The Type of Fat in the Diet Influences Regulatory Aminopeptidases of the Renin-Angiotensin System and Stress in the Hypothalamic-Pituitary-Adrenal Axis in Adult Wistar Rats
Abstract
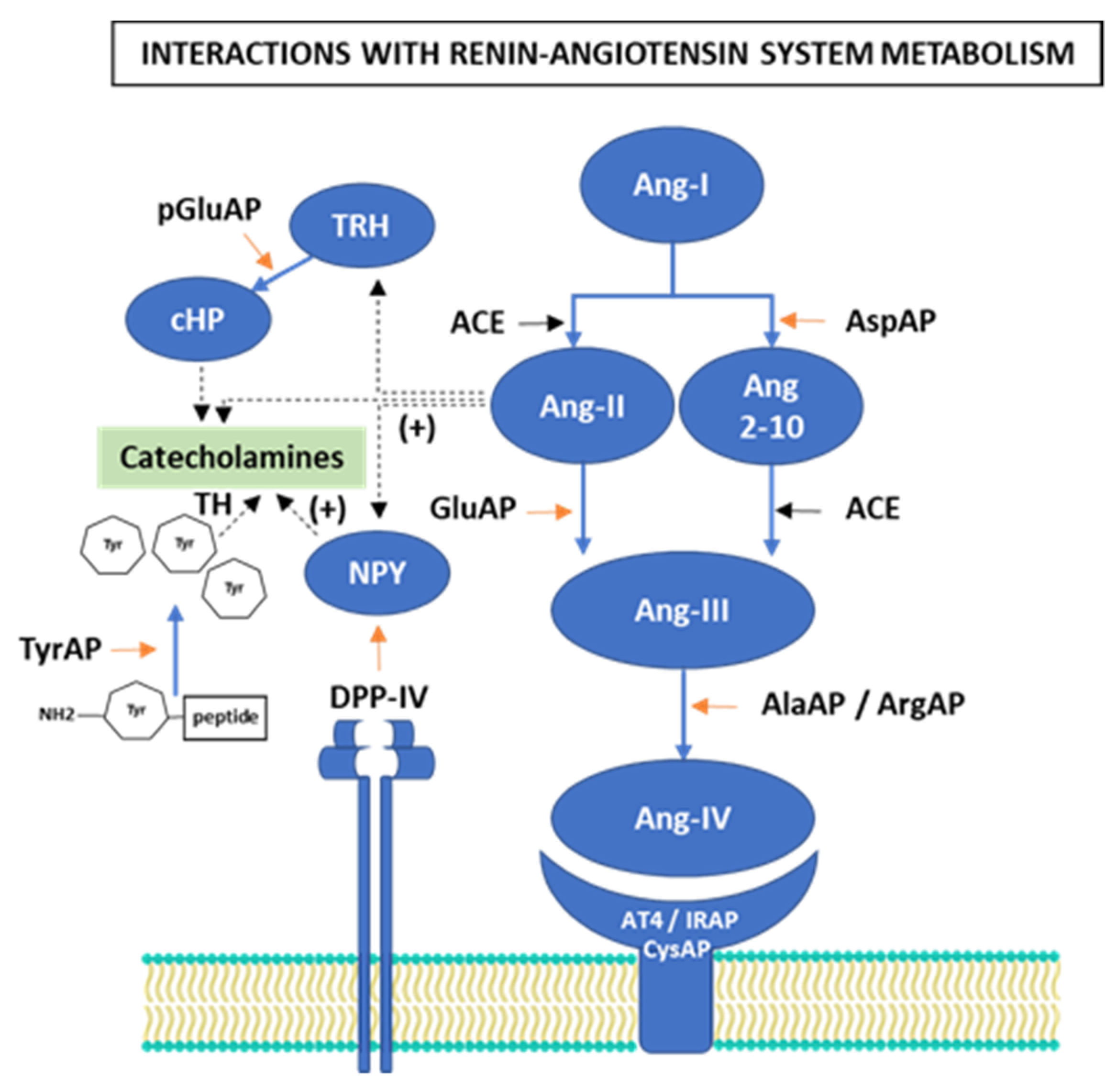
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Assay of Aminopeptidase Activities
2.3. Protein Measurement
2.4. Statistic Analysis
3. Results
3.1. Angiotensinase Activities
3.1.1. Aspartyl-Aminopeptidase Activity
3.1.2. Glutamyl-Aminopeptidase Activity
3.1.3. Alanyl-Aminopeptidase and Arginyl-Aminopeptidase Activities
3.1.4. Cystinyl-Aminopeptidase Activity
3.1.5. Interrelation and Intrarelation of Angiotensinase Activities in the Tissues That Form the Hypothalamic-Pituitary-Adrenal Axis
3.2. Dipeptidyl Peptidase IV, Pyroglutamyl-Aminopeptidase and Tyrosyl-Aminopeptidase Activities
3.3. Interrelation between Stress Activities and Eating Behavior with RAS Regulatory Activities
3.4. Proline-Iminopeptidase Activity like Neuromarker in Hypothalamus and Pituitary
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AlaAP | alanyl-aminopeptidase |
| Ang | angiotensin |
| AP | aminopeptidase |
| ArgAP | arginyl-aminopeptidase |
| AspAP | aspartyl-aminopeptidase |
| Bch | butter plus cholesterol |
| BP | blood pressure |
| CysAP | cystinyl-aminopeptidase |
| CNS | central nervous system |
| DPP-IV | dipeptidyl peptidase IV |
| GluAP | glutamyl-aminopeptidase |
| HFD | high-fat diet |
| IRAP | insulin-regulated aminopeptidase |
| MUFA | monounsaturated fatty acids |
| pGluAP | pyroglutamyl-aminopeptidase |
| PIP | Proline-iminopeptidase |
| RAS | renin–angiotensin system |
| S | standard |
| SAFA | saturated fatty acids |
| TyrAP | Tyrosyl-aminopeptidase |
| VOO | virgin olive oil |
References
- Tafet, G.E.; Nemeroff, C.B. Pharmacological Treatment of Anxiety Disorders: The Role of the HPA Axis. Front. Psychiatry 2020, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Daimon, M.; Kamba, A.; Murakami, H.; Mizushiri, S.; Osonoi, S.; Matsuki, K.; Sato, E.; Tanabe, J.; Takayasu, S.; Matsuhashi, Y.; et al. Dominance of the hypothalamus-pituitary-adrenal axis over the renin-angiotensin-aldosterone system is a risk factor for decreased insulin secretion. Sci. Rep. 2017, 7, 11360. [Google Scholar] [CrossRef]
- Niu, X.; Wu, X.; Ying, A.; Shao, B.; Li, X.; Zhang, W.; Lin, C.; Lin, Y. Maternal high fat diet programs hypothalamic-pituitary-adrenal function in adult rat offspring. Psychoneuroendocrinology 2019, 102, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, H.E.; Romijn, J.A.; Biermasz, N.R.; Pijl, H.; Havekes, L.M.; Smit, J.W.; Rensen, P.C.; Pereira, A.M. The effects of high fat diet on the basal activity of the hypothalamus-pituitary-adrenal axis in mice. J. Endocrinol. 2012, 214, 191–197. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Qian, F.; Lu, C. Hypothalamic-pituitary-adrenal axis hypersensitivity in female rats on a post-weaning high-fat diet after chronic mild stress. Exp. Ther. Med. 2017, 14, 439–446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shin, A.C.; MohanKumar, S.M.J.; Balasubramanian, P.; Sirivelu, M.P.; Linning, K.; Woolcock, A.; James, M.; MohanKumar, P.S. Responsiveness of hypothalamo-pituitary-adrenal axis to leptin is impaired in diet-induced obese rats. Nutr. Diabetes 2019, 9, 10. [Google Scholar] [CrossRef]
- Dutheil, S.; Ota, K.T.; Wohleb, E.S.; Rasmussen, K.; Duman, R.S. High-Fat Diet Induced Anxiety and Anhedonia: Impact on Brain Homeostasis and Inflammation. Neuropsychopharmacology 2016, 41, 1874–1887. [Google Scholar] [CrossRef]
- Yang, J.L.; Liu, D.X.; Jiang, H.; Pan, F.; Ho, C.S.; Ho, R.C. The Effects of High-Fat-Diet Combined with Chronic Unpredictable Mild Stress on Depression-like Behavior and Leptin/LepRb in Male Rats. Sci. Rep. 2016, 6, 35239. [Google Scholar] [CrossRef] [PubMed]
- Sivanathan, S.; Thavartnam, K.; Arif, S.; Elegino, T.; McGowan, P.O. Chronic high fat feeding increases anxiety-like behaviour and reduces transcript abundance of glucocorticoid signalling genes in the hippocampus of female rats. Behav. Brain Res. 2015, 286, 265–270. [Google Scholar] [CrossRef]
- Auvinen, H.E.; Romijn, J.A.; Biermasz, N.R.; Havekes, L.M.; Smit, J.; Rensen, P.C.; Pereira, A.M. Effects of high fat diet on the Basal activity of the hypothalamus-pituitary-adrenal axis in mice: A systematic review. Horm. Metab. Res. 2011, 43, 899–906. [Google Scholar] [CrossRef]
- Weldon, S.M.; Brown, N.F. Inhibitors of Aldosterone Synthase. Vitam. Horm. 2019, 109, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Kawarazaki, W.; Fujita, T. The Role of Aldosterone in Obesity-Related Hypertension. Am. J. Hypertens. 2016, 29, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Aroor, A.R.; Habibi, J.; Nistala, R.; Ramirez-Perez, F.I.; Martinez-Lemus, L.A.; Jaffe, I.Z.; Sowers, J.R.; Jia, G.; Whaley-Connell, A. Diet-Induced Obesity Promotes Kidney Endothelial Stiffening and Fibrosis Dependent on the Endothelial Mineralocorticoid Receptor. Hypertension 2019, 73, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Minemura, T.; Onodera, T.; Shin, J.; Okuno, Y.; Fukuhara, A.; Otsuki, M.; Shimomura, I. Impact of MR on Mature Adipocytes in High-Fat/High-Sucrose Diet-Induced Obesity. J. Endocrinol. 2018, 239, 63–71. [Google Scholar] [CrossRef]
- Michailidou, Z.; Carter, R.N.; Marshall, E.; Sutherland, H.G.; Brownstein, D.G.; Owen, E.; Cockett, K.; Kelly, V.; Ramage, L.; Al-Dujaili, E.A.; et al. Glucocorticoid receptor haploinsufficiency causes hypertension and attenuates hypothalamic-pituitary-adrenal axis and blood pressure adaptions to high-fat diet. FASEB J. 2008, 22, 3896–3907. [Google Scholar] [CrossRef]
- Ferrario, C.M. Role of angiotensin II in cardiovascular disease therapeutic implications of more than a century of research. J. Renin Angiotensin Aldosterone Syst. 2006, 7, 3–14. [Google Scholar] [CrossRef]
- Ivy, J.R.; Evans, L.C.; Moorhouse, R.; Richardson, R.V.; Al-Dujaili, E.A.S.; Flatman, P.W.; Kenyon, C.J.; Chapman, K.E.; Bailey, M.A. Renal and Blood Pressure Response to a High-Salt Diet in Mice with Reduced Global Expression of the Glucocorticoid Receptor. Front. Physiol. 2018, 9, 848. [Google Scholar] [CrossRef]
- Prieto, I.; Segarra, A.B.; de Gasparo, M.; Martínez-Cañamero, M.; Ramírez-Sánchez, M. Divergent profile between hypothalamic and plasmatic aminopeptidase activities in WKY and SHR. Influence of beta-adrenergic blockade. Life Sci. 2018, 192, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.B.; Prieto, I.; Martínez-Cañamero, M.; de Gasparo, M.; Luna, J.d.D.; Ramírez-Sánchez, M. Thyroid Disorders Change the Pattern of Response of Angiotensinase Activities in the Hypothalamus-Pituitary-Adrenal Axis of Male Rats. Front. Endocrinol. 2018, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Daimon, M.; Kamba, A.; Murakami, H.; Takahashi, K.; Otaka, H.; Makita, K.; Yanagimachi, M.; Terui, K.; Kageyama, K.; Nigawara, T.; et al. Association Between Pituitary-Adrenal Axis Dominance Over the Renin-Angiotensin-Aldosterone System and Hypertension. J. Clin. Endocrinol. Metab. 2016, 101, 889–897. [Google Scholar] [CrossRef]
- Park, H.S.; You, M.J.; Yang, B.; Jang, K.B.; Yoo, J.; Choi, H.J.; Lee, S.H.; Bang, M.; Kwon, M.S. Chronically infused angiotensin II induces depressive-like behavior via microglia activation. Sci. Rep. 2020, 10, 22082. [Google Scholar] [CrossRef]
- Domínguez-Vías, G.; Segarra, A.B.; Ramírez-Sánchez, M.; Prieto, I. Effects of Virgin Olive Oil on Blood Pressure and Renal Aminopeptidase Activities in Male Wistar Rats. Int. J. Mol. Sci. 2021, 22, 5388. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Vías, G.; Segarra, A.B.; Ramírez-Sánchez, M.; Prieto, I. The Role of High Fat Diets and Liver Peptidase Activity in the Development of Obesity and Insulin Resistance in Wistar Rats. Nutrients 2020, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Vías, G.; Segarra, A.; Martínez-Cañamero, M.; Ramírez-Sánchez, M.; Prieto, I. Influence of a Virgin Olive Oil versus Butter plus Cholesterol-Enriched Diet on Testicular Enzymatic Activities in Adult Male Rats. Int. J. Mol. Sci. 2017, 18, 1701. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.B.; Domínguez-Vías, G.; Redondo, J.; Martínez-Cañamero, M.; Ramírez-Sánchez, M.; Prieto, I. Hypothalamic Renin–Angiotensin System and Lipid Metabolism: Effects of Virgin Olive Oil versus Butter in the Diet. Nutrients 2021, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Vías, G.; Aretxaga-Maza, G.; Prieto, I.; Luna, J.D.D.; de Gasparo, M.; Ramírez-Sánchez, M. Diurnal opposite variation between angiotensinase activities in photo-neuro-endocrine tissues of rats. Chronobiol. Int. 2017, 34, 1180–1186. [Google Scholar] [CrossRef]
- Morais, R.L.; Hilzendeger, A.M.; Visniauskas, B.; Todiras, M.; Alenina, N.; Mori, M.A.; Araújo, R.C.; Nakaie, C.R.; Chagas, J.R.; Carmona, A.K.; et al. High aminopeptidase A activity contributes to blood pressure control in ob/ob mice by AT2 receptor-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H437–H445. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.B.; Prieto, I.; Banegas, I.; Martínez-Cañamero, M.; de Gasparo, M.; Vanderheyden, P.; Zorad, S.; Ramírez-Sánchez, M. The Type of Fat in the Diet Influences the Behavior and the Relationship Between Cystinyl and Alanyl Aminopeptidase Activities in Frontal Cortex, Liver, and Plasma. Front. Mol. Biosci. 2020, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Grouzmann, E.; Werffeli-George, P.; Fathi, M.; Burnier, M.; Waeber, B.; Waeber, G. Angiotensin-II mediates norepinephrine and neuropeptide-Y secretion in a human pheochromocytoma. J. Clin. Endocrinol. Metab. 1994, 79, 1852–1856. [Google Scholar] [CrossRef]
- Westfall, T.C.; Macarthur, H.; Byku, M.; Yang, C.L.; Murray, J. Interactions of neuropeptide y, catecholamines, and angiotensin at the vascular neuroeffector junction. Adv. Pharmacol. 2013, 68, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Byku, M.; Macarthur, H.; Westfall, T.C. Nerve stimulation induced overflow of neuropeptide Y and modulation by angiotensin II in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2188–H2197. [Google Scholar] [CrossRef]
- Nussdorfer, G.G.; Gottardo, G. Neuropeptide-Y family of peptides in the autocrine-paracrine regulation of adrenocortical function. Horm. Metab. Res. 1998, 30, 368–373. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Pelletier, G. Role of neuropeptide Y in the regulation of tyrosine hydroxylase messenger ribonucleic acid levels in the male rat arcuate nucleus as evaluated by in situ hybridization. J. Neuroendocrinol. 1995, 7, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, S.; Fournier, A.; St-Pierre, S.; Pelletier, G. Role of Neuropeptide Y in the Regulation of Tyrosine Hydroxylase Gene Expression in Rat Adrenal Glands. Neuroendocrinology 1995, 61, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, D.; Hinson, J.P. Neuropeptide Y and the Adrenal Gland: A Review. Peptides 2001, 22, 429–438. [Google Scholar] [CrossRef]
- Sullivan, E.L.; Riper, K.M.; Lockard, R.; Valleau, J.C. Maternal high-fat diet programming of the neuroendocrine system and behavior. Horm. Behav. 2015, 76, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Hryhorczuk, C.; Décarie-Spain, L.; Sharma, S.; Daneault, C.; Rosiers, C.D.; Alquier, T.; Fulton, S. Saturated high-fat feeding independent of obesity alters hypothalamus-pituitary-adrenal axis function but not anxiety-like behaviour. Psychoneuroendocrinology 2017, 83, 142–149. [Google Scholar] [CrossRef]
- van den Heuvel, J.K.; Eggels, L.; van Rozen, A.J.; Luijendijk, M.C.; Fliers, E.; Kalsbeek, A.; Adan, R.A.; la Fleur, S.E. Neuropeptide Y and leptin sensitivity is dependent on diet composition. J. Neuroendocrinol. 2014, 26, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Golub, Y.; Schildbach, E.M.; Touma, C.; Kratz, O.; Moll, G.H.; von Hörsten, S.; Canneva, F. Role of hypothalamus-pituitary-adrenal axis modulation in the stress-resilient phenotype of DPP4-deficient rats. Behav. Brain Res. 2019, 356, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Canneva, F.; Golub, Y.; Distler, J.; Dobner, J.; Meyer, S.; von Hörsten, S. DPP4-Deficient Congenic Rats Display Blunted Stress, Improved Fear Extinction and Increased Central NPY. Psychoneuroendocrinology 2015, 53, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Valerio, C.M.; de Almeida, J.S.; Moreira, R.O.; Aguiar, L.; Siciliano, P.O.; Carvalho, D.P.; Godoy-Matos, A.F. Dipeptidyl peptidase-4 levels are increased and partially related to body fat distribution in patients with familial partial lipodystrophy type 2. Diabetol. Metab. Syndr. 2017, 9, 26. [Google Scholar] [CrossRef]
- Domínguez-Vías, G.; Segarra, A.B.; Ramírez-Sánchez, M.; Prieto, I. High-Fat Diets Modify the Proteolytic Activities of Dipeptidyl-Peptidase IV and the Regulatory Enzymes of the Renin–Angiotensin System in Cardiovascular Tissues of Adult Wistar Rats. Biomedicines 2021, 9, 1149. [Google Scholar] [CrossRef]
- Pérez-Durillo, F.T.; Segarra, A.B.; Villarejo, A.B.; Ramírez-Sánchez, M.; Prieto, I. Influence of Diet and Gender on Plasma DPP4 Activity and GLP-1 in Patients with Metabolic Syndrome: An Experimental Pilot Study. Molecules 2018, 23, 1564. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.; Kaestner, F.; Wolf, R.; Stiller, H.; Heiser, U.; Manhart, S.; Hoffmann, T.; Rahfeld, J.U.; Demuth, H.U.; Rothermundt, M.; et al. Identifying neuropeptide Y (NPY) as the main stress-related substrate of dipeptidyl peptidase 4 (DPP4) in blood circulation. Neuropeptides 2016, 57, 21–34. [Google Scholar] [CrossRef]
- Prasad, C.; Jayaraman, A. Metabolism of thyrotropin-releasing hormone in human cerebrospinal fluid. Isolation and characterization of pyroglutamate aminopeptidase activity. Brain Res. 1986, 364, 331–337. [Google Scholar] [CrossRef]
- Marubashi, S.; Kunii, Y.; Tominaga, M.; Sasaki, H. Modulation of plasma glucose levels by thyrotropin-releasing hormone administered intracerebroventricularly in the rat. Neuroendocrinology 1988, 48, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Iriuchijima, T.; Prasad, C.; Wilber, J.F.; Jayaraman, A.; Rao, J.K.; Robertson, H.J.; Rogers, D.J. Thyrotropin-releasing hormone and cyclo (His-Pro)-like immunoreactivities in the cerebrospinal fluids of ‘normal’ infants and adults, and patients with various neuropsychiatric and neurologic disorders. Life Sci. 1987, 41, 2419–2428. [Google Scholar] [CrossRef]
- Arechaga, G.; Prieto, I.; Segarra, A.B.; Alba, F.; Ruiz-Larrea, M.B.; Ruiz-Sanz, J.I.; de Gasparo, M.; Ramirez, M. Dietary fatty acid composition affects aminopeptidase activities in the testes of mice. Int. J. Androl. 2002, 25, 113–118. [Google Scholar] [CrossRef] [PubMed]
- De Gandarias, J.M.; Irazusta, J.; Echevarria, E.; Lagüera, M.D.; Casis, L. Effect of swimming-to-exhaustion stress on the Tyr-aminopeptidase activity in different brain areas of the rat. Int. J. Neurosci. 1993, 69, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.S. Brain-specific aminopeptidase: From enkephalinase to protector against neurodegeneration. Neurochem. Res. 2007, 32, 2062–2071. [Google Scholar] [CrossRef]
- de Gandarias, J.M.; Astiazaran, J.I.; Varona, A.; Fernández, D.; Gil, J.; Casis, L. Effect of lithium treatments on the tyrosine-aminopeptidase activities in the rat brain and the pituitary gland. Arzneimittelforschung 1999, 49, 816–819. [Google Scholar] [CrossRef]
- Prieto, I.; Segarra, A.B.; Vargas, F.; Alba, F.; de Gasparo, M.; Ramírez, M. Angiotensinase activity in hypothalamus and pituitary of hypothyroid, euthyroid and hyperthyroid adult male rats. Horm. Metab. Res. 2003, 35, 279–281. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Boston, MA, USA, 2007. [Google Scholar]
- Greenberg, L.J. Fluorometric Measurement of Alkaline Phosphatase and Aminopeptidase Activities in the Order of 10-14 Mole. Biochem. Biophys. Res. Commun. 1962, 9, 430–435. [Google Scholar] [CrossRef]
- Cheung, H.S.; Cushman, D.W. A soluble aspartate aminopeptidase from dog kidney. Biochim. Biophys. Acta. 1971, 242, 190–193. [Google Scholar] [CrossRef]
- Tobe, H.; Kojima, F.; Aoyagi, T.; Umezawa, H. Purification by affinity chromatography using amastatin and properties of aminopeptidase A from pig kidney. Biochim. Biophys. Acta. 1980, 613, 459–468. [Google Scholar] [CrossRef]
- Prieto, I.; Martínez, J.M.; Hermoso, F.; Ramírez, M.J.; de Gasparo, M.; Vargas, F.; Alba, F.; Ramírez, M. Effect of valsartan on angiotensin II- and vasopressin-degrading activities in the kidney of normotensive and hypertensive rats. Horm. Metab. Res. 2001, 33, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.; Prieto, I.; Banegas, I.; Segarra, A.B.; Alba, F. Neuropeptidases. Methods Mol. Biol. 2011, 789, 287–294. [Google Scholar] [CrossRef]
- Matsushima, M.; Takahashi, T.; Ichinose, M.; Miki, K.; Kurokawa, K.; Takahashi, K. Structural and immunological evidence for the identity of prolyl aminopeptidase with leucyl aminopeptidase. Biochem. Biophys. Res. Commun. 1991, 178, 1459–1464. [Google Scholar] [CrossRef]
- Turzynski, A.; Mentlein, R. Prolyl aminopeptidase from rat brain and kidney. Action on peptides and identification as leucyl aminopeptidase. Eur. J. Biochem. 1990, 190, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Koll, M.; Ahmed, S.; Mantle, D.; Donohue, T.M.; Palmer, T.N.; Simanowski, U.A.; Seltz, H.K.; Peters, T.J.; Preedy, V.R. Effect of acute and chronic alcohol treatment and their superimposition on lysosomal, cytoplasmic, and proteosomal protease activities in rat skeletal muscle in vivo. Metabolism 2002, 51, 97–104. [Google Scholar] [CrossRef]
- Hiraoka, B.Y.; Harada, M. Use of L-prolyl-L-leucylglycinamide (MIF-1) for the high-performance liquid chromatographic determination of proline iminopeptidase activity in rat liver. J. Chromatogr. 1991, 563, 142–146. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Segarra, A.B.; Prieto, I.; Martinez-Canamero, M.; Ruiz-Sanz, J.I.; Ruiz-Larrea, M.B.; de Gasparo, M.; Banegas, I.; Zorad, S.; Ramirez-Sanchez, M. Enkephalinase activity is modified and correlates with fatty acids in frontal cortex depending on fish, olive or coconut oil used in the diet. Endocr. Regul. 2019, 53, 59–64. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, A.D.; Stewart, C.A.; Sutherland, C.; Balfour, D.J. High fat feeding is associated with stimulation of the hypothalamic-pituitary-adrenal axis and reduced anxiety in the rat. Psychoneuroendocrinology 2015, 52, 272–280. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, Y.; Qin, S.; Liu, H.; Yang, L.; Zhang, X.; Li, G.; Li, Q. Increased Dipeptidyl Peptidase-4 Activity Is Associated with High Prevalence of Depression in Middle-Aged and Older Adults: A Cross-Sectional Study. J. Clin. Psychiatry 2016, 77, e1248–e1255. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Qin, L.; Chen, B.; Hu, X.; Zhang, X.; Liu, Y.; Liu, H.; Qin, S.; Li, G.; Li, Q. Association of Plasma DPP4 Activity with Mild Cognitive Impairment in Elderly Patients with Type 2 Diabetes: Results from the GDMD Study in China. Diabetes Care 2016, 39, 1594–1601. [Google Scholar] [CrossRef]
- Stephan, M.; Radicke, A.; Leutloff, S.; Schmiedl, A.; Pabst, R.; von Hörsten, S.; Dettmer, S.; Lotz, J.; Nave, H. Dipeptidyl peptidase IV (DPP4)-deficiency attenuates diet-induced obesity in rats: Possible implications for the hypothalamic neuropeptidergic system. Behav. Brain Res. 2011, 216, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Maes, M. Evidence for an immune response in major depression: A review and hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 1995, 19, 11–38. [Google Scholar] [CrossRef]
- Dalvi, P.S.; Chalmers, J.A.; Luo, V.; Han, D.Y.; Wellhauser, L.; Liu, Y.; Tran, D.Q.; Castel, J.; Luquet, S.; Wheeler, M.B.; et al. High fat induces acute and chronic inflammation in the hypothalamus: Effect of high-fat diet, palmitate and TNF-α on appetite-regulating NPY neurons. Int. J. Obes. 2017, 41, 149–158. [Google Scholar] [CrossRef]
- Hassan, A.M.; Mancano, G.; Kashofer, K.; Fröhlich, E.E.; Matak, A.; Mayerhofer, R.; Reichmann, F.; Olivares, M.; Neyrinck, A.M.; Delzenne, N.M.; et al. High-fat diet induces depression-like behaviour in mice associated with changes in microbiome, neuropeptide Y, and brain metabolome. Nutr. Neurosci. 2019, 22, 877–893. [Google Scholar] [CrossRef]
- Cavadas, C.; Grand, D.; Mosimann, F.; Cotrim, M.D.; Ribeiro, C.A.F.; Brunner, H.R.; Grouzmann, E. Angiotensin II Mediates Catecholamine and Neuropeptide Y Secretion in Human Adrenal Chromaffin Cells through the AT1 Receptor. Regul. Pept. 2003, 111, 61–65. [Google Scholar] [CrossRef]
- Erdos, B.; Broxson, C.S.; Cudykier, I.; Basgut, B.; Whidden, M.; Landa, T.; Scarpace, P.J.; Tümer, N. Effect of high-fat diet feeding on hypothalamic redox signaling and central blood pressure regulation. Hypertens. Res. 2009, 32, 983–988. [Google Scholar] [CrossRef]
- García, S.I.; Alvarez, A.L.; Porto, P.I.; Garfunkel, V.M.; Finkielman, S.; Pirola, C.J. Antisense inhibition of thyrotropin-releasing hormone reduces arterial blood pressure in spontaneously hypertensive rats. Hypertension 2001, 37, 365–370. [Google Scholar] [CrossRef][Green Version]
- Velardez, M.O.; Benitez, A.H.; Cabilla, J.P.; Bodo, C.C.; Duvilanski, B.H. Nitric oxide decreases the production of inositol phosphates stimulated by angiotensin II and thyrotropin-releasing hormone in anterior pituitary cells. Eur. J. Endocrinol. 2003, 148, 89–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iglesias, A.G.; Suárez, C.; Feierstein, C.; Díaz-Torga, G.; Becu-Villalobos, D. Desensitization of angiotensin II: Effect on [Ca2+]i, inositol triphosphate, and prolactin in pituitary cells. Am. J. Physiol. Endocrinol. Metab. 2001, 280, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Login, I.S.; Judd, A.M.; Kuan, S.I.; MacLeod, R.M. Role of calcium in dopaminergic regulation of TRH- and angiotensin II-stimulated prolactin release. Am. J. Physiol. 1991, 260, E553–E560. [Google Scholar] [CrossRef]
- Prasad, C.; Mori, M.; Pierson, W.; Wilber, J.F.; Ewards, R.M. Developmental Changes in the Distribution of Rat Brain Pyroglutamate Aminopeptidase, a Possible Determinant of Endogenous Cyclo(His-Pro) Concentrations. Neurochem. Res. 1983, 8, 389–399. [Google Scholar] [CrossRef]
- Prasad, C. Cyclo(His-Pro): Its distribution, origin and function in the human. Neurosci. Biobehav. Rev. 1988, 12, 19–22. [Google Scholar] [CrossRef]
- Grottelli, S.; Costanzi, E.; Peirce, M.J.; Minelli, A.; Cellini, B.; Bellezza, I. Potential Influence of Cyclo(His-Pro) on Proteostasis: Impact on Neurodegenerative Diseases. Curr. Protein Pept. Sci. 2018, 19, 805–812. [Google Scholar] [CrossRef]
- Peterkofsky, A.; Battaini, F.; Koch, Y.; Takahara, Y.; Dannies, P. Histidyl-proline diketopiperazine: Its biological role as a regulatory peptide. Mol. Cell. Biochem. 1982, 42, 45–63. [Google Scholar] [CrossRef]
- Aguilar-Valles, A.; Sánchez, E.; de Gortari, P.; García-Vazquez, A.I.; Ramírez-Amaya, V.; Bermúdez-Rattoni, F.; Joseph-Bravo, P. The expression of TRH, its receptors and degrading enzyme is differentially modulated in the rat limbic system during training in the Morris water maze. Neurochem. Int. 2007, 50, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Jikihara, H.; Ikegami, H.; Koike, K.; Wada, K.; Morishige, K.; Kurachi, H.; Hirota, K.; Miyake, A.; Tanizawa, O. Intraventricular administration of histidyl-proline-diketopiperazine [Cyclo(His-Pro)] suppresses prolactin secretion and synthesis: A possible role of Cyclo(His-Pro) as dopamine uptake blocker in rat hypothalamus. Endocrinology 1993, 132, 953–958. [Google Scholar] [CrossRef]
- Miyamori, C.; Murata, A.; Imura, E.; Sato, T. Effect of TRH and histidyl-proline diketopiperazine (cyclo(His-Pro)) on catecholamine secretion. Nihon Naibunpi Gakkai Zasshi 1989, 65, 1226–1238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grottelli, S.; Ferrari, I.; Pietrini, G.; Peirce, M.J.; Minelli, A.; Bellezza, I. The Role of Cyclo(His-Pro) in Neurodegeneration. Int. J. Mol. Sci. 2016, 17, 1332. [Google Scholar] [CrossRef]
- Grottelli, S.; Mezzasoma, L.; Scarpelli, P.; Cacciatore, I.; Cellini, B.; Bellezza, I. Cyclo(His-Pro) inhibits NLRP3 inflammasome cascade in ALS microglial cells. Mol. Cell. Neurosci. 2019, 94, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Kim, I.Y.; Kim, J.H.; Lee, B.E.; Lee, S.H.; Kho, A.R.; Sohn, M.; Suh, S.W. Zinc plus cyclo-(His-Pro) promotes hippocampal neurogenesis in rats. Neuroscience 2016, 339, 634–643. [Google Scholar] [CrossRef]
- Song, M.K.; Bischoff, D.S.; Song, A.M.; Uyemura, K.; Yamaguchi, D.T. Metabolic relationship between diabetes and Alzheimer’s Disease affected by Cyclo(His-Pro) plus zinc treatment. BBA Clin. 2016, 7, 41–54. [Google Scholar] [CrossRef]
- Bataini, F.; Koch, Y.; Takahara, Y.; Peterkofsky, A. Specific binding to adrenal particulate fraction of cyclo(histidyl-proline), a TRH metabolite. Peptides 1983, 4, 89–96. [Google Scholar] [CrossRef]
- Mokrani, M.C.; Duval, F.; Erb, A.; Lopera, F.G.; Danila, V. Are the thyroid and adrenal system alterations linked in depression? Psychoneuroendocrinology 2020, 122, 104831. [Google Scholar] [CrossRef]
- Duval, F.; Mokrani, M.C.; Erb, A.; Danila, V.; Lopera, F.G.; Jeanjean, L. Dopaminergic, Noradrenergic, Adrenal, and Thyroid Abnormalities in Psychotic and Affective Disorders. Front. Psychiatry 2020, 11, 533872. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Vías, G.; Segarra, A.B.; Ramírez-Sánchez, M.; Prieto, I. The Type of Fat in the Diet Influences Regulatory Aminopeptidases of the Renin-Angiotensin System and Stress in the Hypothalamic-Pituitary-Adrenal Axis in Adult Wistar Rats. Nutrients 2021, 13, 3939. https://doi.org/10.3390/nu13113939
Domínguez-Vías G, Segarra AB, Ramírez-Sánchez M, Prieto I. The Type of Fat in the Diet Influences Regulatory Aminopeptidases of the Renin-Angiotensin System and Stress in the Hypothalamic-Pituitary-Adrenal Axis in Adult Wistar Rats. Nutrients. 2021; 13(11):3939. https://doi.org/10.3390/nu13113939
Chicago/Turabian StyleDomínguez-Vías, Germán, Ana Belén Segarra, Manuel Ramírez-Sánchez, and Isabel Prieto. 2021. "The Type of Fat in the Diet Influences Regulatory Aminopeptidases of the Renin-Angiotensin System and Stress in the Hypothalamic-Pituitary-Adrenal Axis in Adult Wistar Rats" Nutrients 13, no. 11: 3939. https://doi.org/10.3390/nu13113939
APA StyleDomínguez-Vías, G., Segarra, A. B., Ramírez-Sánchez, M., & Prieto, I. (2021). The Type of Fat in the Diet Influences Regulatory Aminopeptidases of the Renin-Angiotensin System and Stress in the Hypothalamic-Pituitary-Adrenal Axis in Adult Wistar Rats. Nutrients, 13(11), 3939. https://doi.org/10.3390/nu13113939






