Does Folic Acid Protect Patients with Inflammatory Bowel Disease from Complications?
Abstract
1. Introduction
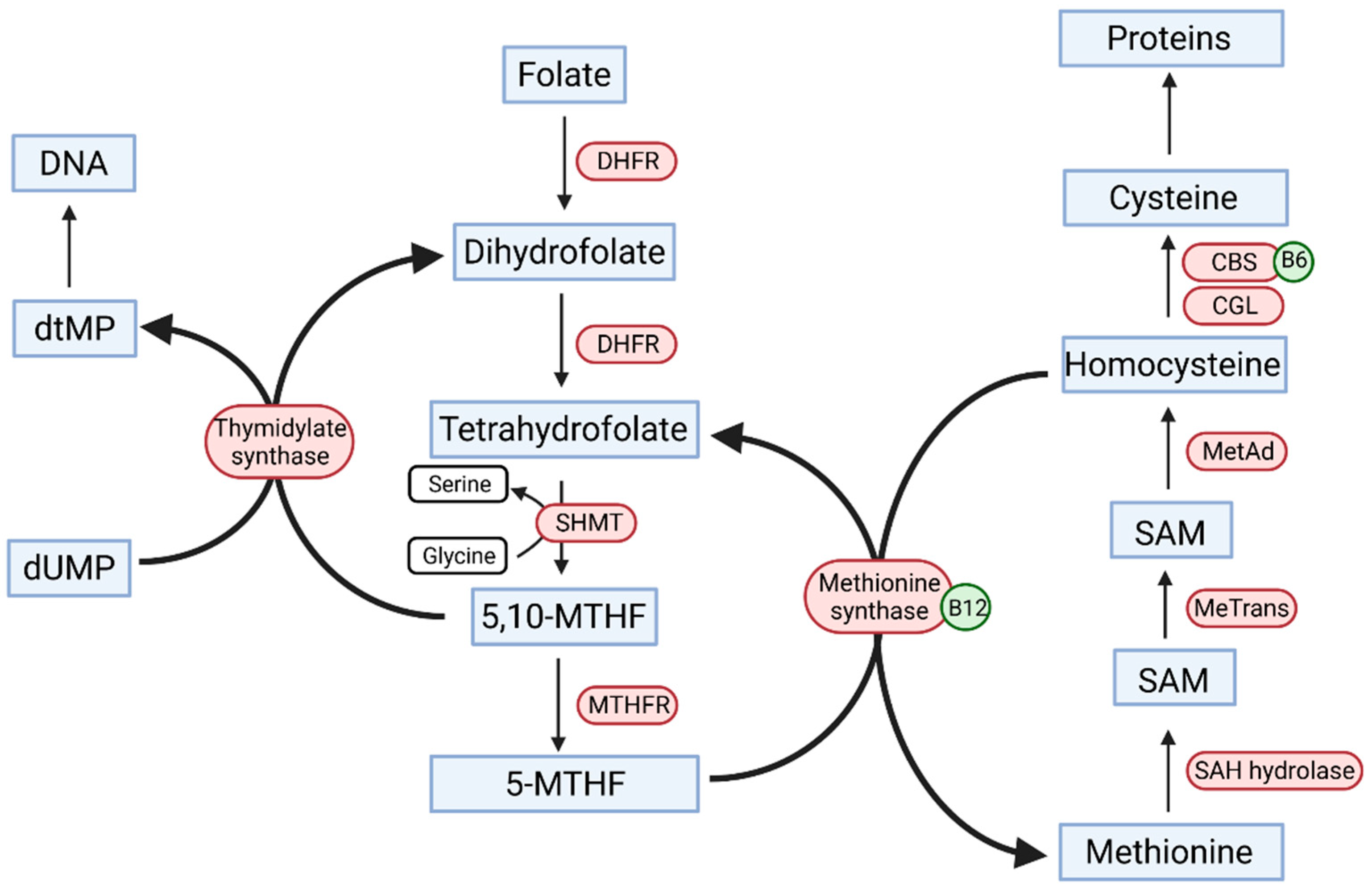
2. The Role of Folic Acid in the Human Body
2.1. Dietary Sources of Folic Acid
2.2. Recommendations Regarding Folic Acid Intake
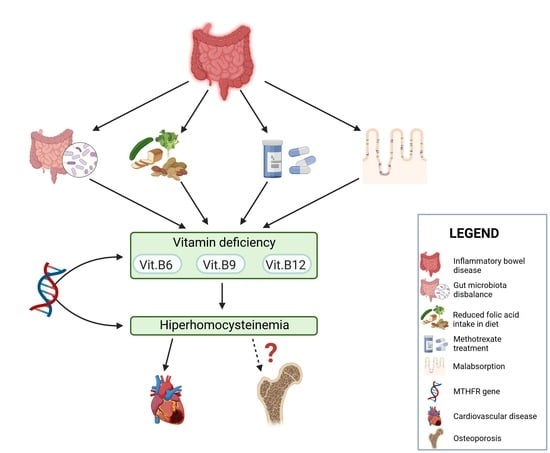
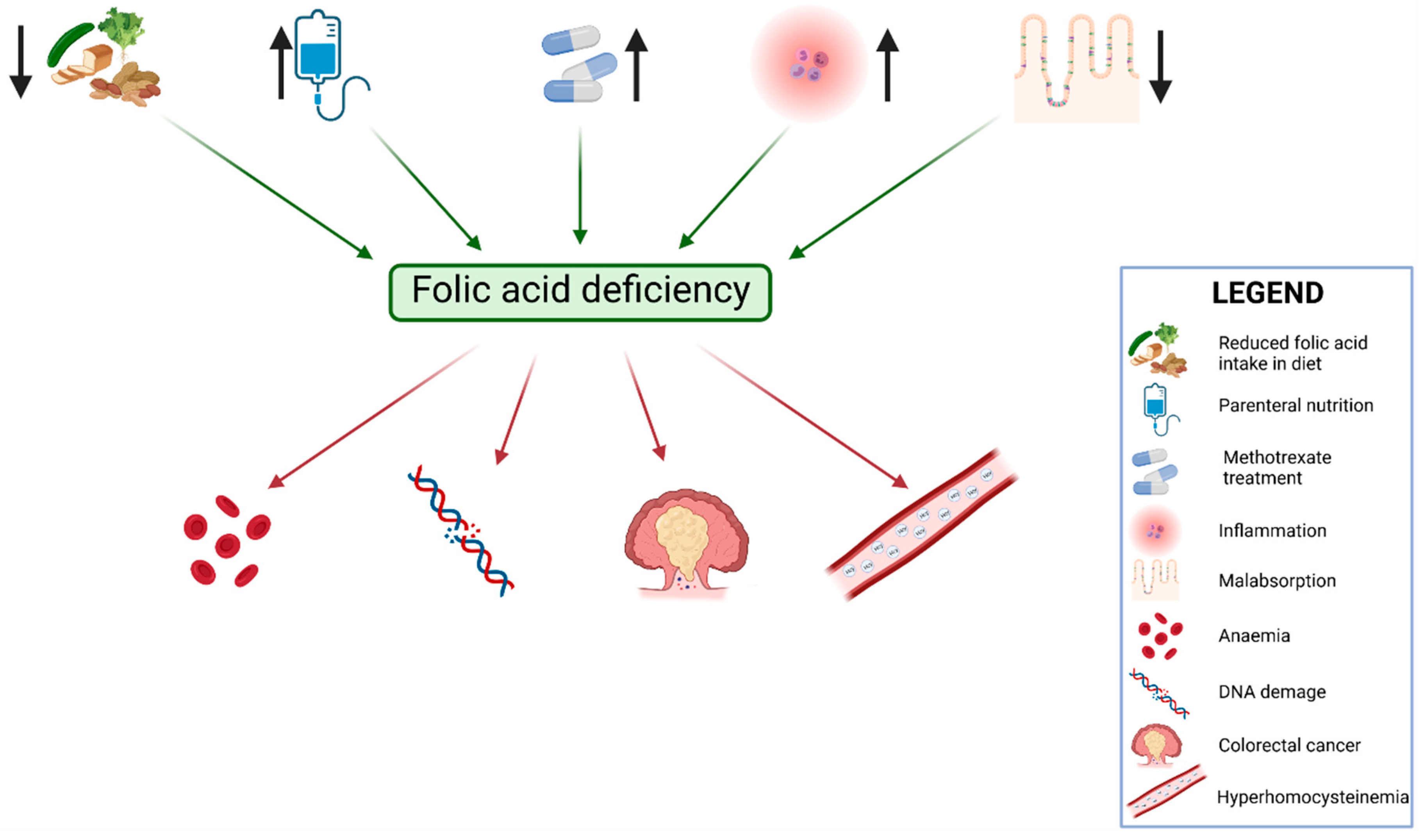
3. The Role of Folic Acid in Inflammatory Bowel Disease
3.1. Methylenotetrahydrofolate Reductase Gene
3.2. Homocysteine and Bone Mineral Density in Inflammatory Bowel Disease Patients
4. Microbiota and Folate Metabolism in IBD Patients
5. Summary and Conclusions
- In patients suffering from IBD, the concentration of folic acid should be evaluated more frequently than once per year, as it will help to diagnose a potential deficiency and macrocytic anemia.
- Following the recommendations of ECCO, IBD patients treated with methotrexate should be supplied with 5 mg of folic acid at two- to three-day intervals during the administration of methotrexate.
- Pregnant women, or women attempting pregnancy, should supplement folic acid. The recommended dosage is 0.4–5 mg/day (depending on the risk of neural birth tube defects).
- The supplementation of folic acid may be a protective factor against the development of CRC. However, this hypothesis requires further research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shelke, N.; Keith, L. Folic Acid Supplementation for Women of Childbearing Age versus Supplementation for the General Population: A Review of the Known Advantages and Risks. Int. J. Fam. Med. 2011, 2011, 173705. [Google Scholar] [CrossRef] [PubMed]
- Merrell, B.J.; McMurry, J.P. Folic Acid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Sijilmassi, O. Folic Acid Deficiency and Vision: A Review. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Enderami, A.; Zarghami, M.; Darvishi-Khezri, H. The Effects and Potential Mechanisms of Folic Acid on Cognitive Function: A Comprehensive Review. Neurol. Sci. 2018, 39, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Panzavolta, G. Folate, Folic Acid and 5-Methyltetrahydrofolate Are Not the Same Thing. Xenobiotica 2014, 44, 480–488. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Guo, H.; Jabir, M.S.; Liu, X.; Cui, W.; Li, D. Associations between Folate and Vitamin B12 Levels and Inflammatory Bowel Disease: A Meta-Analysis. Nutrients 2017, 9, 382. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Nocelli, C.; Fontanella, L.; Ginaldi, L. Hyperhomocysteinemia Is Associated with Inflammation, Bone Resorption, Vitamin B12 and Folate Deficiency and MTHFR C677T Polymorphism in Postmenopausal Women with Decreased Bone Mineral Density. Int. J. Environ. Res. Public Health 2020, 17, 4260. [Google Scholar] [CrossRef]
- Karakoyun, I.; Duman, C.; Demet Arslan, F.; Baysoy, A.; Isbilen Basok, B. Vitamin B12 and Folic Acid Associated Megaloblastic Anemia: Could It Mislead the Diagnosis of Breast Cancer? Int. J. Vitam. Nutr. Res. 2019, 89, 255–260. [Google Scholar] [CrossRef]
- Milman, N. Intestinal Absorption of Folic Acid—New Physiologic & Molecular Aspects. Indian J. Med. Res. 2012, 136, 725–728. [Google Scholar]
- Bernstein, L.H.; Gutstein, S.; Weiner, S.; Efron, G. The Absorption and Malabsorption of Folic Acid and Its Polyglutamates. Am. J. Med. 1970, 48, 570–579. [Google Scholar] [CrossRef]
- Gazzali, A.M.; Lobry, M.; Colombeau, L.; Acherar, S.; Azaïs, H.; Mordon, S.; Arnoux, P.; Baros, F.; Vanderesse, R.; Frochot, C. Stability of Folic Acid under Several Parameters. Eur. J. Pharm. Sci. 2016, 93, 419–430. [Google Scholar] [CrossRef]
- Nuru, M.; Muradashvili, N.; Kalani, A.; Lominadze, D.; Tyagi, N. High Methionine, Low Folate and Low Vitamin B6/B12 (HM-LF-LV) Diet Causes Neurodegeneration and Subsequent Short-Term Memory Loss. Metab. Brain Dis. 2018, 33, 1923–1934. [Google Scholar] [CrossRef]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA Methylation: A Review of Molecular Mechanisms and the Evidence for Folate’s Role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef]
- Duthie, S.J.; Narayanan, S.; Brand, G.M.; Pirie, L.; Grant, G. Impact of Folate Deficiency on DNA Stability. J. Nutr. 2002, 132, 2444S–2449S. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Iwasaki, M.; Hanaoka, T.; Kobayashi, M.; Ishihara, J.; Natsukawa, S.; Shaura, K.; Koizumi, Y.; Kasuga, Y.; Yoshimura, K.; et al. Folate, Vitamin B6, Vitamin B12, and Vitamin B 2 Intake, Genetic Polymorphisms of Related Enzymes, and Risk of Colorectal Cancer in a Hospital-Based Case-Control Study in Japan. Nutr. Cancer 2005, 53, 42–50. [Google Scholar] [CrossRef]
- Duthie, S.J. Folic Acid Deficiency and Cancer: Mechanisms of DNA Instability. Br. Med. Bull. 1999, 55, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, C.; Wang, Q.; Zhang, Z. Supplementation of Folic Acid in Pregnancy and the Risk of Preeclampsia and Gestational Hypertension: A Meta-Analysis. Arch. Gynecol. Obstet. 2018, 298, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Ghishan, F.K.; Kiela, P.R. Vitamins and Minerals in IBD. Gastroenterol. Clin. 2017, 46, 797–808. [Google Scholar] [CrossRef]
- Field, M.S.; Stover, P.J. Safety of Folic Acid. Ann. N. Y. Acad. Sci. 2018, 1414, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Likis, F. Folic Acid. J. Midwifery Women’s Health 2016, 61, 797–798. [Google Scholar] [CrossRef]
- Kunachowicz, H.; Nadolna, I.; Iwanow, K.; Przygoda, B. Wartość Odżywcza Wybranych Produktów Spożywczych i Typowych Potraw; VI uaktualnione i rozszerzone; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2010. [Google Scholar]
- Caudill, M.A. Folate Bioavailability: Implications for Establishing Dietary Recommendations and Optimizing Status1234. Am. J. Clin. Nutr. 2010, 91, 1455S–1460S. [Google Scholar] [CrossRef]
- Kondo, A.; Asada, Y.; Shibata, K.; Kihira, M.; Ninomiya, K.; Suzuki, M.; Oguchi, H.; Hayashi, Y.; Narita, O.; Watanabe, J.; et al. Dietary Folate Intakes and Effects of Folic Acid Supplementation on Folate Concentrations among Japanese Pregnant Women. J. Obstet. Gynaecol. Res. 2011, 37, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.M.; Jee, S.H.; Charleston, J.; Narrett, M.; Appel, L.J. Effects of Folic Acid Supplementation on Serum Folate and Plasma Homocysteine Concentrations in Older Adults: A Dose-Response Trial. Am. J. Epidemiol. 2010, 172, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Bomba-Opoń, D.; Hirnle, L.; Kalinka, J.; Seremak-Mrozikiewicz, A. Folate Supplementation during the Preconception Period, Pregnancy and Puerperium. Polish Society of Gynecologists and Obstetricians Guidelines. Ginekol. Pol. 2017, 88, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.; Mark, A.G.; Rinawi, F.; Shamir, R.; Assa, A. Micronutrient Deficiencies in Children with Inflammatory Bowel Diseases. Nutr. Clin. Pract. 2020, 35, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Hoffbrand, A.V.; Stewart, J.S.; Booth, C.C.; Mollin, D.L. Folate Deficiency in Crohn’s Disease: Incidence, Pathogenesis, and Treatment. Br. Med. J. 1968, 2, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, K.; Łykowska-Szuber, L.; Krela-Kazmierczak, I.; Eder, P.; Szymczak, A.; Stawczyk-Eder, K.; Linke, K. Zastosowanie Metotreksatu w Nieswoistych Chorobach Zapalnych Jelit Na Podstawie Przeglądu Aktualnego Piśmiennictwa. Wiad. Lek. 2016, 69, 262–266. [Google Scholar]
- Schröder, O.; Stein, J. Low Dose Methotrexate in Inflammatory Bowel Disease: Current Status and Future Directions. Am. J. Gastroenterol. 2003, 98, 530–537. [Google Scholar] [CrossRef]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-Based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohns Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology Consensus Guidelines on the Management of Inflammatory Bowel Disease in Adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN Practical Guideline: Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef]
- Restellini, S.; Biedermann, L.; Hruz, P.; Mottet, C.; Moens, A.; Ferrante, M.; Schoepfer, A.M.; on behalf of Swiss IBDnet, an Official Working Group of the S.S. of Gastroenterology. Update on the Management of Inflammatory Bowel Disease during Pregnancy and Breastfeeding. Digestion 2020, 101, 27–42. [Google Scholar] [CrossRef]
- Gasche, C.; Lomer, M.C.E.; Cavill, I.; Weiss, G. Iron, Anaemia, and Inflammatory Bowel Diseases. Gut 2004, 53, 1190–1197. [Google Scholar] [CrossRef]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European Consensus on the Diagnosis and Management of Iron Deficiency and Anaemia in Inflammatory Bowel Diseases. J. Crohns Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef]
- Ebara, S. Nutritional Role of Folate. Congenit. Anom. 2017, 57, 138–141. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial Diagnosis, Monitoring of Known IBD, Detection of Complications. J. Crohns Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
- Samblas, M.; Martínez, J.A.; Milagro, F. Folic Acid Improves the Inflammatory Response in LPS-Activated THP-1 Macrophages. Mediat. Inflamm. 2018, 2018, 1312626. [Google Scholar] [CrossRef]
- Biasco, G.; Zannoni, U.; Paganelli, G.M.; Santucci, R.; Gionchetti, P.; Rivolta, G.; Miniero, R.; Pironi, L.; Calabrese, C.; Di Febo, G.; et al. Folic Acid Supplementation and Cell Kinetics of Rectal Mucosa in Patients with Ulcerative Colitis. Cancer Epidemiol. Biomark. Prev. 1997, 6, 469–471. [Google Scholar]
- Lashner, B.A.; Heidenreich, P.A.; Su, G.L.; Kane, S.V.; Hanauer, S.B. Effect of Folate Supplementation on the Incidence of Dysplasia and Cancer in Chronic Ulcerative Colitis. A Case-Control Study. Gastroenterology 1989, 97, 255–259. [Google Scholar] [CrossRef]
- Lashner, B.A.; Provencher, K.S.; Seidner, D.L.; Knesebeck, A.; Brzezinski, A. The Effect of Folic Acid Supplementation on the Risk for Cancer or Dysplasia in Ulcerative Colitis. Gastroenterology 1997, 112, 29–32. [Google Scholar] [CrossRef]
- Burr, N.E.; Hull, M.A.; Subramanian, V. Folic Acid Supplementation May Reduce Colorectal Cancer Risk in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2017, 51, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Hernández, V.; Myrelid, P.; Kariv, R.; Tsianos, E.; Toruner, M.; Marti-Gallostra, M.; Spinelli, A.; van der Meulen-de Jong, A.E.; Yuksel, E.S.; et al. Colorectal Cancer in Inflammatory Bowel Disease: Results of the 3rd ECCO Pathogenesis Scientific Workshop (I). J. Crohns Colitis 2014, 8, 5–18. [Google Scholar] [CrossRef]
- Wang, N.; Tang, H.; Wang, X.; Wang, W.; Feng, J. Homocysteine Upregulates Interleukin-17A Expression via NSun2-Mediated RNA Methylation in T Lymphocytes. Biochem. Biophys. Res. Commun. 2017, 493, 94–99. [Google Scholar] [CrossRef]
- Lin, X.; Meng, X.; Song, Z. Homocysteine and Psoriasis. Biosci. Rep. 2019, 39, BSR20190867. [Google Scholar] [CrossRef]
- Enneman, A.W.; Swart, K.M.A.; van Wijngaarden, J.P.; van Dijk, S.C.; Ham, A.C.; Brouwer-Brolsma, E.M.; van der Zwaluw, N.L.; Dhonukshe-Rutten, R.A.M.; van der Cammen, T.J.M.; de Groot, L.C.P.G.M.; et al. Effect of Vitamin B12 and Folic Acid Supplementation on Bone Mineral Density and Quantitative Ultrasound Parameters in Older People with an Elevated Plasma Homocysteine Level: B-PROOF, a Randomized Controlled Trial. Calcif. Tissue Int. 2015, 96, 401–409. [Google Scholar] [CrossRef]
- Salari, P.; Abdollahi, M.; Heshmat, R.; Meybodi, H.A.; Razi, F. Effect of Folic Acid on Bone Metabolism: A Randomized Double Blind Clinical Trial in Postmenopausal Osteoporotic Women. DARU J. Pharm. Sci. 2014, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Genetics Home Reference. MTHFR Gene. Available online: https://ghr.nlm.nih.gov/gene/MTHFR (accessed on 11 May 2020).
- Xu, B.; Kong, X.; Xu, R.; Song, Y.; Liu, L.; Zhou, Z.; Gu, R.; Shi, X.; Zhao, M.; Huang, X.; et al. Homocysteine and All-Cause Mortality in Hypertensive Adults without Pre-Existing Cardiovascular Conditions: Effect Modification by MTHFR C677T Polymorphism. Medicine 2017, 96, e5862. [Google Scholar] [CrossRef] [PubMed]
- Hanks, J.; Ayed, I.; Kukreja, N.; Rogers, C.; Harris, J.; Gheorghiu, A.; Liu, C.L.; Emery, P.; Pufulete, M. The Association between MTHFR 677C>T Genotype and Folate Status and Genomic and Gene-Specific DNA Methylation in the Colon of Individuals without Colorectal Neoplasia. Am. J. Clin. Nutr. 2013, 98, 1564–1574. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahmud, N.; Molloy, A.; McPartlin, J.; Corbally, R.; Whitehead, A.S.; Scott, J.M.; Weir, D.G. Increased Prevalence of Methylenetetrahydrofolate Reductase C677T Variant in Patients with Inflammatory Bowel Disease, and Its Clinical Implications. Gut 1999, 45, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Stocco, G.; Martelossi, S.; Sartor, F.; Toffoli, G.; Lionetti, P.; Barabino, A.; Fontana, M.; Decorti, G.; Bartoli, F.; Giraldi, T.; et al. Prevalence of Methylenetetrahydrofolate Reductase Polymorphisms in Young Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2006, 51, 474–479. [Google Scholar] [CrossRef]
- Senhaji, N.; Serbati, N.; Diakité, B.; Arazzakou, S.; Hamzi, K.; Badre, W.; Nadifi, S. Methylenetetrahydrofolate Reductase C677T Variant in Moroccan Patients with Inflammatory Bowel Disease. Gene 2013, 521, 45–49. [Google Scholar] [CrossRef]
- Lu, L.; Wang, W.; Li, L.; Pang, X.; Fei, S. Risk of Inflammatory Bowel Disease Associated with MTHFRC677T and Prothrombin G20210A Mutation: A Meta-Analysis. Int. J. Clin. Exp. Med. 2017, 10, 743–7442. [Google Scholar]
- Chen, M.; Peyrin-Biroulet, L.; Xia, B.; Guéant-Rodriguez, R.-M.; Bronowicki, J.-P.; Bigard, M.-A.; Guéant, J.-L. Methionine Synthase A2756G Polymorphism May Predict Ulcerative Colitis and Methylenetetrahydrofolate Reductase C677T Pancolitis, in Central China. BMC Med. Genet. 2008, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Hustad, S.; Midttun, Ø.; Schneede, J.; Vollset, S.E.; Grotmol, T.; Ueland, P.M. The Methylenetetrahydrofolate Reductase 677C-->T Polymorphism as a Modulator of a B Vitamin Network with Major Effects on Homocysteine Metabolism. Am. J. Hum. Genet. 2007, 80, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-X.; Dai, S.-X.; Zheng, J.-J.; Liu, J.-Q.; Huang, J.-F. Homocysteine Metabolism Gene Polymorphisms (MTHFR C677T, MTHFR A1298C, MTR A2756G and MTRR A66G) Jointly Elevate the Risk of Folate Deficiency. Nutrients 2015, 7, 6670–6687. [Google Scholar] [CrossRef] [PubMed]
- Guéant-Rodriguez, R.-M.; Guéant, J.-L.; Debard, R.; Thirion, S.; Hong, L.X.; Bronowicki, J.-P.; Namour, F.; Chabi, N.W.; Sanni, A.; Anello, G.; et al. Prevalence of Methylenetetrahydrofolate Reductase 677T and 1298C Alleles and Folate Status: A Comparative Study in Mexican, West African, and European Populations. Am. J. Clin. Nutr. 2006, 83, 701–707. [Google Scholar] [CrossRef]
- Gjesdal, C.G.; Vollset, S.E.; Ueland, P.M.; Refsum, H.; Meyer, H.E.; Tell, G.S. Plasma Homocysteine, Folate, and Vitamin B 12 and the Risk of Hip Fracture: The Hordaland Homocysteine Study. J. Bone Miner. Res. 2007, 22, 747–756. [Google Scholar] [CrossRef]
- Li, D.; Wu, J. Association of the MTHFR C677T Polymorphism and Bone Mineral Density in Postmenopausal Women: A Meta-Analysis. J. Biomed. Res. 2010, 24, 417–423. [Google Scholar] [CrossRef][Green Version]
- Villadsen, M.M.; Bünger, M.H.; Carstens, M.; Stenkjaer, L.; Langdahl, B.L. Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism Is Associated with Osteoporotic Vertebral Fractures, but Is a Weak Predictor of BMD. Osteoporos. Int. 2005, 16, 411–416. [Google Scholar] [CrossRef]
- Cd, S.; Pm, E.; Sj, L.; Gd, S.; Jh, T. Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism Is Associated with Spinal BMD in 9-Year-Old Children. J. Bone Miner. Res. 2009, 24, 117–124. [Google Scholar] [CrossRef]
- Krela-Kaźmierczak, I.; Michalak, M.; Szymczak-Tomczak, A.; Łykowska-Szuber, L.; Stawczyk-Eder, K.; Waszak, K.; Kucharski, M.A.; Dobrowolska, A.; Eder, P. Prevalence of Osteoporosis and Osteopenia in a Population of Patients with Inflammatory Bowel Diseases from the Wielkopolska Region. Pol. Arch. Intern. Med. 2018, 128, 447–454. [Google Scholar] [CrossRef]
- Adriani, A.; Pantaleoni, S.; Luchino, M.; Ribaldone, D.G.; Reggiani, S.; Sapone, N.; Sguazzini, C.; Isaia, G.; Pellicano, R.; Astegiano, M. Osteopenia and Osteoporosis in Patients with New Diagnosis of Inflammatory Bowel Disease. Panminerva Med. 2014, 56, 145–149. [Google Scholar]
- Oussalah, A.; Guéant, J.-L.; Peyrin-Biroulet, L. Meta-Analysis: Hyperhomocysteinaemia in Inflammatory Bowel Diseases. Aliment. Pharm. Ther. 2011, 34, 1173–1184. [Google Scholar] [CrossRef]
- Owczarek, D.; Cibor, D.; Sałapa, K.; Jurczyszyn, A.; Mach, T. Homocysteine in Patients with Inflammatory Bowel Diseases. Przegląd Lek. 2014, 71, 189–192. [Google Scholar]
- Akbulut, S.; Altiparmak, E.; Topal, F.; Ozaslan, E.; Kucukazman, M.; Yonem, O. Increased Levels of Homocysteine in Patients with Ulcerative Colitis. World J. Gastroenterol. 2010, 16, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Zezos, P.; Papaioannou, G.; Nikolaidis, N.; Vasiliadis, T.; Giouleme, O.; Evgenidis, N. Hyperhomocysteinemia in Ulcerative Colitis Is Related to Folate Levels. World J. Gastroenterol. 2005, 11, 6038–6042. [Google Scholar] [CrossRef] [PubMed]
- Škovierová, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červeňová, T.; Halašová, E.; Lehotský, J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef] [PubMed]
- Moll, S.; Varga, E.A. Homocysteine and MTHFR Mutations. Circulation 2015, 132, e6–e9. [Google Scholar] [CrossRef]
- Bahtiri, E.; Islami, H.; Rexhepi, S.; Qorraj-Bytyqi, H.; Thaçi, K.; Thaçi, S.; Karakulak, C.; Hoxha, R. Relationship of Homocysteine Levels with Lumbar Spine and Femur Neck BMD in Postmenopausal Women. Acta Reum. Port. 2015, 40, 355–362. [Google Scholar]
- Behera, J.; Bala, J.; Nuru, M.; Tyagi, S.C.; Tyagi, N. Homocysteine as a Pathological Biomarker for Bone Disease. J. Cell. Physiol. 2017, 232, 2704–2709. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, N.; Álvarez-Ríos, A.I.; Guerrero, J.M.; García-García, F.J.; Rodríguez-Mañas, L.; Cruz-Chamorro, I.; Lardone, P.J.; Carrillo-Vico, A. Homocysteine Levels Are Associated with Bone Resorption in Pre-Frail and Frail Spanish Women: The Toledo Study for Healthy Aging. Exp. Gerontol. 2018, 108, 201–208. [Google Scholar] [CrossRef]
- Garcia Lopez, M.; Baron, J.A.; Omsland, T.K.; Søgaard, A.J.; Meyer, H.E. Homocysteine-Lowering Treatment and the Risk of Fracture: Secondary Analysis of a Randomized Controlled Trial and an Updated Meta-Analysis. JBMR Plus 2018, 2, 295–303. [Google Scholar] [CrossRef]
- Saoji, R.; Das, R.S.; Desai, M.; Pasi, A.; Sachdeva, G.; Das, T.K.; Khatkhatay, M.I. Association of High-Density Lipoprotein, Triglycerides, and Homocysteine with Bone Mineral Density in Young Indian Tribal Women. Arch. Osteoporos. 2018, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Looker, A.C.; Lu, Z.; Fan, R.; Eicher-Miller, H.A.; Fakhouri, T.H.; Gahche, J.J.; Weaver, C.M.; Mills, J.L. B-Vitamin Status and Bone Mineral Density and Risk of Lumbar Osteoporosis in Older Females in the United States. Am. J. Clin. Nutr. 2015, 102, 687–694. [Google Scholar] [CrossRef]
- Tariq, S.; Lone, K.P.; Tariq, S. Comparison of Parameters of Bone Profile and Homocysteine in Physically Active and Non-Active Postmenopausal Females. Pak. J. Med. Sci. 2016, 32, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Verma, R.; Mishra, A.; Singh, A.; Kumar, V.; Sawlani, K.K.; Ahmad, M.K.; Mishra, P.; Gaur, R. Relation of Bone Mineral Density with Homocysteine and Cathepsin K Levels in Postmenopausal Women. Indian J. Endocrinol. Metab. 2018, 22, 261. [Google Scholar] [CrossRef] [PubMed]
- Haliloglu, B.; Aksungar, F.B.; Ilter, E.; Peker, H.; Akin, F.T.; Mutlu, N.; Ozekici, U. Relationship between Bone Mineral Density, Bone Turnover Markers and Homocysteine, Folate and Vitamin B12 Levels in Postmenopausal Women. Arch. Gynecol. Obstet. 2010, 281, 663–668. [Google Scholar] [CrossRef]
- Van Wijngaarden, J.P.; Doets, E.L.; Szczecińska, A.; Souverein, O.W.; Duffy, M.E.; Dullemeijer, C.; Cavelaars, A.E.J.M.; Pietruszka, B.; Van’t Veer, P.; Brzozowska, A.; et al. Vitamin B12, Folate, Homocysteine, and Bone Health in Adults and Elderly People: A Systematic Review with Meta-Analyses. J. Nutr. Metab. 2013, 2013, 486186. [Google Scholar] [CrossRef]
- Stone, K.L.; Lui, L.-Y.; Christen, W.G.; Troen, A.M.; Bauer, D.C.; Kado, D.; Schambach, C.; Cummings, S.R.; Manson, J.E. Effect of Combination Folic Acid, Vitamin B6, and Vitamin B12 Supplementation on Fracture Risk in Women: A Randomized, Controlled Trial. J. Bone Miner. Res. 2017, 32, 2331–2338. [Google Scholar] [CrossRef]
- Ruan, J.; Gong, X.; Kong, J.; Wang, H.; Zheng, X.; Chen, T. Effect of B Vitamin (Folate, B6, and B12) Supplementation on Osteoporotic Fracture and Bone Turnover Markers: A Meta-Analysis. Med. Sci. Monit. 2015, 21, 875–881. [Google Scholar] [CrossRef]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The Microbiome and Inflammatory Bowel Disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Czeczot, H. Kwas foliowy w fizjologii i patologii. Postepy Hig. Med. Dosw. 2008, 62, 405–419. [Google Scholar]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate Production by Probiotic Bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef]
- Sugahara, H.; Odamaki, T.; Hashikura, N.; Abe, F.; Xiao, J. Differences in Folate Production by Bifidobacteria of Different Origins. Biosci. Microbiota Food Health 2015, 34, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Pompei, A.; Cordisco, L.; Amaretti, A.; Zanoni, S.; Matteuzzi, D.; Rossi, M. Folate Production by Bifidobacteria as a Potential Probiotic Property. Appl. Environ. Microbiol. 2007, 73, 179–185. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as Vitamin Suppliers to Their Host: A Gut Microbiota Perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, C.; Chen, Z.; Chen, Z.; Zhang, W.; Ma, X.; Wang, L.; Yang, X.; Jiang, Z. Protective Effects of Lactobacillus Plantarum on Epithelial Barrier Disruption Caused by Enterotoxigenic Escherichia Coli in Intestinal Porcine Epithelial Cells. Vet. Immunol. Immunopathol. 2016, 172, 55–63. [Google Scholar] [CrossRef]
- MacFarlane, A.J.; Behan, N.A.; Matias, F.M.G.; Green, J.; Caldwell, D.; Brooks, S.P.J. Dietary Folate Does Not Significantly Affect the Intestinal Microbiome, Inflammation or Tumorigenesis in Azoxymethane-Dextran Sodium Sulphate-Treated Mice. Br. J. Nutr. 2013, 109, 630–638. [Google Scholar] [CrossRef]
- Choi, S.-W.; Mason, J.B. Folate Status: Effects on Pathways of Colorectal Carcinogenesis. J. Nutr. 2002, 132, 2413S–2418S. [Google Scholar] [CrossRef]
- Laiño, J.E.; Leblanc, J.G.; Savoy de Giori, G. Production of Natural Folates by Lactic Acid Bacteria Starter Cultures Isolated from Artisanal Argentinean Yogurts. Can. J. Microbiol. 2012, 58, 581–588. [Google Scholar] [CrossRef]
- Rossi, M.; Amaretti, A. Probiotic Properties of Bifidobacteria. In Bifidobacteria: Genomics and Molecular Aspects; van Synderen, D., Mayo, B., Eds.; Caister Academic Press: Norfolk, UK, 2010. [Google Scholar]
- Strozzi, G.P.; Mogna, L. Quantification of Folic Acid in Human Feces after Administration of Bifidobacterium Probiotic Strains. J. Clin. Gastroenterol. 2008, 42, S179–S184. [Google Scholar] [CrossRef] [PubMed]
- Storelli, G.; Téfit, M.; Leulier, F. Metformin, Microbes, and Aging. Cell Metab. 2013, 17, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.F. Jawetz, Melnick & Adelberg’s Medical Microbiology; McGraw-Hill Medical: New York, NY, USA, 2007; ISBN 978-0-07-147666-9. [Google Scholar]
| Product | Folate Content in 100 g of a Product (μg) |
|---|---|
| Milk | 5 |
| Quark | 27 |
| Egg yolk | 152 |
| Chicken liver | 590 |
| Beef liver | 330 |
| Rice | 29 |
| Broccoli | 119 |
| Parsley | 170 |
| Spinach | 193 |
| Avocado | 62 |
| Apple | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczak, A.E.; Szymczak-Tomczak, A.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Does Folic Acid Protect Patients with Inflammatory Bowel Disease from Complications? Nutrients 2021, 13, 4036. https://doi.org/10.3390/nu13114036
Ratajczak AE, Szymczak-Tomczak A, Rychter AM, Zawada A, Dobrowolska A, Krela-Kaźmierczak I. Does Folic Acid Protect Patients with Inflammatory Bowel Disease from Complications? Nutrients. 2021; 13(11):4036. https://doi.org/10.3390/nu13114036
Chicago/Turabian StyleRatajczak, Alicja Ewa, Aleksandra Szymczak-Tomczak, Anna Maria Rychter, Agnieszka Zawada, Agnieszka Dobrowolska, and Iwona Krela-Kaźmierczak. 2021. "Does Folic Acid Protect Patients with Inflammatory Bowel Disease from Complications?" Nutrients 13, no. 11: 4036. https://doi.org/10.3390/nu13114036
APA StyleRatajczak, A. E., Szymczak-Tomczak, A., Rychter, A. M., Zawada, A., Dobrowolska, A., & Krela-Kaźmierczak, I. (2021). Does Folic Acid Protect Patients with Inflammatory Bowel Disease from Complications? Nutrients, 13(11), 4036. https://doi.org/10.3390/nu13114036