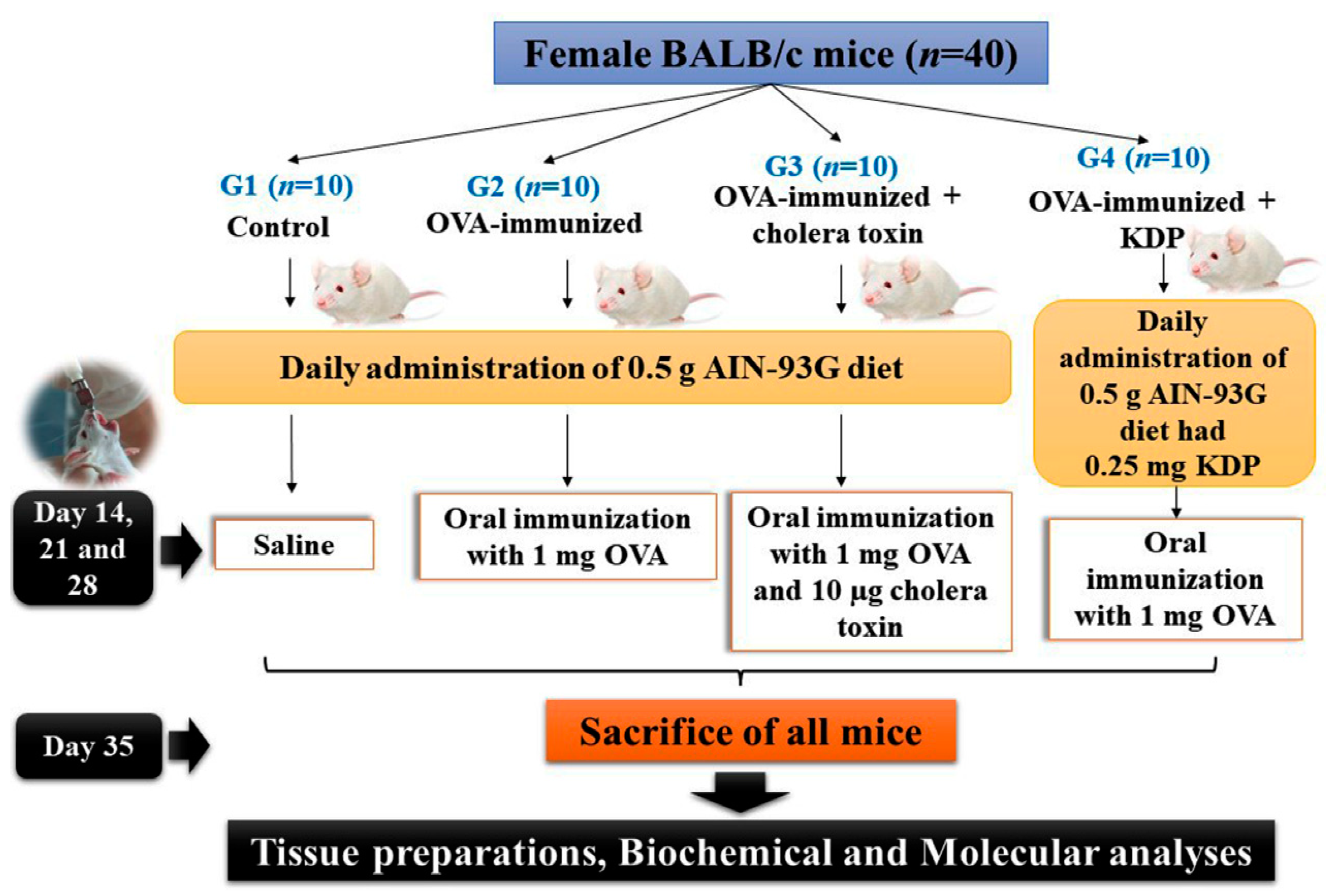
2.4. Experimental Design
2.5. Preparation of Tissues
2.6. Cytokine and IgA Measurements
2.6.1. Determination of Total IgA
2.6.2. Determination of OVA-IgA
2.6.3. RNA Extraction and RT-PCR Analysis
2.7. Antioxidant Activity
2.7.1. Determination of DPPH Scavenging Activity
2.7.2. Determination of NO Radical Scavenging Activity
2.7.3. Reducing Power Assay
2.7.4. Total Antioxidant Capacity
2.8. Antimicrobial Activity
2.9. Statistical Analysis
3. Results
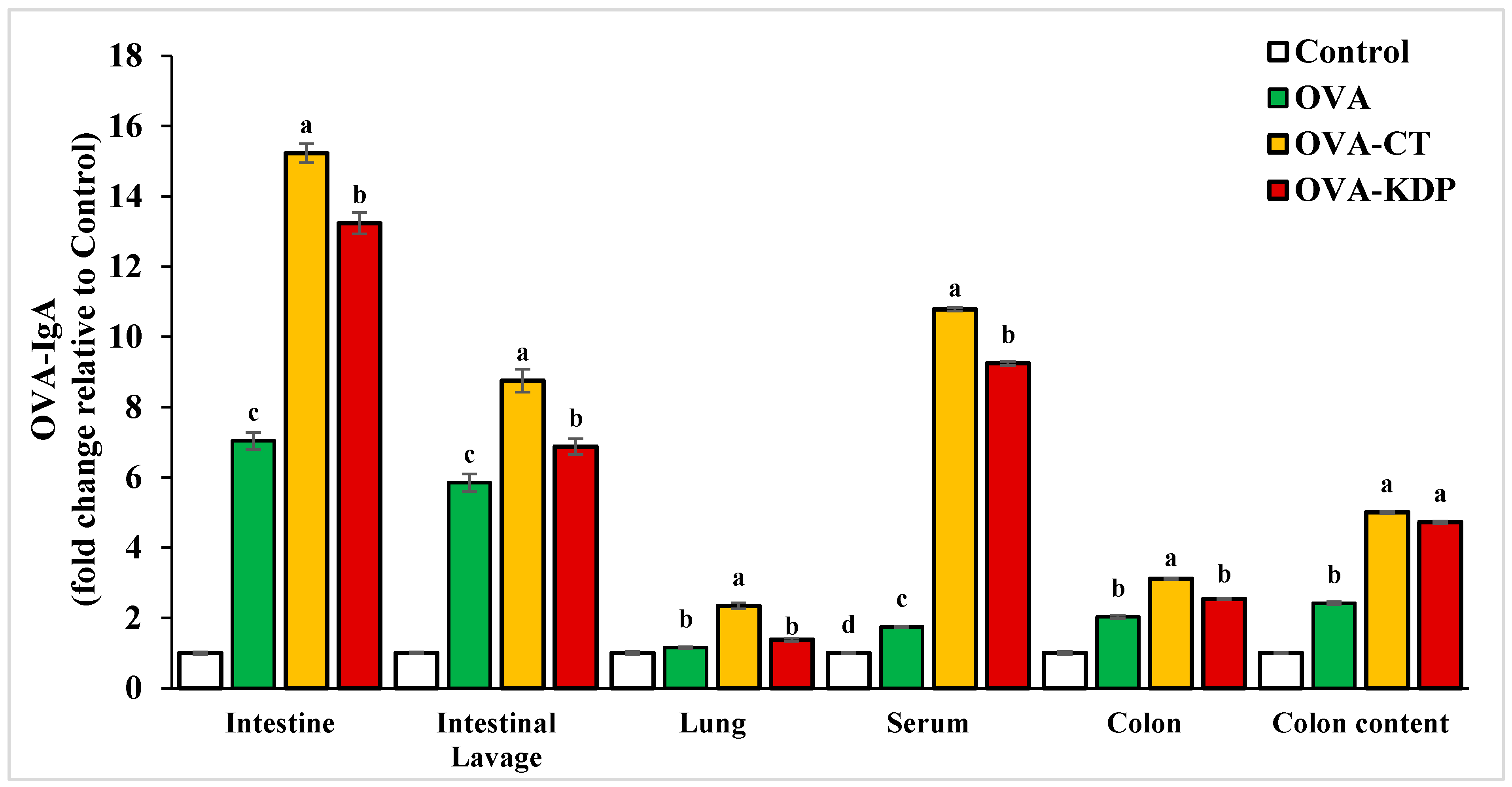
3.1. KDP Induced Antigen-Specific IgA Production
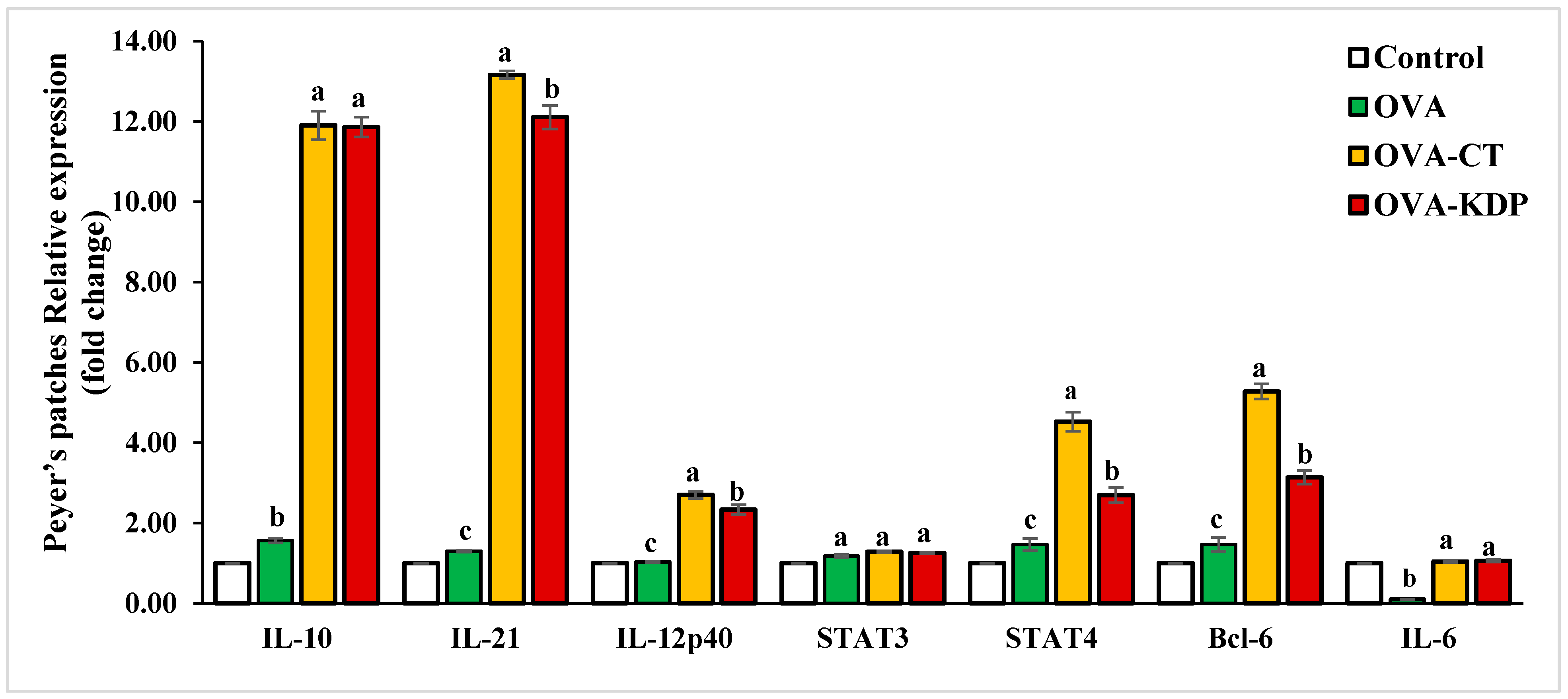
3.2. KDP Increased Gene Expression Levels of IL-10, IL-12p40, IL-21, STAT4, and Bcl-6 in Peyer’s Patches
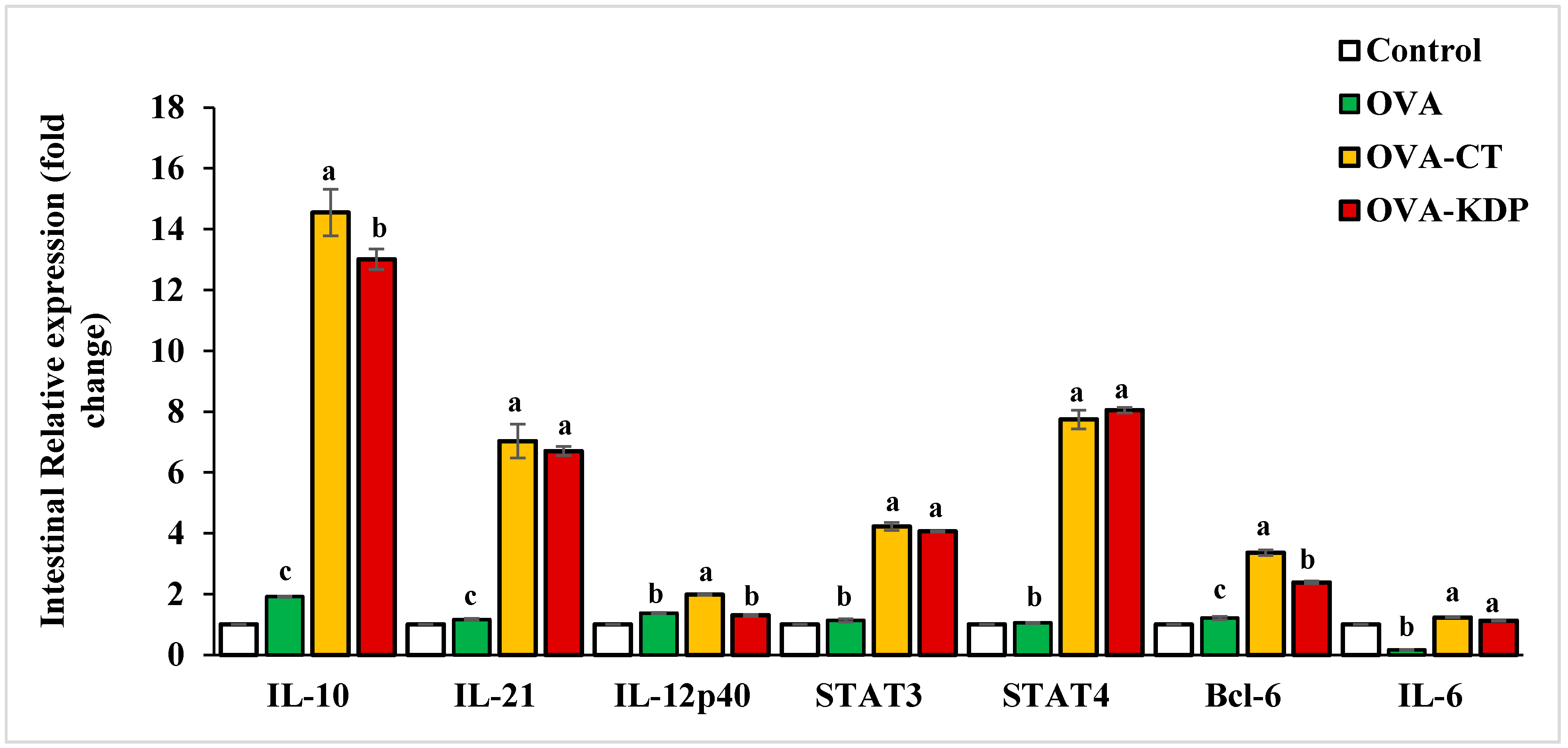
3.3. KDP Increased the Gene Expression Levels of IL-10, IL-12p40, IL-21, STAT4, and Bcl-6 in the Small Intestine
3.4. KDP Enhances Antioxidant Activity
3.5. KDP Exhibits Antimicrobial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lycke, N.Y.; Bemark, M. The role of Peyer’s patches in synchronizing gut IgA responses. Front. Immunol. 2012, 3, 329. [Google Scholar] [CrossRef] [Green Version]
- Fagarasan, S.; Honjo, T. Intestinal IgA synthesis: Regulation of front-line body defences. Nat. Rev. Immunol. 2003, 3, 63–72. [Google Scholar] [CrossRef]
- Kiyono, H.; Fukuyama, S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat. Rev. Immunol. 2004, 4, 699–710. [Google Scholar] [CrossRef]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef]
- Fazilleau, N.; Mark, L.; McHeyzer-Williams, L.J.; McHeyzer-Williams, M.G. Follicular helper T cells: Lineage and location. Immunity 2009, 30, 324–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazemi, A.; Soltani, S.; Nasri, F.; Clark, C.C.T.; Kolahdouz-Mohammadi, R. The effect of probiotics, parabiotics, synbiotics, fermented foods and other microbial forms on immunoglobulin production: A systematic review and meta-analysis of clinical trials. Int. J. Food Sci. Nutr. 2020, 72, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Hayashi, K.; Kosaka, A.; Kawashima, M.; Igarashi, T.; Tsutsui, H.; Tsuji, N.M.; Nishimura, I.; Hayashi, T.; Obata, A. Lactobacillus plantarum strain YU from fermented foods activates Th1 and protective immune responses. Int. Immunopharmacol. 2011, 11, 2017–2024. [Google Scholar] [CrossRef]
- Jang, H.J.; Yu, H.S.; Lee, N.K.; Paik, H.D. Immune-stimulating effect of Lactobacillus plantarum Ln1 isolated from the traditional Korean fermented food, Kimchi. J. Microbiol. Biotechnol. 2020, 30, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Gill, H.S. Immune protection mediated by the probiotic Lactobacillus rhamnosus HN001 (DR20) against Escherichia coli O157:H7 infection in mice. FEMS Immunol. Med. Microbiol. 2020, 34, 59–64. [Google Scholar] [CrossRef]
- Corr, S.C.; Gahan, C.G.; Hill, C. Impact of selected Lactobacillus and Bifidobacterium species on Listeria monocytogenes infection and the mucosal immune response. FEMS Immunol. Med. Microbiol. 2007, 50, 380–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.K.; Park, S.Y.; An, H.M.; Kim, J.R.; Kim, M.J.; Lee, S.W.; Cha, M.K.; Kim, S.A.; Chung, M.J.; Lee, K.O.; et al. Antimicrobial activity of Bifidobacterium spp. isolated from healthy adult Koreans against cariogenic microflora. Arch. Oral Biol. 2011, 56, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Chattha, K.S.; Kandasamy, S.; Liu, Z.; Esseili, M.; Shao, L.; Rajashekara, G.; Saif, L.J. Lactobacilli and bifidobacteria promote immune homeostasis by modulating innate immune responses to human rotavirus in neonatal gnotobiotic pigs. PLoS ONE 2013, 8, e76962. [Google Scholar] [CrossRef]
- Gagnon, M.; Vimont, A.; Darveau, A.; Fliss, I.; Jean, J. Study of the ability of bifidobacteria of human origin to prevent and treat rotavirus infection using colonic cell and mouse models. PLoS ONE 2016, 11, e0164512. [Google Scholar] [CrossRef] [PubMed]
- Badr El-Din, N.K.; Shabana, S.M.; Abdulmajeed, B.A.; Ghoneum, M. A novel kefir product (PFT) inhibits Ehrlich ascites carcinoma in mice via induction of apoptosis and immunomodulation. BMC Complement. Med. Ther. 2020, 20, 127. [Google Scholar] [CrossRef]
- Lim, H.J.; Shin, H.S. Antimicrobial and Immunomodulatory Effects of Bifidobacterium Strains: A Review. J. Microbiol. Biotechnol. 2020, 30, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Garrat, D.C. The Quantitative Analysis of Drugs Japan, 3rd ed.; Chapman and Hall Ltd.: Tokyo, Japan, 1964; Volume 3, pp. 456–458. [Google Scholar]
- Yildirim, A.; Mani, A.; Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- Umamaheswari, M.; Chatterjee, T.K. In vitro antioxidant activities of the fractions of Coccinnia grandis L. leaf extract. Afr. J. Tradit. Complement. Altern. Med. 2007, 5, 61–73. [Google Scholar]
- Kadaikunnan, S.; Rejiniemon, T.S.; Khaled, J.M.; Alharbi, N.S.; Mothana, R. In-vitro antibacterial, antifungal, antioxidant and functional properties of Bacillus amyloliquefaciens. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 9. [Google Scholar] [CrossRef] [Green Version]
- Pierre, P.; Denis, O.; Bazin, H.; Mbongolo Mbella, E.; Vaerman, J.P. Modulation of oral tolerance to ovalbumin by cholera toxin and its B subunit. Eur. J. Immunol. 1992, 22, 3179–3182. [Google Scholar] [CrossRef]
- Mattsson, J.; Schön, K.; Ekman, L.; Fahlén-Yrlid, L.; Yrlid, U.; Lycke, N.Y. Cholera toxin adjuvant promotes a balanced Th1/Th2/Th17 response independently of IL-12 and IL-17 by acting on Gsα in CD11b+ DCs. Mucosal Immunol. 2015, 8, 815–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, S.; Iwabuchi, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Hachimura, S. Orally administered heat-killed Lactobacillus paracasei MCC1849 enhances antigen-specific IgA secretion and induces follicular helper T cells in mice. PLoS ONE 2018, 13, e0199018. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Tan, Z.L.; Abu Hafsa, S.H.; Adegbeye, M.J.; Greiner, R.; Ugbogu, E.A.; Cedillo Monroy, J.; Salem, A.Z.M. Saccharomyces cerevisiae as a probiotic feed additive to non and pseudo-ruminant feeding: A review. J. Appl. Microbiol. 2020, 128, 658–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Terahara, M.; Iwamoto, T.; Yamada, K.; Asano, M.; Kakuta, S.; Iwakura, Y.; Totsuka, M. Upregulation of polymeric immunoglobulin receptor expression by the heat-inactivated potential probiotic Bifidobacterium bifidum OLB6378 in a mouse intestinal explant model. Scand. J. Immunol. 2012, 75, 176–183. [Google Scholar] [CrossRef]
- Lee, A.; Lee, Y.J.; Yoo, H.J.; Kim, M.; Chang, Y.; Lee, D.S.; Lee, J.H. Consumption of dairy yogurt containing Lactobacillus paracasei ssp. paracasei, Bifidobacterium animalis ssp. lactis and heat-treated Lactobacillus plantarum improves immune function including natural killer cell activity. Nutrients 2017, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Akutko, K.; Stawarski, A. Probiotics, Prebiotics and Synbiotics in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 2466. [Google Scholar] [CrossRef]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef]
- Kawashima, T.; Ikari, N.; Kouchi, T.; Kowatari, Y.; Kubota, Y.; Shimojo, N.; Tsuji, N.M. The molecular mechanism for activating IgA production by Pediococcus acidilactici K15 and the clinical impact in a randomized trial. Sci Rep. 2018, 8, 5065. [Google Scholar] [CrossRef]
- Hutchins, A.P. The IL-10/STAT3-mediated anti-inflammatory response: Recent developments and future challenges. Brief Funct. Genom. 2013, 12, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Spolski, R.; Leonard, W.J. Interleukin-21: A double-edged sword with therapeutic potential. Nat. Rev. Drug Discov. 2014, 13, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Fina, D.; Sarra, M.; Fantini, M.C.; Rizzo, A.; Caruso, R.; Caprioli, F.; Stolfi, C.; Cardolini, I.; Dottori, M.; Boirivant, M.; et al. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology 2008, 134, 1038–1048. [Google Scholar] [CrossRef]
- Stolfi, C.; Rizzo, A.; Franzè, E.; Rotondi, A.; Fantini, M.C.; Sarra, M.; Caruso, R.; Monteleone, I.; Sileri, P.; Franceschilli, L.; et al. Involvement of interleukin-21 in the regulation of colitis associated colon cancer. J. Exp. Med. 2011, 208, 2279–2290. [Google Scholar] [CrossRef]
- Cho, H.; Jaime, H.; Oliveira, R.P.; Kang, B.; Spolski, R. Defective IgA response to atypical intestinal commensals in IL-21 receptor deficiency reshapes immune cell homeostasis and mucosal immunity. Mucosal Immunol. 2019, 12, 85–96. [Google Scholar] [CrossRef]
- Avery, D.T.; Bryant, V.L.; Ma, C.S.; de Waal Malefyt, R.; Tangye, S.G. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J. Immunol. 2008, 181, 1767–1779. [Google Scholar] [CrossRef] [Green Version]
- Seo, G.Y.; Youn, J.; Kim, P.H. IL-21 ensures TGF-beta 1-induced IgA isotype expression in mouse Peyer’s patches. J. Leukoc. Biol. 2009, 85, 744–750. [Google Scholar] [CrossRef]
- Cao, A.T.; Yao, S.; Gong, B.; Nurieva, R.I.; Elson, C.O.; Cong, Y. Interleukin (IL)-21 promotes intestinal IgA response to microbiota. Mucosal Immunol. 2015, 8, 1072–1082. [Google Scholar] [CrossRef] [Green Version]
- Nurieva, R.I.; Chung, Y.; Martinez, G.J.; Yang, X.O.; Tanaka, S.; Matskevitch, T.D.; Wang, Y.; Dong, C. Bcl6 mediates the development of T follicular helper cells. Science 2009, 325, 1001–1005. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Chen, J.; Chen, Q.; Yang, L.; Yin, J.; Li, Y.; Huang, Y. Lactobacillus delbrueckii Protected Intestinal Integrity, Alleviated Intestinal Oxidative Damage, and Activated Toll-Like Receptor-Bruton’s Tyrosine Kinase-Nuclear Factor Erythroid 2-Related Factor 2 Pathway in Weaned Piglets Challenged with Lipopolysaccharide. Antioxidants 2021, 10, 468. [Google Scholar] [PubMed]
- Petrova, P.; Ivanov, I.; Tsigoriyna, L.; Valcheva, N.; Vasileva, E.; Parvanova-Mancheva, T.; Arsov, A.; Petrov, K. Traditional Bulgarian dairy products: Ethnic foods with health benefits. Microorganisms 2021, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Cizeikiene, D.; Gaide, I.; Basinskiene, L. Effect of lactic acid fermentation on quinoa characteristics and quality of quinoa-wheat composite bread. Foods 2021, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.; Abdulmalek, S.; Pan, D. Reversal of age-associated oxidative stress in mice by PFT, a novel kefir product. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420950149. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Sakai, Y.; Tsukahara, T.; Bukawa, W.; Matsubara, N.; Ushida, K. Cell preparation of enterococcus faecalis strain EC-12 prevents vancomycin-resistant enterococci colonization in the cecum of newly hatched chicks. Poult. Sci. 2006, 85, 273–277. [Google Scholar] [CrossRef]
- Kanasugi, H.; Hasegawa, T.; Goto, Y.; Ohtsuka, H.; Makimura, S.; Yamamoto, T. Single administration of enterococcal preparation (FK-23) augments nonspecific immune responses in healthy dogs. Int. J. Immunopharmacol. 1997, 19, 655–659. [Google Scholar] [CrossRef]
- Lahtinen, S.J. Probiotic viability—Does it matter? Microb. Ecol. Health Dis. 2012, 23, 18567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, S.D.; Zhang, D.Z.; Lu, H.; Jiang, S.H.; Liu, H.Y.; Wang, G.S.; Zhang, Z.B.; Lin, G.J.; Wang, G.l. Multicenter, randomized, controlled trial of heat-killed Lactobacillus acidophilus LB in patients with chronic diarrhea. Adv. Ther. 2003, 20, 253–260. [Google Scholar] [CrossRef]
- Marin, M.L.; Lee, J.H.; Murtha, J.; Ustunol, Z.; Pestka, J.J. Differential cytokine production in clonal macrophage and T-cell lines cultured with bifidobacteria. J. Dairy Sci. 1997, 80, 2713–2720. [Google Scholar] [CrossRef]

| Primer | Forward | Reverse |
|---|---|---|
| IL-6 (NM_012589.2) | 5′-TGGAGTCACAGAAGGAGTGGCTAAG-3′ | 5′-TCTGACCACAGTGAGGAATGTCAAC-3′ |
| IL-10 (NM_012854.2) | 5′-CCAAGCCTTATCGGAAATGA-3′ | 5′-TTTTCACAGGGGAGAAATCG-3′ |
| IL-12p40 (NM_022611.1) | 5′- TGGTTTGCCATCGTTTTGCTG -3′ | 5′-ACAGGTGAGGTTCACTGTTTCT-3′ |
| IL-21 (NM_001108943.2) | 5′-CGCCTCCTGATTAGACTTCG-3′ | 5′-TGTTTCTTTCCTCCCCTCCT-3′ |
| IFN-γ (NM_008337.4) | 5′-CAGCAACAGCAAGGCGAAAAAGG-3′ | 5′-TTTCCGCTTCCTGAGGCTGGAT-3′ |
| STAT3 (NM_012747.2) | 5′-GACCCGCCAACAAATTAAGA-3′ | 5′-TCGTGGTAAACTGGACACCA-3′ |
| STAT4 (NM_001012226.1) | 5′-CATCCCTGAAAACCCTCTGA-3′ | 5′-GACATGGGGAGAAGGTCTGA-3′ |
| Bcl-6 (NM_001107084.1) | 5′-CCTGAGGGAAGGCAATATCA-3′ | 5′-CGGCTGTTCAGGAACTCTTC-3′ |
| GAPDH (NM_017008.4) | 5′-TGTGTCCGTCGTGGATCTGA-3′ | 5′-TTGCTGTTGAAGTCGCAGGAG-3′ |
| % Inhibition | ||||||||
|---|---|---|---|---|---|---|---|---|
| DPPH | Nitric Oxide | Total Antioxidant Capacity | Reducing Power Assay | |||||
| Concentration (μg/mL) | Ascorbic Acid | KDP | Ascorbic Acid | KDP | Ascorbic Acid | KDP | Ascorbic Acid | KDP |
| 5 | 32.50 e | 11.56 f | 35.05 e | 3.50 f | 39.30 e | 11.85 f | 29.55 f | 8.00 f |
| 10 | 50.63 d | 24.06 e | 47.03 d | 15.80 e | 51.33 d | 23.03 e | 44.63 e | 17.01 e |
| 20 | 78.75 c | 31.25 d | 68.95 c | 29.09 d | 71.55 c | 35.85 d | 63.65 d | 31.90 d |
| 40 | 84.69 b | 53.13 c | 87.09 b | 42.06 c | 83.56 b | 44.79 c | 79.09 c | 43.00 c |
| 80 | 96.40 a | 66.88 b | 95.44 a | 61.94 b | 91.65 a | 56.44 b | 88.14 b | 52.04 b |
| 100 | 99.10 a | 75.56 a | 98.91 a | 72.05 a | 97.32 a | 69.01 a | 97.36 a | 66.50 a |
| IC50 Value (μg/mL) | ||||
|---|---|---|---|---|
| DPPH | Nitric Oxide | Total Antioxidant Capacity | Reducing Power | |
| Ascorbic acid | 1.74 ± 0.01 b | 5.98 ± 0.02 b | 0.11 ± 0.001 b | 14.70 ± 0.01 b |
| KDP | 52.90 ± 0.03 a | 62.46 ± 0.35 a | 61.15 ± 0.29 a | 67.92 ± 0.31 a |
| Sample Concentration (mg/mL) | ||||||
|---|---|---|---|---|---|---|
| Pathogenic Strain | 200 * | 100 * | 50 * | 25 * | 12.50 * | MIC |
| Gram-Negative Bacteria | Inhibition Zone Diameter (mm) ** | |||||
| Escherichia coli ATCC25922 | 25 ± 0.12 | 23 ± 0.21 | 20 ± 0.23 | 19 ± 0.23 | 17 ± 0.21 | 12.5 |
| Salmonella typhi ATCC14028 | 17 ± 0.03 | 11 ± 0.15 | ND | ND | ND | 100 |
| Klebsiella pneumoniaATCC700603 | 21 ± 0.09 | 17 ± 0.18 | 13 ± 0.2 | ND | ND | 50 |
| Gram-Positive Bacteria | Inhibition Zone Diameter (mm) ** | |||||
| Streptococcus pyogenes EMCC1772 | 10 ± 0.05 | 6 ± 0.03 | ND | ND | ND | 100 |
| Staphylococcus aureus ATCC25923 | 6 ± 0.02 | 3 ± 0.06 | ND | ND | ND | 100 |
| Bacillus cereus ATCC10876 | 15 ± 0.08 | 13 ± 0.14 | 8 ± 0.17 | ND | ND | 50 |
| Yeast | Inhibition Zone Diameter (mm) ** | |||||
| Candida albicans EMCC105 | 7 ± 0.07 | 3 ± 0.03 | ND | ND | 50 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoneum, M.; Abdulmalek, S. KDP, a Lactobacilli Product from Kimchi, Enhances Mucosal Immunity by Increasing Secretory IgA in Mice and Exhibits Antimicrobial Activity. Nutrients 2021, 13, 3936. https://doi.org/10.3390/nu13113936
Ghoneum M, Abdulmalek S. KDP, a Lactobacilli Product from Kimchi, Enhances Mucosal Immunity by Increasing Secretory IgA in Mice and Exhibits Antimicrobial Activity. Nutrients. 2021; 13(11):3936. https://doi.org/10.3390/nu13113936
Chicago/Turabian StyleGhoneum, Mamdooh, and Shaymaa Abdulmalek. 2021. "KDP, a Lactobacilli Product from Kimchi, Enhances Mucosal Immunity by Increasing Secretory IgA in Mice and Exhibits Antimicrobial Activity" Nutrients 13, no. 11: 3936. https://doi.org/10.3390/nu13113936
APA StyleGhoneum, M., & Abdulmalek, S. (2021). KDP, a Lactobacilli Product from Kimchi, Enhances Mucosal Immunity by Increasing Secretory IgA in Mice and Exhibits Antimicrobial Activity. Nutrients, 13(11), 3936. https://doi.org/10.3390/nu13113936






