Antiglycoxidative Properties of Extracts and Fractions from Reynoutria Rhizomes
Abstract
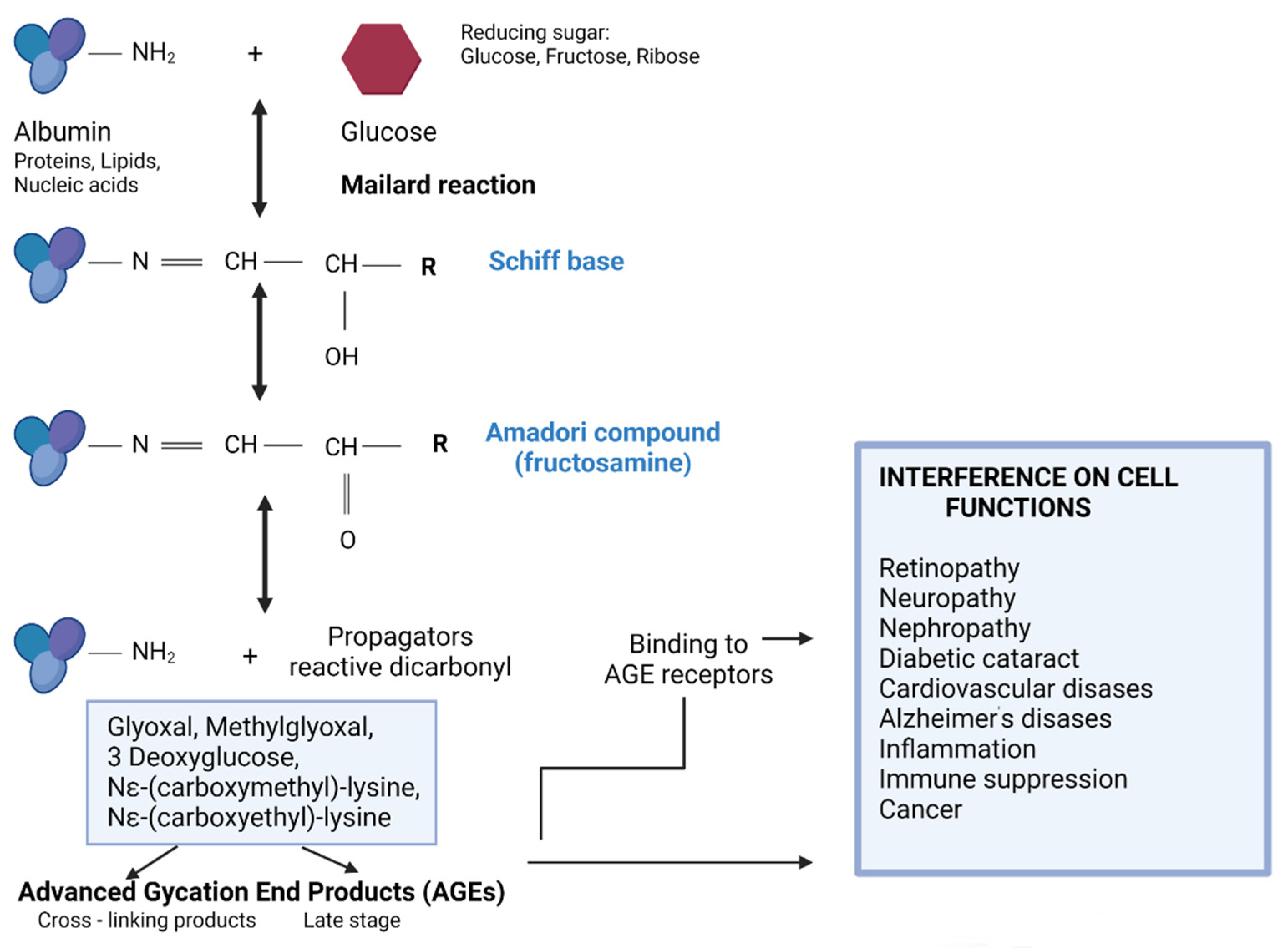
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Plant Extracts and Fractions
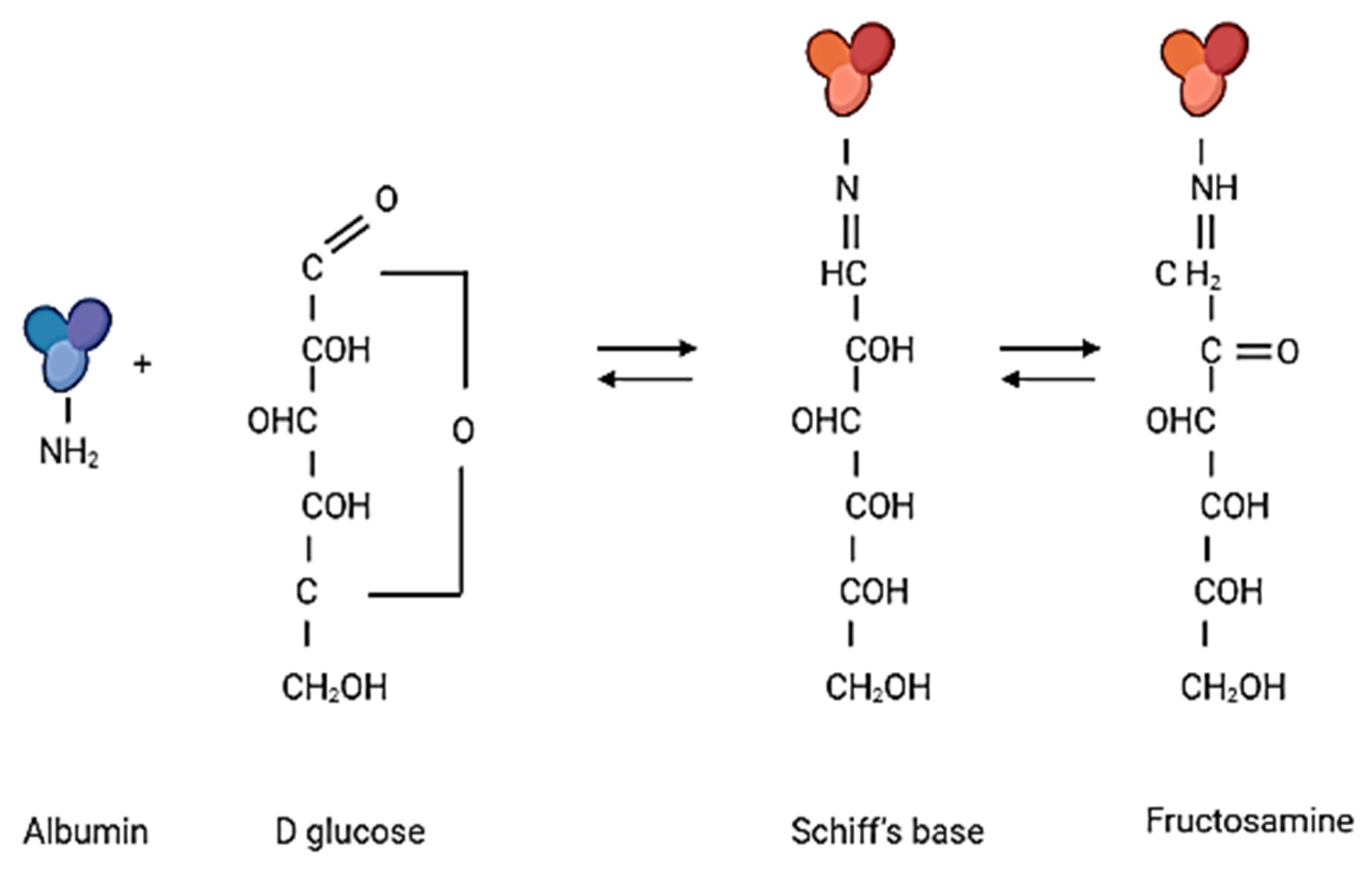
2.3. In Vitro Glycation of Albumin
2.4. Measurement of Fructosamine Levels
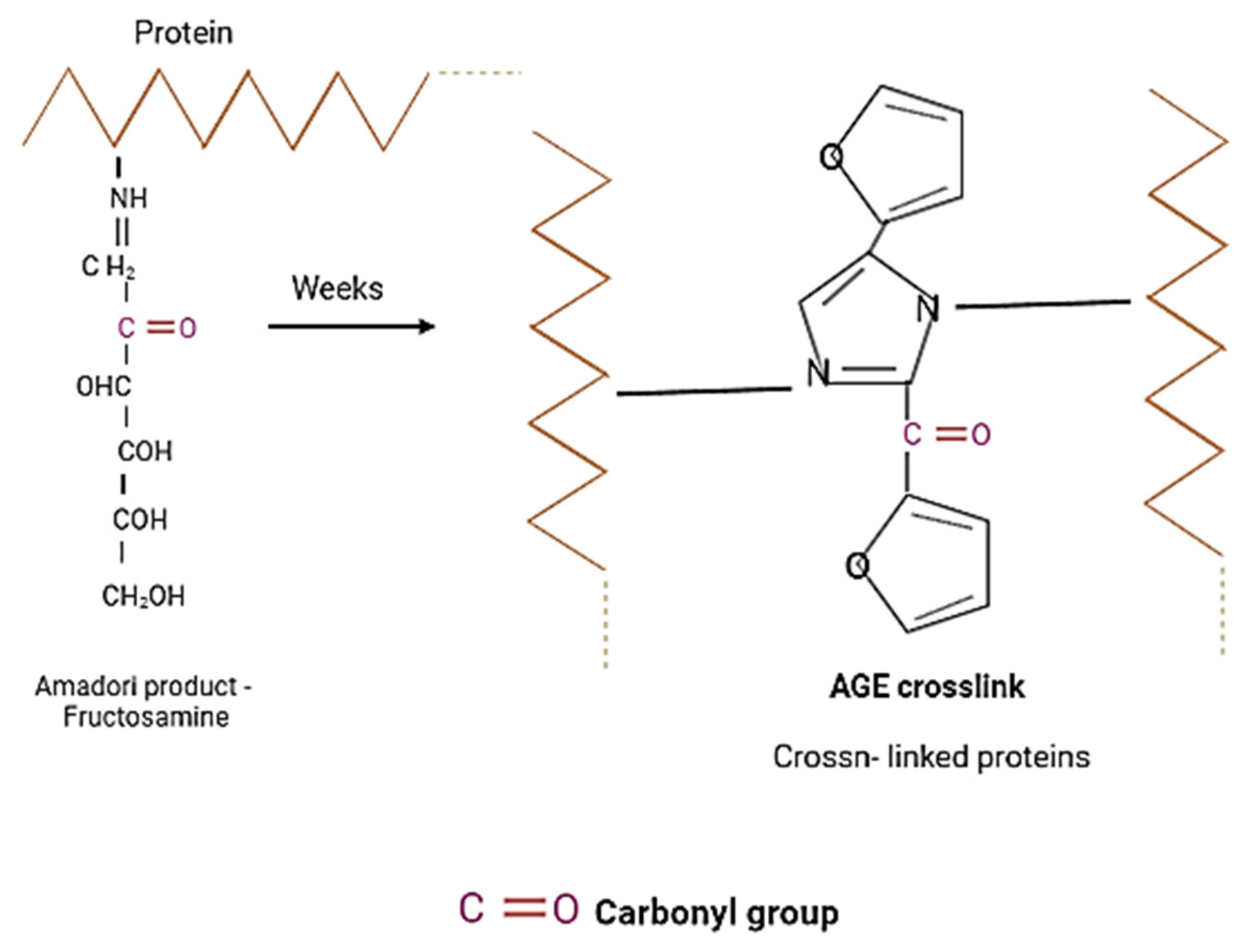
2.5. Measurement of AGEs by Fluorescence
2.6. Measurement of Protein Carbonyl Groups
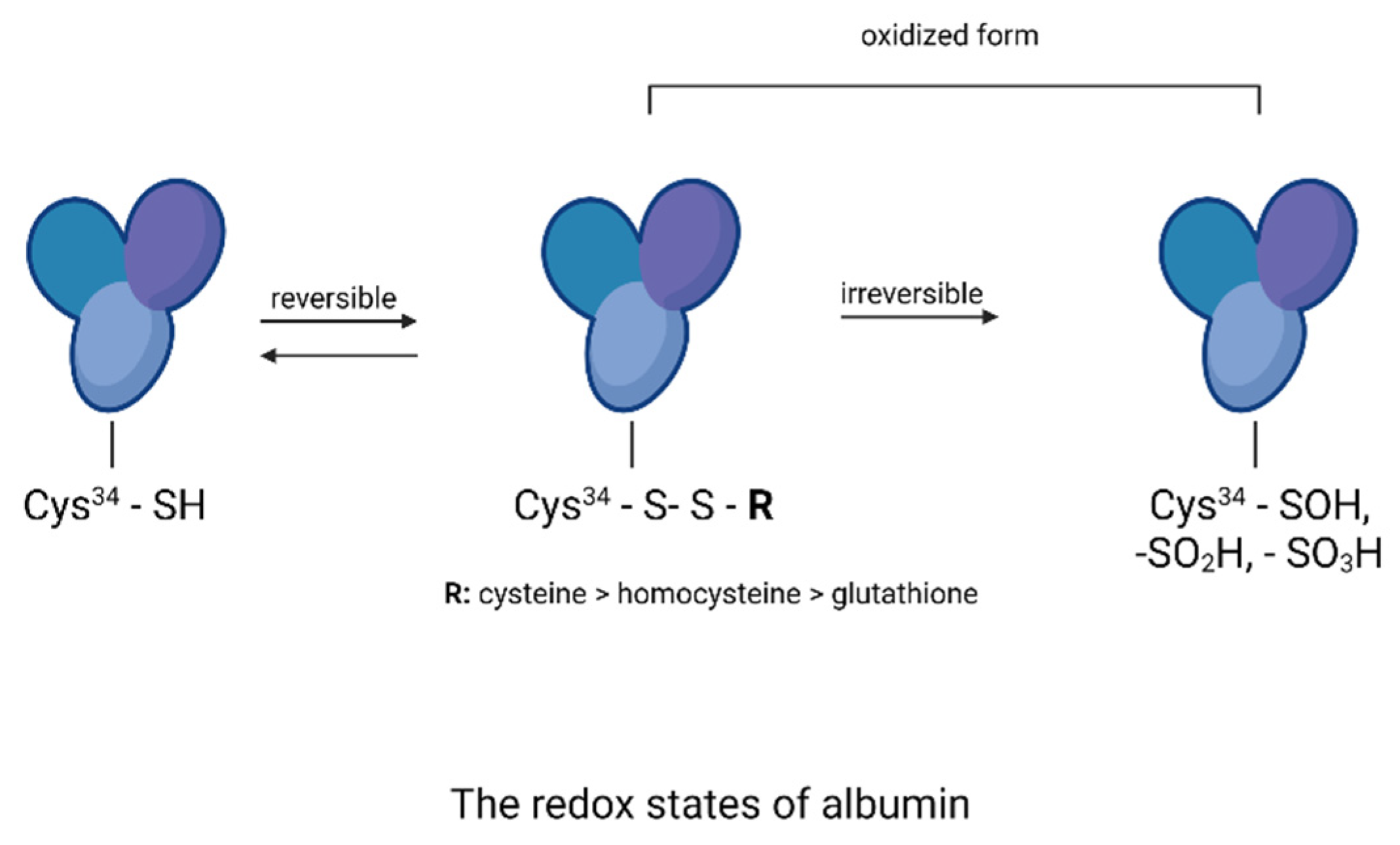
2.7. Thiol Group Estimation
2.8. Determination of Amyloid-β Aggregation by Thioflavin T
2.9. Determination of Amyloid-β Aggregation by Congo Red
2.10. Statistical Analysis
3. Results
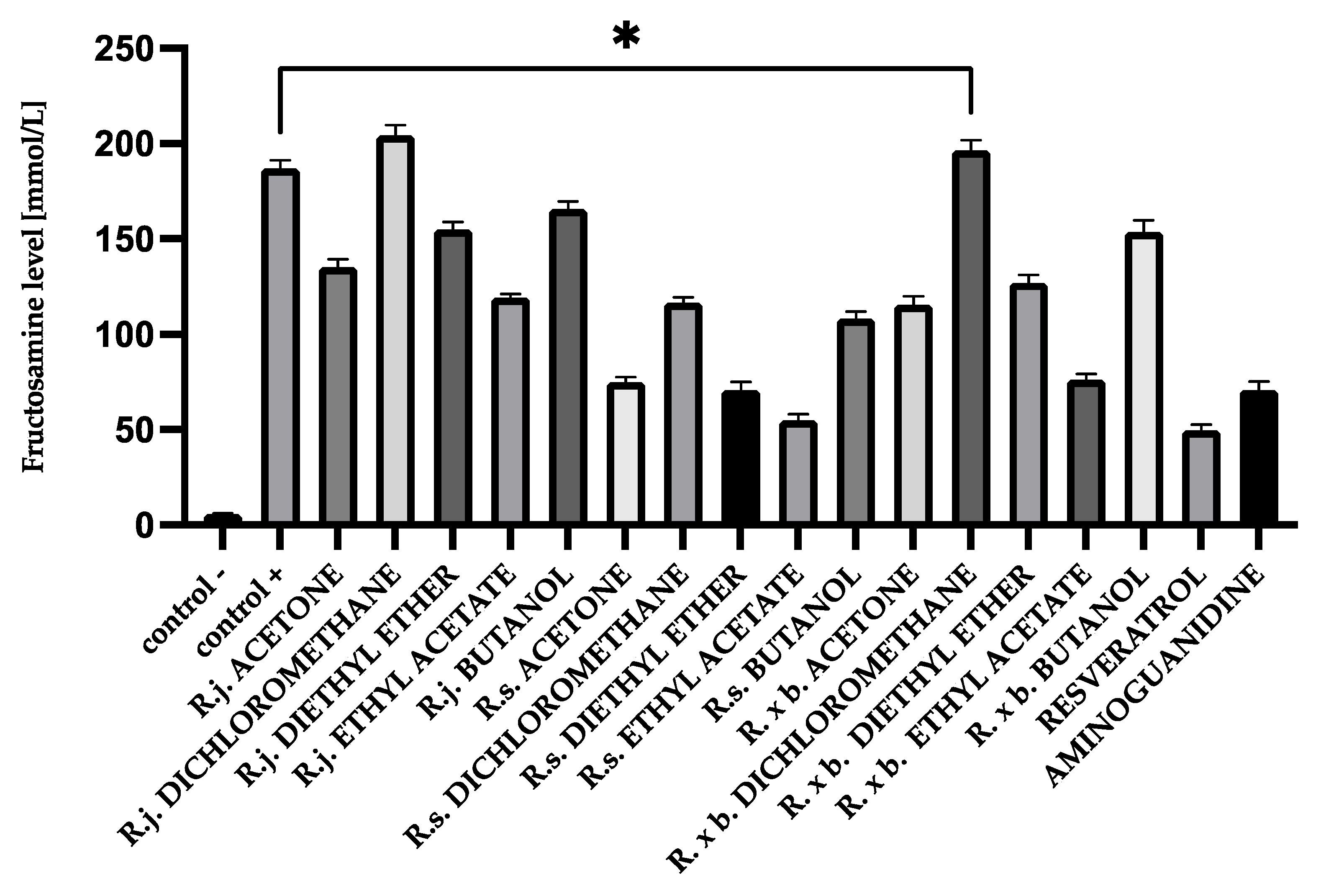
3.1. Fructosamine Levels
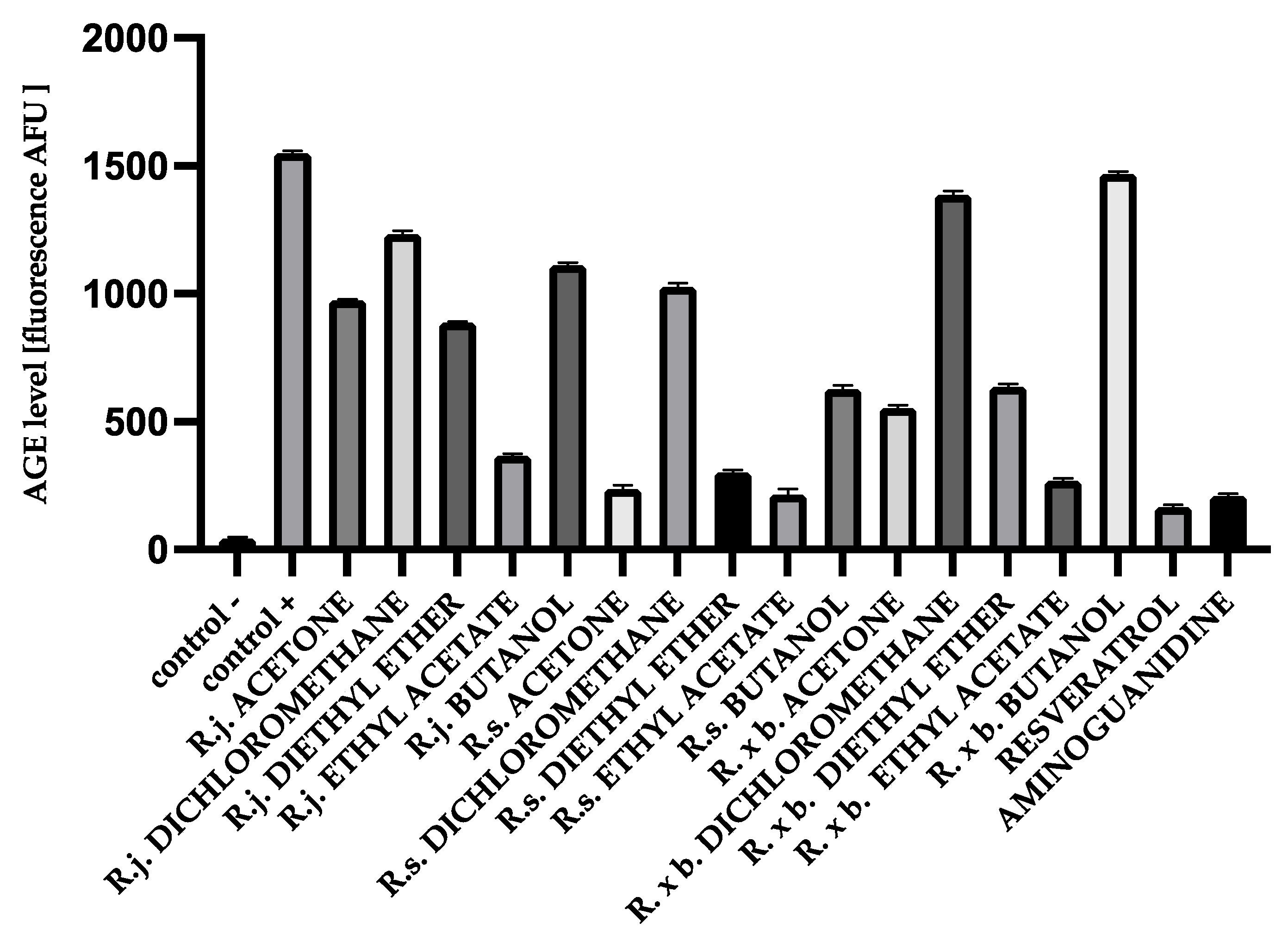
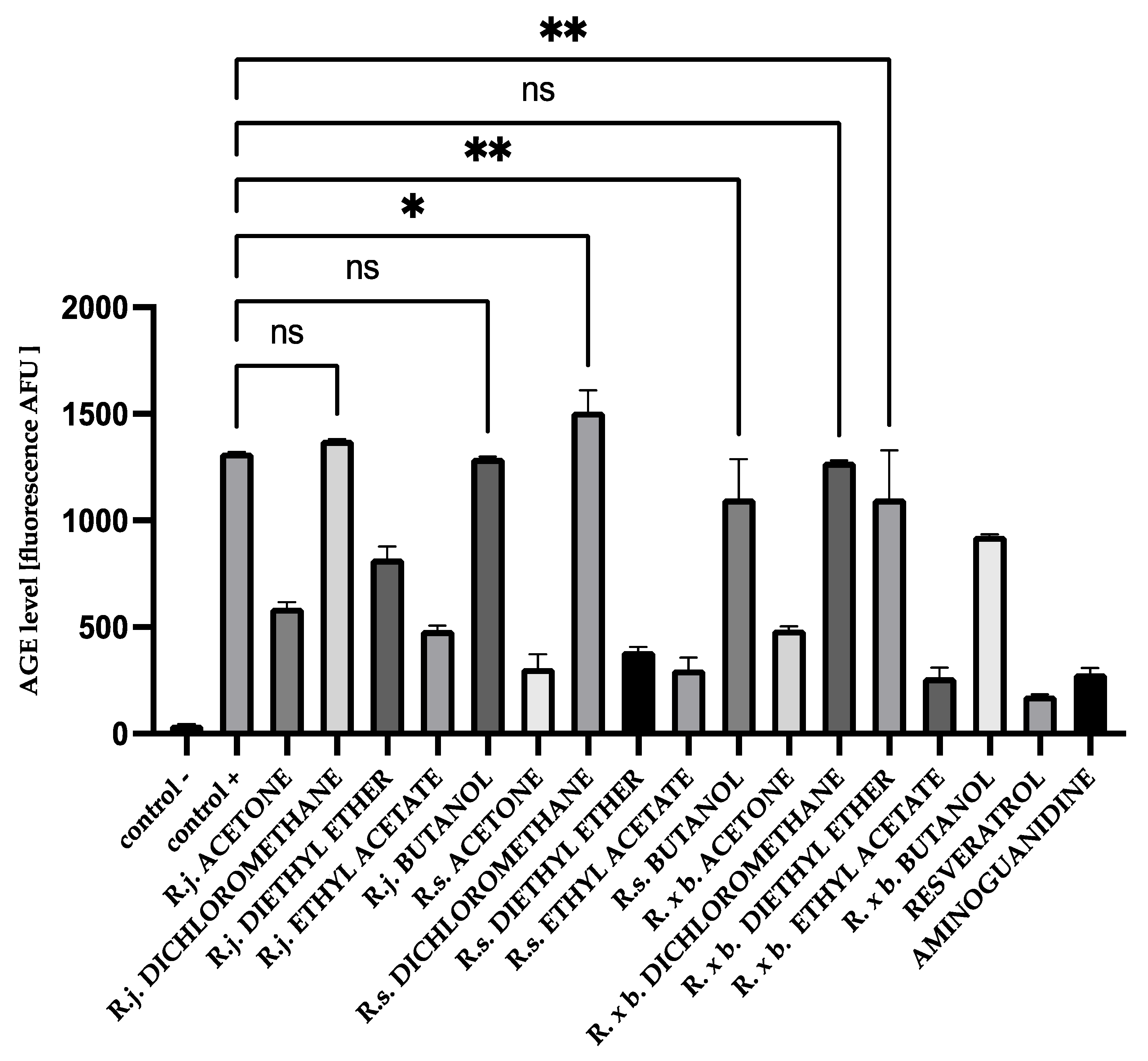
3.2. AGEs Levels
3.3. Protein Carbonyl Group Levels
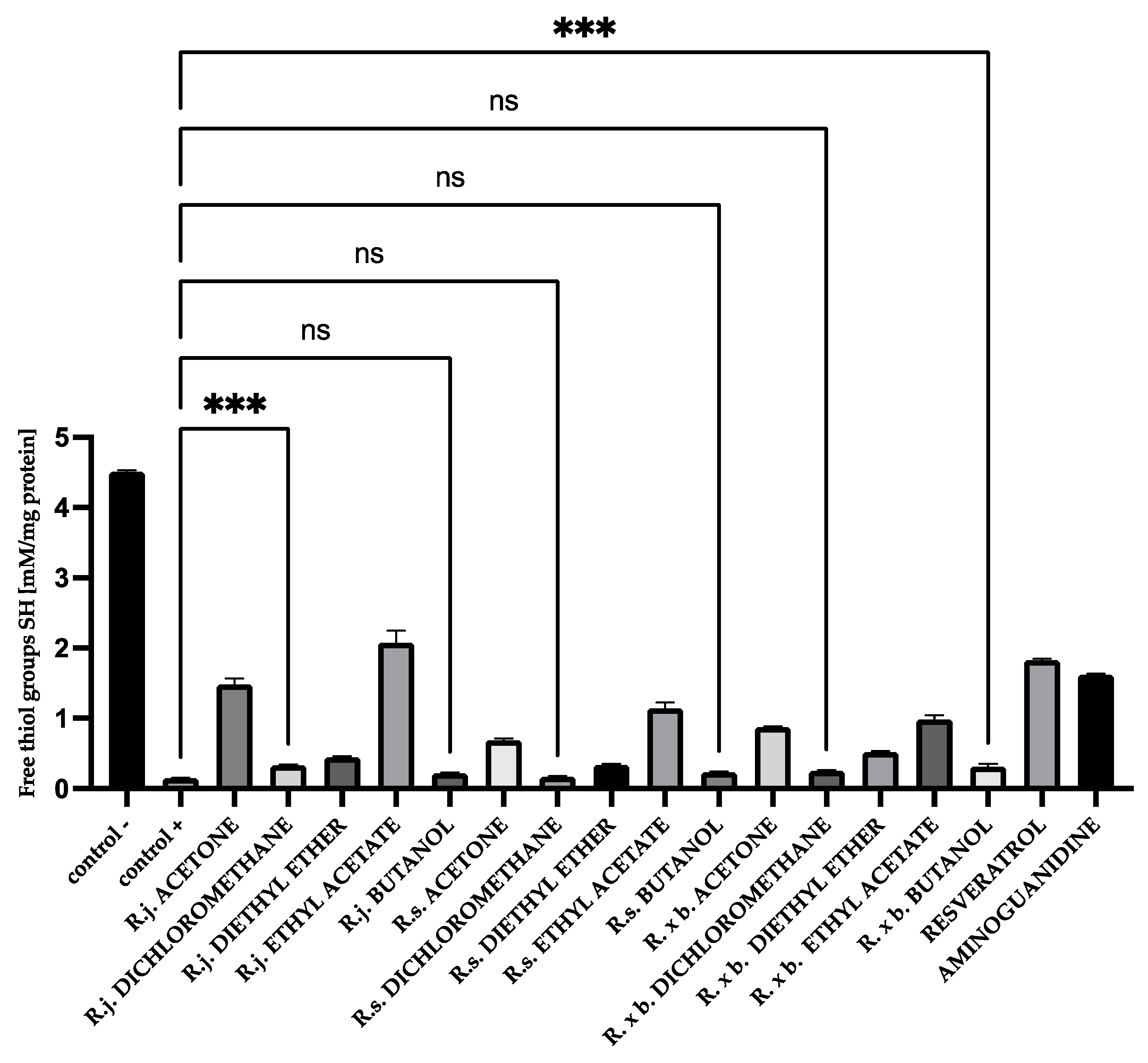
3.4. Thiol Group Levels
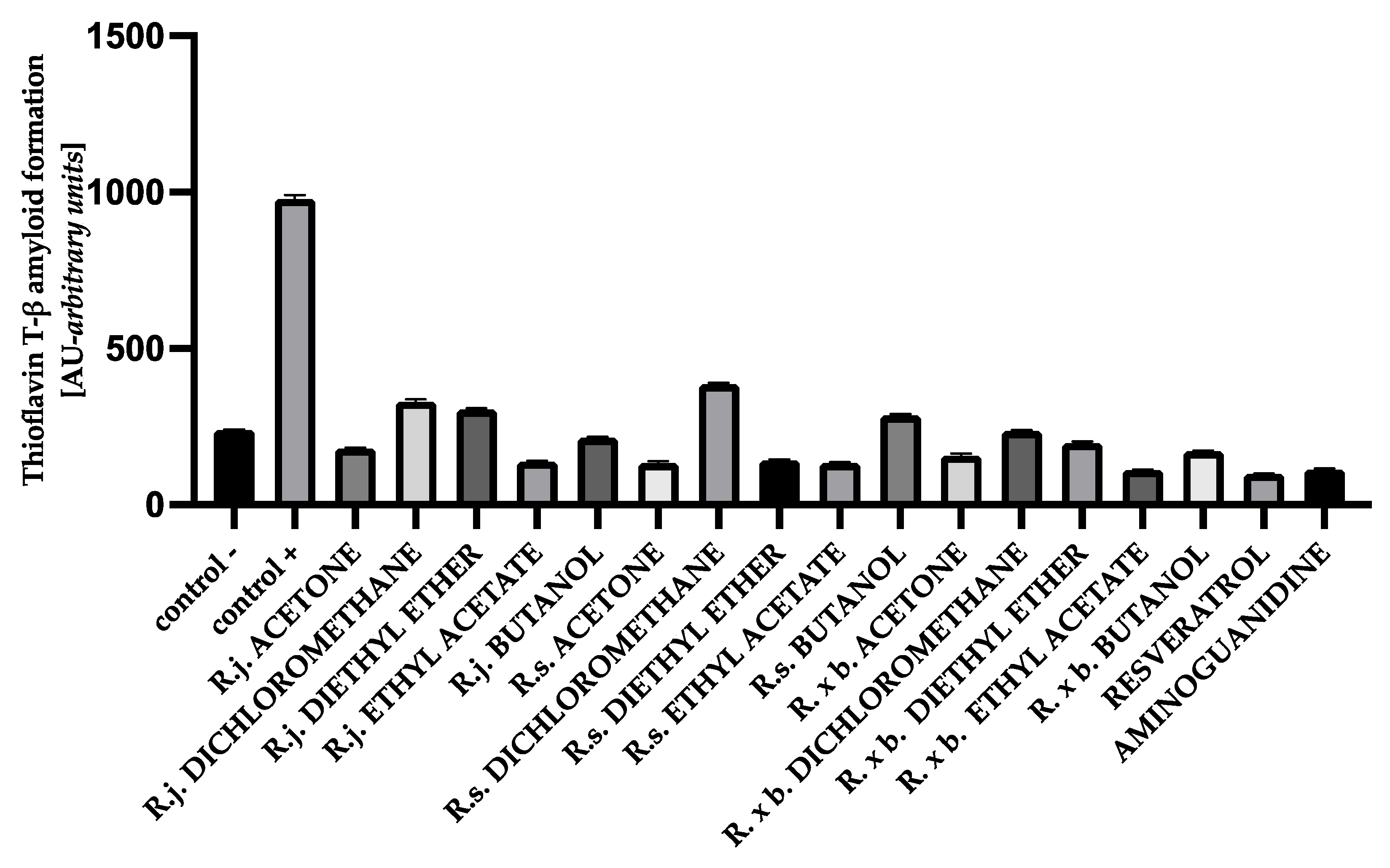
3.5. The Effect of Extracts on Amyloid-β Aggregation Thioflavin T Assay
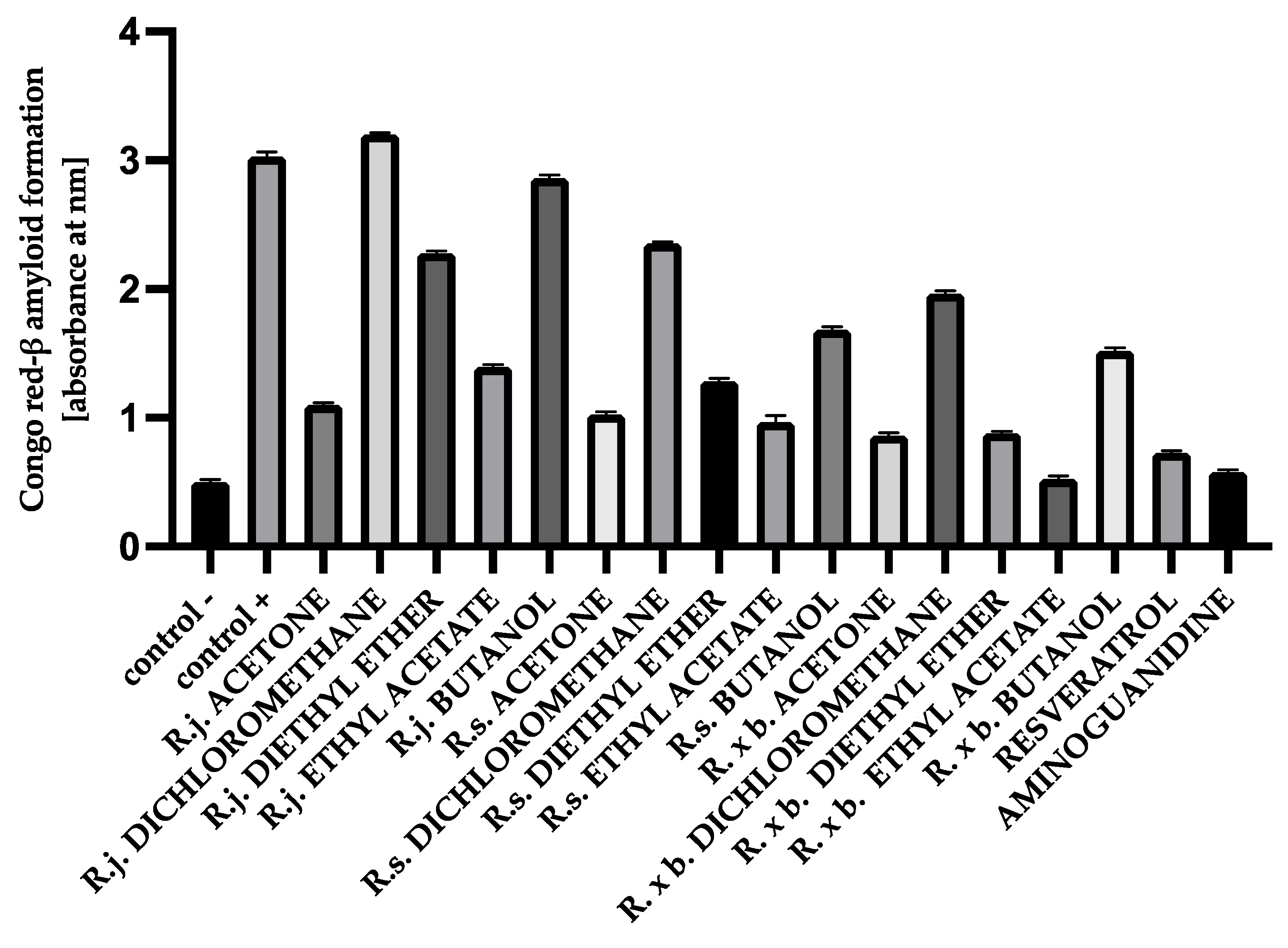
3.6. The Effect of Extracts on Amyloid-β Aggregation Congo Red Assay
3.7. Total Antiglycation Potential of Plant Extracts
4. Discussion
5. Conclusions
- −
- simple flavan-3-ols, such as epicatechin, catechin, and epicatechin-3-O-gallate;
- −
- procyanidins with a low degree of polymerization;
- −
- phenylpropanoid disaccharide esters that dominated in the rhizomes of R. sachalinensis.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Saeedi, P.; Petersohn, I.; Paraskevi, I.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. IDF Diabetes Atlas Committee.Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Spasov, A.A.; Solov’eva, O.A.; Kuznetsova, V.A. Protein Glycation during Diabetes Mellitus and the Possibility of its Pharmacological Correction (Review). Pharm. Chem. J. 2017, 51, 429–433. [Google Scholar] [CrossRef]
- Koga, M.; Kasayama, S. Clinical impast of glycated albumin as another glycemic control marker. Endocr. J. 2010, 57, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell. Longev. 2020, 3818196. [Google Scholar] [CrossRef] [Green Version]
- Rondeau, P.; Bourdon, E. The glycation of albumin: Structural and functional impacts. Biochimie 2011, 93, 645–658. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; van Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Luo, Y.; Shiming, L.; Ho, C.T. Key Aspects of Amadori Rearrangement Products as Future Food Additives. Molecules 2021, 26, 4314. [Google Scholar] [CrossRef]
- Kinoshita, S.; Furusawa, C.; Sugawa, H.; Shirakawa, J.; Ohno, R.; Ichimaru, K.; Nagai, M.; Nagai, R. Inhibitory effect of natural products on the formation of advanced glycation end products. Glycative Stress Res. 2017, 4, 109–116. [Google Scholar]
- Lee, D.; Kim, C.Y. Inhibition of advanced glycation end product formation by burdock root extract. J. Nutr. Health 2016, 49, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Abbas, G.; Al-Harrasi, A.S.; Hussain, H.; Hussain, J.; Rashid, R.; Choudhary, H.I. Antiglycation therapy: Discovery of promising antiglycation agents for the management of diabetic complications. Pharm. Biol. 2016, 54, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Gomez, B.; Nitride, C.; Ullate, M.; Mamore, G.; Pasquale, F.; del Castillo, M.D. Inhibitors of advanced glycation end products from coffee bean roasting by-product. Eur. Food Res. Tech. 2018, 244, 1101–1110. [Google Scholar] [CrossRef]
- Sekhon-Loodu, S.; Rupasinghe, H.P.V. Evaluation of Antioxidant, Antidiabetic and Antiobesity Potential of Selected Traditional Medicinal Plants. Front. Nutr. 2019, 6, 53. [Google Scholar] [CrossRef]
- Spínola, V.; Llorent-Martínez, E.J.; Castilho, P.C. Polyphenols of Myrica faya inhibit key enzymes linked to type II diabetes and obesity and formation of advanced glycation end-products (in vitro): Potential role in the prevention of diabetic complications. Food Res. Int. 2019, 116, 1229–1238. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Ślusarczyk, S.; Granica, S.; Hadzik, J.; Matkowski, A. Phytochemical Diversity in Rhizomes of Three Reynoutria Species and their Antioxidant Activity Correlations Elucidated by LC-ESI-MS/MS Analysis. Molecules 2019, 24, 1136. [Google Scholar] [CrossRef] [Green Version]
- Nawrot-Hadzik, I.; Granica, S.; Domaradzki, K.; Pecio, Ł.; Matkowski, A. Isolation and Determination of Phenolic Glycosides and Anthraquinones from Rhizomes of Various Reynoutria Species. Planta Med. 2018, 84, 1118–1126. [Google Scholar] [CrossRef] [Green Version]
- Pirożnikow, E. Japanese knotweed (Reynoutria japonica Houtt.)—A food plant used in the Białowieża Forest. Etnobiol. Pol. 2012, 2, 27–32. [Google Scholar]
- Sheng, Z.; Ai, B.; Zheng, L.; Zheng, X.; Yang, Y.; Shen, Y. Capability of Polygonum cuspidatum extract in inhibiting AGEs and preventing diabetes. Food Sci. Nutr. 2019, 7, 2006–2016. [Google Scholar] [CrossRef]
- Sohn, E.; Kim, J.; Kim, C.S.; Jo, K.; Kim, J.S. Extract of Rhizoma Polygonum cuspidatum reduces early renal podocyte injury in streptozotocin-induced diabetic rats and its active compound emodin inhibits methylglyoxal-mediated glycation of proteins. Mol. Med. Rep. 2015, 12, 5837–5845. [Google Scholar] [CrossRef] [Green Version]
- Münch, G.; Keis, R.; Wessels, A.; Riederer, P.; Bahner, U.; Heidland, A.; Niwa, T.; Lemke, H.D.; Schinzel, R. Determination of advanced glycation end products in serum by fluorescence spectroscopy and competitive ELISA. Eur. J. Clin. Chem. Clin. Biochem. 1997, 35, 669–677. [Google Scholar] [CrossRef]
- Johnson, R.N.; Metcalf, P.A.; Baker, J.R. Fructosamine: A new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin. Chim. Acta 1983, 127, 87–95. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Diplock, A.T.; Symons, M.C.R. Techniques in Free Radical Research; Elsevier: Amsterdam, The Netherlands; London, UK; New York, NY, USA; Tokyo, Japan, 1991; Volume 22, pp. 207–230. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Le Vine, H., III. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Meth. Enzymol. 1999, 309, 274–284. [Google Scholar]
- Klunk, W.E.; Jacob, R.F.; Mason, R.P. Quantifying amyloid by Congo red spectral shift assay. Meth. Enzymol. 1999, 309, 285–305. [Google Scholar]
- Colombo, G.; Clerici, M.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Redox albuminomics: Oxidized albumin in human diseases. Antioxid. Redox Signal. 2012, 17, 1515–1527. [Google Scholar] [CrossRef]
- Turpin, C.; Catan, A.; Guerin-Dubourg, A.; Debussche, X.; Bravo, S.B.; Álvarez, E.; Van Den Elsen, J.; Meilhac, O.; Rondeau, R.; Bourdon, E. Enhanced oxidative stress and damage in glycated erythrocytes. PLoS ONE 2020, 15, e0235335. [Google Scholar] [CrossRef]
- Thornalley, P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Castilho, P.C. Hypoglycemic, Anti-glycation and antioxidant in vitro properties of two Vaccinium species from Macaronesia: A relation to their phenolic composition. J. Funct. Foods 2018, 40, 595–605. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Prevention of protein glycation by natural compounds. Molecules 2015, 20, 3309–3334. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, X. Structures Required of Polyphenols for Inhibiting Advanced Glycation End Products Formation. Curr. Drug Metab. 2013, 14, 414–431. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, S.; Chen, Y.; Zhang, R.; Zhou, M.; Wang, C.; Feng, N.; Wu, Q. Structure-Activity relationship of procyanidins on advanced glycation end products formation and corresponding mechanisms. Food Chem. 2019, 272, 679–687. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Wang, W.; Khoo, C.; Taylor, J.; Gu, L. Cranberry phytochemicals inhibit glycation of human hemoglobin and serum albumin by scavenging reactive carbonyls. Food Funct. 2011, 2, 475–482. [Google Scholar] [CrossRef]
- Sun, C.; McIntyre, K.; Saleem, A.; Haddad, P.S.; Arnason, J.T. The relationship between antiglycation activity and procyanidin and phenolic content in commercial grape seed products. Can. J. Physiol. Pharmacol. 2012, 90, 167–174. [Google Scholar]
- Rivera-Velez, S.M.; Hwang, J.; Navas, J.; Villarino, N.F. Identification of differences in the formation of plasma glycated proteins between dogs and humans under diabetes-like glucose concentration conditions. Int. J. Biol. Macromol. 2019, 123, 1197–1203. [Google Scholar] [CrossRef]
- Watanabe, H.; Imafuku, T.; Otagiri, M.; Maryama, T. Clinical Implications Associated with the Posttranslational Modification–Induced Functional Impairment of Albumin in Oxidative Stress-Related Diseases. J. Pharm. Sci. 2017, 106, 2195–2203. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.X.; Knecht, K.J.; Thorpe, S.R.; Baynes, J.W. Role of oxygen in cross-linking and chemical modification of collagen by glucose. Diabetes 1992, 41, 42–48. [Google Scholar] [CrossRef]
- Yeboah, F.K.; Alli, I.; Yaylayan, V.A. Reactivities of D-glucose and D-fructose during glycation of bovine serum albumin. J. Agric. Food Chem. 1999, 47, 3164–3172. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, Z.; Sheng, Z. Ability of resveratrol to inhibit advanced glycation end product formation and carbohydrate-hydrolyzing enzyme activity, and to conjugate methylglyoxal. Food Chem. 2017, 216, 153–160. [Google Scholar] [CrossRef]
- Arcanjo, N.M.O.; Luna, C.; Madruga, M.S.; Estévez, M. Antioxidant and pro-oxidant actions of resveratrol on human serum albumin in the presence of toxic diabetes metabolites: Glyoxal and methyl-glyoxal. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1938–1947. [Google Scholar] [CrossRef]
- Yilmaz, Z.; Kalaz, E.B.; Aydin, A.F.; Olgaç, V.; Doğru-Abbasoğlu, S.; Uysal, M.; Koçak-Toker, N. The effect of resveratrol on glycation and oxidation products in plasma and liver of chronic methylglyoxal-treated rats. Pharmacol. Rep. 2018, 70, 584–590. [Google Scholar] [CrossRef]
- Hajizadeh-Sharafabad, F.; Sahebkar, A.; Zabetian-Targhi, F.; Maleki, V. The impact of resveratrol on toxicity and related complications of advanced glycation end products: A systematic review. Biofactors 2019, 45, 651–665. [Google Scholar] [CrossRef]
- De Souza Farias, S.A.; da Costa, K.S.; Martins, J.B.L. Analysis of Conformational, Structural, Magnetic, and Electronic Properties Related to Antioxidant Activity: Revisiting Flavan, Anthocyanidin, Flavanone, Flavonol, Isoflavone, Flavone, and Flavan-3-ol. ACS Omega 2021, 6, 8908–8918. [Google Scholar] [CrossRef]
- Mizuno, M.; Nakanishi, I.; Matsubayashi, S.; Imai, K.; Arai, T.; Matsumoto, K.I.; Fukuhara, K. Synthesis and antioxidant activity of a procyanidin B3 analogue. Bioorg. Med. Chem. Lett. 2017, 27, 1041–1044. [Google Scholar] [CrossRef]
- Dastmalchi, K.; Dorman, D.H.J.; Oinonen, P.P.; Darwis, Y.; Laakso, J.; Hiltunen, R. Chemical composition and in vitro antioxidative activity of a lemon balm (Melissa officinalis L.) extract. LWT Food Sci. Technol. 2008, 41, 391–400. [Google Scholar] [CrossRef]
- Wolff, S.P.; Dean, R.T. Glucose autoxidation and protein modification: The potential role of “autooxidative glycosylation” in diabetes. Biochem. J. 1987, 245, 243–250. [Google Scholar] [CrossRef]
- Price, D.L.; Rhett, P.M.; Thorpe, S.R.; Baynes, J.W. Chelating activity of advanced glycation end-product inhibitors. J. Biol. Chem. 2001, 276, 48967–48972. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Weber, D.; Raupbach, J.; Dakal, T.C.; Fließbach, K.; Ramirez, A.; Grune, T.; Wüllner, U. Advanced glycation end products and protein carbonyl levels in plasma reveal sex-specific differences in Parkinson’s and Alzheimer’s disease. Redox Biol. 2020, 34, 101546. [Google Scholar] [CrossRef]
- Miroliaei, M.; Khazaei, S.; Moshkelgosha, S.; Shirvani, M. Inhibitory effects of Lemon balm (Melissa officinalis, L.) extract on the formation of advanced glycation end products. Food Chem. 2011, 129, 267–271. [Google Scholar] [CrossRef]
- Rahmanifar, E.; Miroliaei, M. Differential effect of biophenols on attenuation of AGE-induced hemoglobin aggregation. Int. J. Biol. Macromol. 2020, 151, 797–805. [Google Scholar] [CrossRef]
- Froldi, G.; Baronchelli, F.; Marin, E.; Grison, M. Antiglycation Activity and HT-29 Cellular Uptake of Aloe-Emodin, Aloin and Aloe arborescens Leaf Extracts. Molecules 2019, 24, 2128. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Zhu, J.X.; Wu, A.G.; Xu, T.X.; Duan, T.T.; Zheng, Z.G.; Wang, R.S.; Li, D.; Zhu, Q. Pre-column incubation followed by fast liquid chromatography analysis for rapid screening of natural methylglyoxal scavengers directly from herbal medicines: Case study of Polygonum cuspidatum. J. Chromatogr. A 2013, 1286, 102–110. [Google Scholar] [CrossRef]
- Liu, W.; Cai, A.; Carley, R.; Rocchio, R.; Petrovas, Z.M.; Chartier, C.A.; Meng, X.; Su, J.; Cho, B.P.; Dain, J.A.; et al. Bioactive anthraquinones found in plant foods interact with human serum albumin and inhibit the formation of advanced glycation endproducts. J. Food Bioact. 2018, 4, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Zhao, X.; Yue, L.; Liu, L. Main anthraquinone components in Aloe vera and their inhibitory effects on the formation of advanced glycation end-products. J. Food Process. Preserv. 2016, 41, e13160. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, H.; Lv, Z.; Li, S.; Hu, B.; Guan, Y.; Xie, B.; Sun, Z. Oligomeric procyanidins of lotus seedpod inhibits the formation of advanced glycation end-products by scavenging reactive carbonyls. Food Chem. 2013, 138, 1493–1502. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, K.W.; Ma, J.; Chen, B.; Ho, C.T.; Lo, C.; Chen, F.; Wang, M. Cinnamon bark proanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation endproducts. J. Agric. Food Chem. 2008, 56, 1907–1911. [Google Scholar] [CrossRef]
- Liao, X.; Brock, A.A.; Jackson, B.T.; Greenspan, P.; Pegg, R.B. The cellular antioxidant and anti-glycation capacities of phenolics from Georgia peaches. Food Chem. 2020, 316, 126234. [Google Scholar] [CrossRef]
- Matsuda, H.; Wang, T.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorg. Med. Chem. 2003, 11, 5317–5323. [Google Scholar] [CrossRef]
- Yokozawa, T.; Nakagawa, T. Inhibitory effects of Luobuma tea and its components against glucose-mediated protein damage. Food Chem. Toxicol. 2004, 42, 975–981. [Google Scholar] [CrossRef]
- Castellain, R.C.L.; Gesser, M.; Tonini, F.; Schulte, R.V.; Demessiano, K.Z.; Wolff, F.R.; Delle-Monache, F.; Netz, D.J.A.; Cechinel-Filho, V.; de Freitas, R.A.; et al. Chemical composition, antioxidant and antinociceptive properties of Litchi chinensis leaves. J. Pharm. Pharmacol. 2014, 66, 1796–1807. [Google Scholar] [CrossRef]
- Cos, P.; De Bruyne, T.; Hermans, N.; Apers, S.; Vanden Berghe, D.; Vlietinck, A.J. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2014, 11, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Cho, E.J.; Tanaka, T.; Yokozawa, T. Inhibitory Activities of Proanthocyanidins from Persimmon against Oxidative Stress and Digestive Enzymes Related to Diabetes. J. Nutr. Sci. Vitaminol. 2007, 53, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.A.; Kim, J.Y.; Cho, E.J.; Yokozawa, T. Ameliorative Effects of Proanthocyanidin on Oxidative Stress and Inflammation in Streptozotocin-Induced Diabetic Rats. J. Agric. Food Chem. 2007, 55, 9395–9400. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Hay, A.E.; Marston, A.; Lou, H.; Hostettmann, K. Chemical variability of the invasive neophytes Polygonum cuspidatum Sieb. And Zucc. and Polygonum sachalinensis F. Schmidt ex Maxim. Biochem. Syst. Ecol. 2009, 37, 24–34. [Google Scholar] [CrossRef]
- Fan, P.; Terrier, L.; Hay, A.E.; Marston, A.; Hostettmann, K. Antioxidant and enzyme inhibition activities and chemical profiles of Polygonum sachalinensis F. Schmidt ex Maxim (Polygonaceae). Fitoterapia 2010, 81, 124–131. [Google Scholar] [CrossRef]
- Pogačnik, L.; Bergant, T.; Skrt, M.; Poklar Ulrih, N.; Viktorová, J.; Ruml, T. In Vitro Comparison of the Bioactivities of Japanese and Bohemian Knotweed Ethanol Extracts. Foods 2020, 9, 544. [Google Scholar] [CrossRef]





| Inhibition of Glycation | Inhibition of Protein Oxidation | Inhibition of Amyloid Aggregation | ||||
|---|---|---|---|---|---|---|
| Extracts and Fractions | Average Value (Fructosamines and AGEs) | Rank | Average Value (Protein Carbonyls and Thiols) | Rank | Average Value (Congo Red and Thioflavin T) | Rank |
| R. japonica acetone | 391.7 | 12 | 34.3 | 4 | 62.6 | 10 |
| R. japonica dichloromethane | 720.3 | 17 | 68.4 | 16 | 115.6 | 16 |
| R. japonica diethyl ether | 366.1 | 11 | 42.4 | 6 | 107.1 | 15 |
| R. japonica ethyl acetate | 161.7 | 4 | 33.5 | 3 | 47.8 | 6 |
| R. japonica butanol | 452.9 | 14 | 61.1 | 15 | 75.73 | 12 |
| R. sachalinensis acetone | 112.4 | 7 | 46.4 | 8 | 46.6 | 5 |
| R. sachalinensis dichloromethane | 433.1 | 16 | 71.2 | 17 | 134.5 | 17 |
| R. sachalinensis diethyl ether | 147.1 | 6 | 48.5 | 9 | 49.9 | 7 |
| R. sachalinensis ethyl acetate | 80.8 | 2 | 36.5 | 5 | 46.1 | 4 |
| R. sachalinensis butanol | 281.2 | 10 | 56.9 | 14 | 99.8 | 14 |
| R. × bohemica acetone | 241.4 | 8 | 49.1 | 10 | 55.2 | 8 |
| R. × bohemica dichloromethane | 661.7 | 16 | 54.2 | 13 | 83.1 | 13 |
| R. × bohemica diethyl ether | 271.7 | 9 | 49.9 | 11 | 68.5 | 11 |
| R. × bohemica ethyl acetate | 129.1 | 5 | 43.6 | 7 | 42.8 | 3 |
| R. × bohemica butanol | 570.3 | 15 | 51.8 | 12 | 60.2 | 9 |
| Resveratrol | 65.6 | 1 | 29.7 | 1 | 34.1 | 1 |
| Aminoguanidine | 102.3 | 3 | 32.1 | 2 | 39.3 | 2 |
| Inhibition of Glycation | Inhibition of Protein Oxidation | Inhibition of Amyloid Aggregation | ||||
|---|---|---|---|---|---|---|
| Extracts and Fractions | Average Value (Fructosamines and AGEs) | Rank | Average Value (Protein Carbonyls and Thiols) | Rank | Average Value (Congo Red and Thioflavin T) | Rank |
| R. japonica acetone | 259.2 | 10 | 34.7 | 4 | 57.3 | 10 |
| R. japonica dichloromethane | 553.1 | 17 | 53.1 | 13 | 93.0 | 17 |
| R. japonica diethyl ether | 277.8 | 11 | 38.2 | 7 | 63.2 | 11 |
| R. japonica ethyl acetate | 199.8 | 8 | 31.8 | 3 | 41.6 | 6 |
| R. japonica butanol | 502.9 | 16 | 45.4 | 11 | 68.1 | 13 |
| R. sachalinensis acetone | 90.1 | 5 | 41.4 | 9 | 46.3 | 8 |
| R. sachalinensis dichloromethane | 353.3 | 14 | 70.7 | 17 | 90.1 | 16 |
| R. sachalinensis diethyl ether | 116.9 | 6 | 48.6 | 12 | 50.9 | 9 |
| R. sachalinensis ethyl acetate | 80.8 | 4 | 36.1 | 5 | 23.7 | 1 |
| R. sachalinensis butanol | 324.4 | 12 | 61.1 | 15 | 74.0 | 14 |
| R. × bohemica acetone | 184.9 | 7 | 39.4 | 8 | 39.6 | 5 |
| R. × bohemica dichloromethane | 456.8 | 15 | 61.1 | 16 | 79.6 | 15 |
| R. × bohemica diethyl ether | 206.5 | 9 | 42.5 | 10 | 42.4 | 7 |
| R. × bohemica ethyl acetate | 80.4 | 3 | 36.5 | 6 | 34.2 | 2 |
| R. × bohemica butanol | 336.6 | 13 | 55.7 | 14 | 63.9 | 12 |
| Resveratrol | 60.5 | 1 | 20.6 | 1 | 35.5 | 3 |
| Aminoguanidine | 76.1 | 2 | 25.4 | 2 | 36.3 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dołowacka-Jóźwiak, A.; Matkowski, A.; Nawrot-Hadzik, I. Antiglycoxidative Properties of Extracts and Fractions from Reynoutria Rhizomes. Nutrients 2021, 13, 4066. https://doi.org/10.3390/nu13114066
Dołowacka-Jóźwiak A, Matkowski A, Nawrot-Hadzik I. Antiglycoxidative Properties of Extracts and Fractions from Reynoutria Rhizomes. Nutrients. 2021; 13(11):4066. https://doi.org/10.3390/nu13114066
Chicago/Turabian StyleDołowacka-Jóźwiak, Arleta, Adam Matkowski, and Izabela Nawrot-Hadzik. 2021. "Antiglycoxidative Properties of Extracts and Fractions from Reynoutria Rhizomes" Nutrients 13, no. 11: 4066. https://doi.org/10.3390/nu13114066
APA StyleDołowacka-Jóźwiak, A., Matkowski, A., & Nawrot-Hadzik, I. (2021). Antiglycoxidative Properties of Extracts and Fractions from Reynoutria Rhizomes. Nutrients, 13(11), 4066. https://doi.org/10.3390/nu13114066







