Effect of Standardized Grape Powder Consumption on the Gut Microbiome of Healthy Subjects: A Pilot Study
Abstract
:1. Introduction
2. Method and Materials
2.1. Study Design
2.2. Fecal 16S rRNA Gene Sequencing and Taxonomic Analysis
2.3. Serum Lipid Measurement
2.4. Serum Bile Acid (BA) Measurement
2.5. Statistical Analyses
3. Results
3.1. Study Subjects
3.2. Effect of Grape Powder Intake on Gut Microbiota
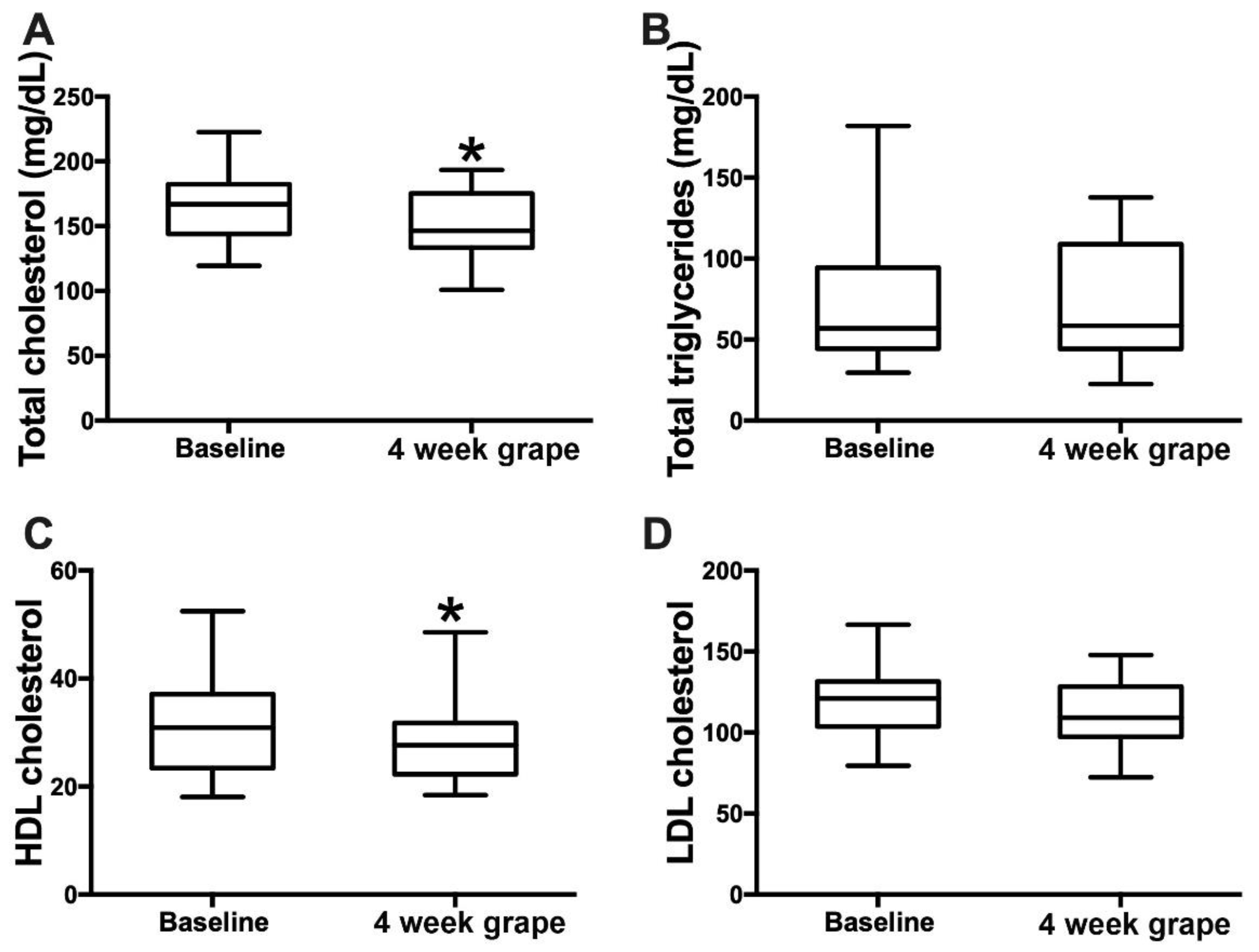
3.3. Grape Powder Intake and Serum Lipids and Bile Acids
3.4. Correlation between Gut Microbiota and Serum Lipids and BAs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giovinazzo, G.; Grieco, F. Functional Properties of Grape and Wine Polyphenols. Plant Foods Hum. Nutr. (Dordr. Neth.) 2015, 70, 454–462. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Zheng, L.; Li, J. Advance on the bioactivity and potential applications of dietary fibre from grape pomace. Food Chem. 2015, 186, 207–212. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef]
- Yanni, A.E.; Efthymiou, V.; Lelovas, P.; Agrogiannis, G.; Kostomitsopoulos, N.; Karathanos, V.T. Effects of dietary Corinthian currants (Vitis vinifera L., var. Apyrena) on atherosclerosis and plasma phenolic compounds during prolonged hypercholesterolemia in New Zealand White rabbits. Food Funct. 2015, 6, 963–971. [Google Scholar] [CrossRef]
- Baldwin, J.; Collins, B.; Wolf, P.G.; Martinez, K.; Shen, W.; Chuang, C.C.; Zhong, W.; Cooney, P.; Cockrell, C.; Chang, E.; et al. Table grape consumption reduces adiposity and markers of hepatic lipogenesis and alters gut microbiota in butter fat-fed mice. J. Nutr. Biochem. 2016, 27, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Chang, E.B. Exploring gut microbes in human health and disease: Pushing the envelope. Genes Dis. 2014, 1, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Le Roy, T.; Lecuyer, E.; Chassaing, B.; Rhimi, M.; Lhomme, M.; Boudebbouze, S.; Ichou, F.; Haro Barcelo, J.; Huby, T.; Guerin, M.; et al. The intestinal microbiota regulates host cholesterol homeostasis. BMC Biol. 2019, 17, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriaa, A.; Bourgin, M.; Potiron, A.; Mkaouar, H.; Jablaoui, A.; Gerard, P.; Maguin, E.; Rhimi, M. Microbial impact on cholesterol and bile acid metabolism: Current status and future prospects. J. Lipid Res. 2019, 60, 323–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, S.; Henning, S.M.; Lee, R.; Hsu, M.; Grojean, E.; Pisegna, R.; Ly, A.; Heber, D.; Li, Z. Cholesterol-lowering effects of dietary pomegranate extract and inulin in mice fed an obesogenic diet. J. Nutr. Biochem. 2018, 52, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Heidker, R.M.; Caiozzi, G.C.; Ricketts, M.L. Grape Seed Procyanidins and Cholestyramine Differentially Alter Bile Acid and Cholesterol Homeostatic Gene Expression in Mouse Intestine and Liver. PLoS ONE 2016, 11, e0154305. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.Y.; Rasmussen, A.M.; Yang, J.; Lee, R.P.; Huang, J.; Shao, P.; Carpenter, C.L.; Gilbuena, I.; Thames, G.; Henning, S.M.; et al. Mixed Spices at Culinary Doses Have Prebiotic Effects in Healthy Adults: A Pilot Study. Nutrients 2019, 11, 1425. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, J.P.; Lin, L.; Goudarzi, M.; Ruegger, P.; McGovern, D.P.; Fornace, A.J., Jr.; Borneman, J.; Xia, L.; Braun, J. Microbial, metabolomic, and immunologic dynamics in a relapsing genetic mouse model of colitis induced by T-synthase deficiency. Gut Microbes 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Guo, Y.; Lee, R.; Henning, S.M.; Wang, J.; Pan, Y.; Qing, T.; Hsu, M.; Nguyen, A.; Prabha, S.; et al. Pomegranate Metabolites Impact Tryptophan Metabolism in Humans and Mice. Curr. Dev. Nutr. 2020, 4, nzaa165. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Alnouti, Y.; Csanaky, I.L.; Klaassen, C.D. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J. Chromatogr. B 2008, 873, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andres-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- Laitinen, K.; Mokkala, K. Overall Dietary Quality Relates to Gut Microbiota Diversity and Abundance. Int. J. Mol. Sci. 2019, 20, 1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Gallego, C.; Pohl, S.; Salminen, S.; De Vos, W.M.; Kneifel, W. Akkermansia muciniphila: A novel functional microbe with probiotic properties. Benef. Microbes 2016, 7, 571–584. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J. Funct. Foods 2017, 33, 194–201. [Google Scholar] [CrossRef]
- Tabasco, R.; Sanchez-Patan, F.; Monagas, M.; Bartolome, B.; Victoria Moreno-Arribas, M.; Pelaez, C.; Requena, T. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiol. 2011, 28, 1345–1352. [Google Scholar] [CrossRef]
- Garcia-Diez, E.; Cuesta-Hervas, M.; Veses-Alcobendas, A.M.; Alonso-Gordo, O.; Garcia-Maldonado, E.; Martinez-Suarez, M.; Herranz, B.; Vaquero, M.P.; Alvarez, M.D.; Perez-Jimenez, J. Acute supplementation with grapes in obese subjects did not affect postprandial metabolism: A randomized, double-blind, crossover clinical trial. Eur. J. Nutr. 2021, 60, 2671–2681. [Google Scholar] [CrossRef]
- Ogita, T.; Yamamoto, Y.; Mikami, A.; Shigemori, S.; Sato, T.; Shimosato, T. Oral Administration of Flavonifractor plautii Strongly Suppresses Th2 Immune Responses in Mice. Front. Immunol. 2020, 11, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Coffey, M.J.; Nielsen, S.; Wemheuer, B.; Kaakoush, N.O.; Garg, M.; Needham, B.; Pickford, R.; Jaffe, A.; Thomas, T.; Ooi, C.Y. Gut Microbiota in Children With Cystic Fibrosis: A Taxonomic and Functional Dysbiosis. Sci. Rep.-UK 2019, 9, 18593. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.R., Jr.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Daniels, T.F.; Killinger, K.M.; Michal, J.J.; Wright, R.W., Jr.; Jiang, Z. Lipoproteins, cholesterol homeostasis and cardiac health. Int. J. Biol. Sci. 2009, 5, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.G.; David, B.; Alan, C.; Luther, T.C.; Margo, D.; Richard, J.H.; William, R.H.; Stephen, B.H.; Donald, B.H.; Robert, A.K.; et al. Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). JAMA 1993, 269, 3015–3023. [Google Scholar]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Brinton, E.A.; Eisenberg, S.; Breslow, J.L. A low-fat diet decreases high density lipoprotein (HDL) cholesterol levels by decreasing HDL apolipoprotein transport rates. J. Clin. Investig. 1990, 85, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asztalos, B.; Lefevre, M.; Wong, L.; Foster, T.A.; Tulley, R.; Windhauser, M.; Zhang, W.W.; Roheim, P.S. Differential response to low-fat diet between low and normal HDL-cholesterol subjects. J. Lipid Res. 2000, 41, 321–328. [Google Scholar] [CrossRef]


| Baseline | 4-Week Grape | |
|---|---|---|
| Age | 33.5 (10.5) | |
| Sex, % women | 70% | |
| BMI (kg/m2) | 28.5 (10.1) | 28.5 (10.3) |
| Weight (lb) | 164.8 (47.7) | 165.0 (49.4) |
| Baseline | 4-Week Grape | |
|---|---|---|
| Bacteroidetes | 45.34 (18.08) | 40.69 (18.41) |
| Firmicutes | 47.34 (16.42) | 51.39 (16.82) |
| Actinobacteria | 3.71 (3.68) | 2.49 (2.02) |
| Verrucomicrobia | 0.5 (1.02) | 3.54 (6.27) * |
| Proteobacteria | 2.54 (5.02) | 1.5 (1.49) |
| Euryarchaeota | 0.52 (1.4) | 0.26 (0.65) |
| Tenericutes | 0.05 (0.13) | 0.13 (0.48) |
| Baseline | 4 Week Grape | p Value | |
|---|---|---|---|
| Primary BAs plus conjugates | |||
| CA (nM) | 142.9 (228.7) | 177.7 (274) | 0.98 |
| GCA (nM) | 356.5 (691.8) | 315.5 (820.8) | 0.39 |
| TCA (nM) | 60.2 (136.6) | 60.2 (228.3) | 0.45 |
| CDCA (nM) | 200.7 (184.9) | 205.4 (194.5) | 0.90 |
| GCDCA (nM) | 1344.4 (1903.4) | 700.5 (920.7) | 0.00 |
| TCDCA (nM) | 168.8 (231.5) | 97.8 (199.5) | 0.03 |
| Secondary/Tertiary BAs plus conjugates | |||
| DCA (nM) | 485.4 (391.1) | 508.6 (422.2) | 0.49 |
| GDCA (nM) | 491.4 (627.4) | 354.8 (466.9) | 0.02 |
| TDCA (nM) | 50.5 (55.4) | 32.4 (54.6) | 0.05 |
| LCA (nM) | 9.9 (23.6) | 6.9 (14.6) | 1.00 |
| GLCA (nM) | 18.5 (29.6) | 14.3 (19.5) | 0.41 |
| UDCA (nM) | 137 (332.7) | 95.9 (169.1) | 1.00 |
| Total BAs | |||
| Total | 3466.3 (4053.8) | 2569.9 (3190.2) | 0.04 |
| Ratio: Unconjugated/Total | 0.36 (0.22) | 0.44 (0.17) | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Kurnia, P.; Henning, S.M.; Lee, R.; Huang, J.; Garcia, M.C.; Surampudi, V.; Heber, D.; Li, Z. Effect of Standardized Grape Powder Consumption on the Gut Microbiome of Healthy Subjects: A Pilot Study. Nutrients 2021, 13, 3965. https://doi.org/10.3390/nu13113965
Yang J, Kurnia P, Henning SM, Lee R, Huang J, Garcia MC, Surampudi V, Heber D, Li Z. Effect of Standardized Grape Powder Consumption on the Gut Microbiome of Healthy Subjects: A Pilot Study. Nutrients. 2021; 13(11):3965. https://doi.org/10.3390/nu13113965
Chicago/Turabian StyleYang, Jieping, Patrick Kurnia, Susanne M. Henning, Rupo Lee, Jianjun Huang, Michael C. Garcia, Vijaya Surampudi, David Heber, and Zhaoping Li. 2021. "Effect of Standardized Grape Powder Consumption on the Gut Microbiome of Healthy Subjects: A Pilot Study" Nutrients 13, no. 11: 3965. https://doi.org/10.3390/nu13113965
APA StyleYang, J., Kurnia, P., Henning, S. M., Lee, R., Huang, J., Garcia, M. C., Surampudi, V., Heber, D., & Li, Z. (2021). Effect of Standardized Grape Powder Consumption on the Gut Microbiome of Healthy Subjects: A Pilot Study. Nutrients, 13(11), 3965. https://doi.org/10.3390/nu13113965






