Single Nucleotide Polymorphisms in Close Proximity to the Fibroblast Growth Factor 21 (FGF21) Gene Found to Be Associated with Sugar Intake in a Swedish Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Data
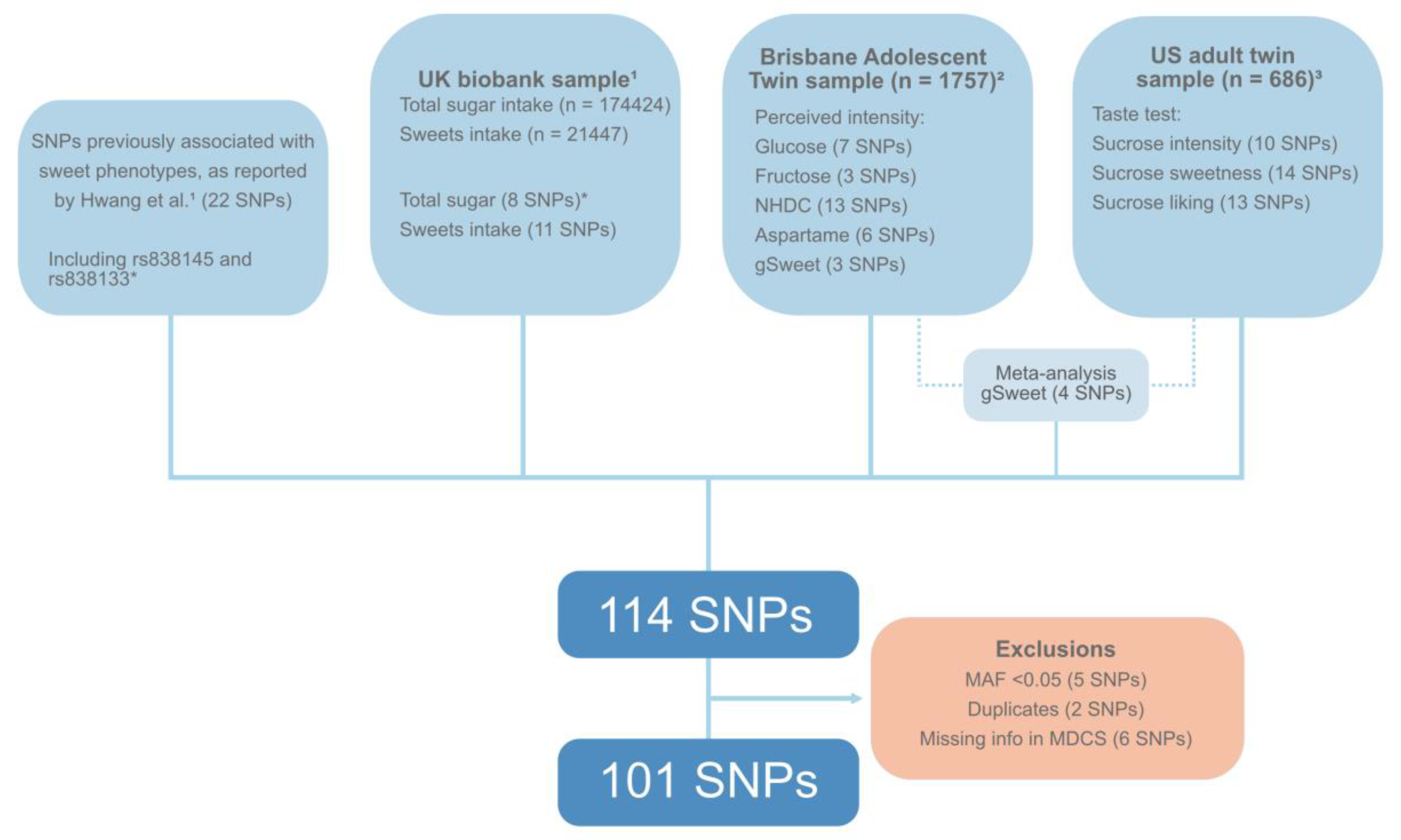
2.3. Genotyping and Selection of SNPs
2.4. Statistical Analyses
3. Results
3.1. Study Population Characteristics
3.2. Associations between Primary SNPs and Main Outcomes
3.3. Associations between Primary SNPs and Secondary Outcomes
3.4. Associations between Secondary SNPs and All Outcomes
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, T.A.; Tayyiba, M.; Agarwal, A.; Mejia, S.B.; de Souza, R.J.; Wolever, T.M.S.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Sievenpiper, J.L. Relation of Total Sugars, Sucrose, Fructose, and Added Sugars with the Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Mayo Clin. Proc. 2019, 94, 2399–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsilas, C.S.; de Souza, R.J.; Mejia, S.B.; Mirrahimi, A.; Cozma, A.I.; Jayalath, V.H.; Ha, V.; Tawfik, R.; Di Buono, M.; Jenkins, A.L.; et al. Relation of total sugars, fructose and sucrose with incident type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. CMAJ 2017, 189, E711–E720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, V.S.; Schulze, M.B.; Hu, F.B. Intake of sugar-sweetened beverages and weight gain: A systematic review. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [CrossRef]
- Vartanian, L.R.; Schwartz, M.B.; Brownell, K.D. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am. J. Public Health 2007, 97, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Despres, J.P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organisation. Guideline: Sugars Intake for Adults and Children; World Health Organisation: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organisation/Food and Agriculture Organisation. Joint WHO/FAO Expert Consultation on Diet., Nutrition and the Prevention of Chronic Diseases; World Health Organisation/Food and Agriculture Organisation: Geneva, Switzerland, 2003. [Google Scholar]
- Tabor, H.K.; Risch, N.J.; Myers, R.M. Candidate-gene approaches for studying complex genetic traits: Practical considerations. Nat. Rev. Genet. 2002, 3, 391–397. [Google Scholar] [CrossRef]
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.G.; Eny, K.M.; Cockburn, M.; Chiu, W.; Nielsen, D.E.; Duizer, L.; El-Sohemy, A. Variation in the TAS1R2 Gene, Sweet Taste Perception and Intake of Sugars. J. Nutr. Nutr. 2015, 8, 81–90. [Google Scholar] [CrossRef]
- Fushan, A.A.; Simons, C.T.; Slack, J.P.; Manichaikul, A.; Drayna, D. Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr. Biol. 2009, 19, 1288–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fushan, A.A.; Simons, C.T.; Slack, J.P.; Drayna, D. Association between common variation in genes encoding sweet taste signaling components and human sucrose perception. Chem. Senses 2010, 35, 579–592. [Google Scholar] [CrossRef]
- Søberg, S.; Sandholt, C.H.; Jespersen, N.Z.; Toft, U.; Madsen, A.L.; von Holstein-Rathlou, S.; Grevengoed, T.J.; Christensen, K.B.; Bredie, W.L.P.; Potthoff, M.J.; et al. FGF21 Is a Sugar-Induced Hormone Associated with Sweet Intake and Preference in Humans. Cell Metab. 2017, 25, 1045–1053.e1046. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Keast, R.S.J.; Roura, E. Salivary leptin and TAS1R2/TAS1R3 polymorphisms are related to sweet taste sensitivity and carbohydrate intake from a buffet meal in healthy young adults. Br. J. Nutr. 2017, 118, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Eny, K.M.; Wolever, T.M.; Fontaine-Bisson, B.; El-Sohemy, A. Genetic variant in the glucose transporter type 2 is associated with higher intakes of sugars in two distinct populations. Physiol. Genom. 2008, 33, 355–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, L.D.; Lin, C.; Gharahkhani, P.; Cuellar-Partida, G.; Ong, J.S.; An, J.; Gordon, S.D.; Zhu, G.; MacGregor, S.; Lawlor, D.A.; et al. New insight into human sweet taste: A genome-wide association study of the perception and intake of sweet substances. Am. J. Clin. Nutr. 2019, 109, 1724–1737. [Google Scholar] [CrossRef] [Green Version]
- Chu, A.Y.; Workalemahu, T.; Paynter, N.P.; Rose, L.M.; Giulianini, F.; Tanaka, T.; Ngwa, J.S.; Qi, Q.; Curhan, G.C.; Rimm, E.B.; et al. Novel locus including FGF21 is associated with dietary macronutrient intake. Hum. Mol. Genet. 2013, 22, 1895–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frayling, T.M.; Beaumont, R.N.; Jones, S.E.; Yaghootkar, H.; Tuke, M.A.; Ruth, K.S.; Casanova, F.; West, B.; Locke, J.; Sharp, S.; et al. A Common Allele in FGF21 Associated with Sugar Intake Is Associated with Body Shape, Lower Total Body-Fat Percentage, and Higher Blood Pressure. Cell Rep. 2018, 23, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Ngwa, J.S.; van Rooij, F.J.; Zillikens, M.C.; Wojczynski, M.K.; Frazier-Wood, A.C.; Houston, D.K.; Kanoni, S.; Lemaitre, R.N.; Luan, J.; et al. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am. J. Clin. Nutr. 2013, 97, 1395–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berglund, G.; Elmstahl, S.; Janzon, L.; Larsson, S.A. The Malmo Diet and Cancer Study. Design and feasibility. J. Intern. Med. 1993, 233, 45–51. [Google Scholar] [CrossRef]
- Manjer, J.; Carlsson, S.; Elmstahl, S.; Gullberg, B.; Janzon, L.; Lindstrom, M.; Mattisson, I.; Berglund, G. The Malmo Diet and Cancer Study: Representativity, cancer incidence and mortality in participants and non-participants. Eur. J. Cancer Prev. 2001, 10, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Wirfalt, E.; Mattisson, I.; Johansson, U.; Gullberg, B.; Wallstrom, P.; Berglund, G. A methodological report from the Malmo Diet and Cancer study: Development and evaluation of altered routines in dietary data processing. Nutr. J. 2002, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Riboli, E.; Elmståhl, S.; Saracci, R.; Gullberg, B.; Lindgärde, F. The Malmö Food Study: Validity of two dietary assessment methods for measuring nutrient intake. Int. J. Epidemiol. 1997, 26 (Suppl. S1), S161–S173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramne, S.; Alves Dias, J.; Gonzalez-Padilla, E.; Olsson, K.; Lindahl, B.; Engstrom, G.; Ericson, U.; Johansson, I.; Sonestedt, E. Association between added sugar intake and mortality is nonlinear and dependent on sugar source in 2 Swedish population-based prospective cohorts. Am. J. Clin. Nutr. 2019, 109, 411–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, S.; Das, S.; Kretzschmar, W.; Delaneau, O.; Wood, A.R.; Teumer, A.; Kang, H.M.; Fuchsberger, C.; Danecek, P.; Sharp, K.; et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016, 48, 1279–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knaapila, A.; Hwang, L.D.; Lysenko, A.; Duke, F.F.; Fesi, B.; Khoshnevisan, A.; James, R.S.; Wysocki, C.J.; Rhyu, M.; Tordoff, M.G.; et al. Genetic analysis of chemosensory traits in human twins. Chem. Senses 2012, 37, 869–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, L.D.; Zhu, G.; Breslin, P.A.; Reed, D.R.; Martin, N.G.; Wright, M.J. A common genetic influence on human intensity ratings of sugars and high-potency sweeteners. Twin Res. Hum. Genet. 2015, 18, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Warnes, G.; Gorjanc, G.; Leisch, F.; Man, M. Genetics: Population Genetics. R Package Version 1.3.8.1.3. 2021. Available online: https://CRAN.R-project.org/package=genetics (accessed on 15 September 2021).
- Moore, C.; Jacobson, S. genpwr: Power Calculations under Genetic Model Misspecification. R Package Version 1.0.4. 2021. Available online: https://CRAN.R-project.org/package=genpwr (accessed on 15 September 2021).
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2000, 24, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Habberstad, C.; Drake, I.; Sonestedt, E. Variation in the Sweet Taste Receptor Gene and Dietary Intake in a Swedish Middle-Aged Population. Front. Endocrinol. 2017, 8, 348. [Google Scholar] [CrossRef] [Green Version]
- Brunkwall, L.; Ericson, U.; Hellstrand, S.; Gullberg, B.; Orho-Melander, M.; Sonestedt, E. Genetic variation in the fat mass and obesity-associated gene (FTO) in association with food preferences in healthy adults. Food Nutr. Res. 2013, 57. [Google Scholar] [CrossRef]
- Loos, R.J.; Yeo, G.S. The bigger picture of FTO: The first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 2014, 10, 51–61. [Google Scholar] [CrossRef]
- Frye, R.E.; Schwartz, B.S.; Doty, R.L. Dose-related effects of cigarette smoking on olfactory function. JAMA 1990, 263, 1233–1236. [Google Scholar]
- Pepino, M.Y.; Mennella, J.A. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol. Clin. Exp. Res. 2007, 31, 1891–1899. [Google Scholar] [CrossRef]
- Meddens, S.F.W.; de Vlaming, R.; Bowers, P.; Burik, C.A.P.; Linner, R.K.; Lee, C.; Okbay, A.; Turley, P.; Rietveld, C.A.; Fontana, M.A.; et al. Genomic analysis of diet composition finds novel loci and associations with health and lifestyle. Mol. Psychiatry 2021, 26, 2056–2069. [Google Scholar] [CrossRef]
- Merino, J.; Dashti, H.S.; Sarnowski, C.; Lane, J.M.; Todorov, P.V.; Udler, M.S.; Song, Y.; Wang, H.; Kim, J.; Tucker, C.; et al. Genetic analysis of dietary intake identifies new loci and functional links with metabolic traits. Nat. Hum. Behav. 2021. [Google Scholar] [CrossRef]
- Migdal, A.; Comte, S.; Rodgers, M.; Heineman, B.; Maratos-Flier, E.; Herman, M.; Dushay, J. Fibroblast growth factor 21 and fructose dynamics in humans. Obes. Sci. Pract. 2018, 4, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, Y.; Xiao, J.; Liu, L.; Chen, S.; Mohammadi, M.; McClain, C.J.; Li, X.; Feng, W. FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. J. Lipid Res. 2015, 56, 1481–1491. [Google Scholar] [CrossRef] [Green Version]
- Laeger, T.; Henagan, T.M.; Albarado, D.C.; Redman, L.M.; Bray, G.A.; Noland, R.C.; Munzberg, H.; Hutson, S.M.; Gettys, T.W.; Schwartz, M.W.; et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Investig. 2014, 124, 3913–3922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maida, A.; Zota, A.; Sjoberg, K.A.; Schumacher, J.; Sijmonsma, T.P.; Pfenninger, A.; Christensen, M.M.; Gantert, T.; Fuhrmeister, J.; Rothermel, U.; et al. A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. J. Clin. Investig. 2016, 126, 3263–3278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Holstein-Rathlou, S.; BonDurant, L.D.; Peltekian, L.; Naber, M.C.; Yin, T.C.; Claflin, K.E.; Urizar, A.I.; Madsen, A.N.; Ratner, C.; Holst, B.; et al. FGF21 Mediates Endocrine Control of Simple Sugar Intake and Sweet Taste Preference by the Liver. Cell Metab. 2016, 23, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Jensen-Cody, S.O.; Flippo, K.H.; Claflin, K.E.; Yavuz, Y.; Sapouckey, S.A.; Walters, G.C.; Usachev, Y.M.; Atasoy, D.; Gillum, M.P.; Potthoff, M.J. FGF21 Signals to Glutamatergic Neurons in the Ventromedial Hypothalamus to Suppress Carbohydrate Intake. Cell Metab. 2020, 32, 273–286.e276. [Google Scholar] [CrossRef]
- Hill, C.M.; Qualls-Creekmore, E.; Berthoud, H.R.; Soto, P.; Yu, S.; McDougal, D.H.; Munzberg, H.; Morrison, C.D. FGF21 and the Physiological Regulation of Macronutrient Preference. Endocrinology 2020, 161. [Google Scholar] [CrossRef] [Green Version]
- Talukdar, S.; Owen, B.M.; Song, P.; Hernandez, G.; Zhang, Y.; Zhou, Y.; Scott, W.T.; Paratala, B.; Turner, T.; Smith, A.; et al. FGF21 Regulates Sweet and Alcohol Preference. Cell Metab. 2016, 23, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Baruch, A.; Wong, C.; Chinn, L.W.; Vaze, A.; Sonoda, J.; Gelzleichter, T.; Chen, S.; Lewin-Koh, N.; Morrow, L.; Dheerendra, S.; et al. Antibody-mediated activation of the FGFR1/Klothoβ complex corrects metabolic dysfunction and alters food preference in obese humans. Proc. Natl. Acad. Sci. USA 2020, 117, 28992–29000. [Google Scholar] [CrossRef] [PubMed]
- Flippo, K.H.; Jensen-Cody, S.O.; Claflin, K.E.; Potthoff, M.J. FGF21 signaling in glutamatergic neurons is required for weight loss associated with dietary protein dilution. Sci. Rep. 2020, 10, 19521. [Google Scholar] [CrossRef] [PubMed]
- Eny, K.M.; Wolever, T.M.; Corey, P.N.; El-Sohemy, A. Genetic variation in TAS1R2 (Ile191Val) is associated with consumption of sugars in overweight and obese individuals in 2 distinct populations. Am. J. Clin. Nutr. 2010, 92, 1501–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.H. TAS1R2 sweet taste receptor genetic variation and dietary intake in Korean females. Appetite 2021, 164, 105281. [Google Scholar] [CrossRef] [PubMed]
- Chamoun, E.; Liu, A.S.; Duizer, L.M.; Feng, Z.; Darlington, G.; Duncan, A.M.; Haines, J.; Ma, D.W.L. Single nucleotide polymorphisms in sweet, fat, umami, salt, bitter and sour taste receptor genes are associated with gustatory function and taste preferences in young adults. Nutr. Res. 2021, 85, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jiang, Y.; Wedow, R.; Li, Y.; Brazel, D.M.; Chen, F.; Datta, G.; Davila-Velderrain, J.; McGuire, D.; Tian, C.; et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 2019, 51, 237–244. [Google Scholar] [CrossRef]


| Characteristic | Mean (SD) |
|---|---|
| Age (years) | 58.1 (7.68) |
| BMI (kg/m2) | 25.5 (3.87) |
| Total sugar (E%) | 20.4 (5.24) |
| Added sugar (E%) | 10.2 (4.21) |
| Sugars with sweet taste (E%) 1 | 16.0 (4.93) |
| Sucrose (E%) | 8.62 (3.46) |
| Monosaccharides (E%) | 7.36 (2.82) |
| Disaccharides (E%) | 13.0 (3.98) |
| Sweets and chocolate (g/day) | 15.1 (19.9) |
| Sugar-sweetened beverages (g/day) | 74.9 (143) |
| Ice cream (g/day) | 12.1 (18.6) |
| Cakes (g/day) | 38.1 (31.0) |
| Total energy (kcal/day) | 2281 (644) |
| Carbohydrates (E%) | 45.0 (5.97) |
| Fat (E%) | 39.2 (6.06) |
| Protein (E%) | 15.8 (2.50) |
| n (%) | |
| BMI < 25 2 | 11,188 (49.1) |
| BMI ≥ 25 2 | 11,579 (50.8) |
| Women | 13,992 (61.4) |
| Smoking status 3 | |
| Non-smokers | 8754 (38.4) |
| Former smokers | 7682 (33.7) |
| Current smokers | 6352 (27.9) |
| SNP | CHR:BP | Associated Gene | EA 1 | NEA | EAF | p-Value for Total Sugar Intake from Hwang et al. 2 |
|---|---|---|---|---|---|---|
| rs11577403 | 1:43989773 | PTPRF | A | G | 0.36 | 1.60 × 10−7 |
| rs7424551 | 2:216079163 | AC073284.4 | G | A | 0.35 | 6.70 × 10−8 |
| rs35267617 | 5:146693114 | STK32A | T | C | 0.47 | 3.60 × 10−7 |
| rs6911544 | 6:51477640 | RP3-335N17.2 | A | C | 0.18 | 1.00 × 10−6 |
| rs559904 | 12:121029354 | POP5 | A | G | 0.29 | 2.90 × 10−7 |
| rs11642841 | 16:53845487 | FTO | C | A | 0.41 | 3.80 × 10−8 |
| rs60764613 | 18:1839911 | CTD-2015H3.1 | G | T | 0.15 | 1.20 × 10−7 |
| rs838145 | 19:48745473 | IZUMO1, FGF21 | G | A | 0.40 | 2.70 × 10−6 |
| rs8103840 | 19:49254955 | FUT1, FGF21 | C | T | 0.50 | 5.90 × 10−7 |
| rs838133 | 19:49261368 | FGF21 | A | G | 0.43 | 4.80 × 10−7 |
| Total Sugar | Added Sugar | Sugars with Sweet Taste 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP EA | Associated Gene | β | SE | p | β | SE | p | β | SE | p |
| rs11577403 A | PTPRF | 0.04 | 0.05 | 0.42 | 0.01 | 0.04 | 0.82 | 0.04 | 0.05 | 0.40 |
| rs7424551 G | AC073284.4 | −0.02 | 0.05 | 0.71 | 0.03 | 0.04 | 0.45 | −0.01 | 0.05 | 0.83 |
| rs35267617 T | STK32A | −0.01 | 0.05 | 0.76 | 0.00 | 0.04 | 0.90 | −0.04 | 0.05 | 0.34 |
| rs6911544 A | RP3-335N17.2 | 0.08 | 0.06 | 0.22 | 0.07 | 0.05 | 0.20 | 0.08 | 0.06 | 0.19 |
| rs559904 A | POP5 | 0.08 | 0.05 | 0.13 | 0.01 | 0.04 | 0.74 | 0.06 | 0.05 | 0.21 |
| rs11642841 C | FTO | 0.09 | 0.05 | 0.07 | 0.10 | 0.04 | 0.01 | 0.06 | 0.05 | 0.17 |
| rs60764613 G | CTD-2015H3.1 | 0.06 | 0.07 | 0.33 | 0.16 | 0.05 | 2.89 × 10−3 | 0.12 | 0.06 | 0.06 |
| rs838145 G | IZUMO1, FGF21 | 0.18 | 0.05 | 2.32 × 10−4 | 0.13 | 0.04 | 1.53 × 10−3 | 0.16 | 0.05 | 6.52 × 10−4 |
| rs8103840 C | FUT1, FGF21 | 0.20 | 0.05 | 2.04 × 10−5 | 0.13 | 0.04 | 4.85 × 10−4 | 0.20 | 0.04 | 1.06 × 10−5 |
| rs838133 A | FGF21 | 0.22 | 0.05 | 2.42 × 10−6 | 0.15 | 0.04 | 1.87 × 10−4 | 0.22 | 0.05 | 6.82 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janzi, S.; González-Padilla, E.; Najafi, K.; Ramne, S.; Ahlqvist, E.; Borné, Y.; Sonestedt, E. Single Nucleotide Polymorphisms in Close Proximity to the Fibroblast Growth Factor 21 (FGF21) Gene Found to Be Associated with Sugar Intake in a Swedish Population. Nutrients 2021, 13, 3954. https://doi.org/10.3390/nu13113954
Janzi S, González-Padilla E, Najafi K, Ramne S, Ahlqvist E, Borné Y, Sonestedt E. Single Nucleotide Polymorphisms in Close Proximity to the Fibroblast Growth Factor 21 (FGF21) Gene Found to Be Associated with Sugar Intake in a Swedish Population. Nutrients. 2021; 13(11):3954. https://doi.org/10.3390/nu13113954
Chicago/Turabian StyleJanzi, Suzanne, Esther González-Padilla, Kevin Najafi, Stina Ramne, Emma Ahlqvist, Yan Borné, and Emily Sonestedt. 2021. "Single Nucleotide Polymorphisms in Close Proximity to the Fibroblast Growth Factor 21 (FGF21) Gene Found to Be Associated with Sugar Intake in a Swedish Population" Nutrients 13, no. 11: 3954. https://doi.org/10.3390/nu13113954
APA StyleJanzi, S., González-Padilla, E., Najafi, K., Ramne, S., Ahlqvist, E., Borné, Y., & Sonestedt, E. (2021). Single Nucleotide Polymorphisms in Close Proximity to the Fibroblast Growth Factor 21 (FGF21) Gene Found to Be Associated with Sugar Intake in a Swedish Population. Nutrients, 13(11), 3954. https://doi.org/10.3390/nu13113954







