Effect of a Remotely Delivered Weight Loss Intervention in Early-Stage Breast Cancer: Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
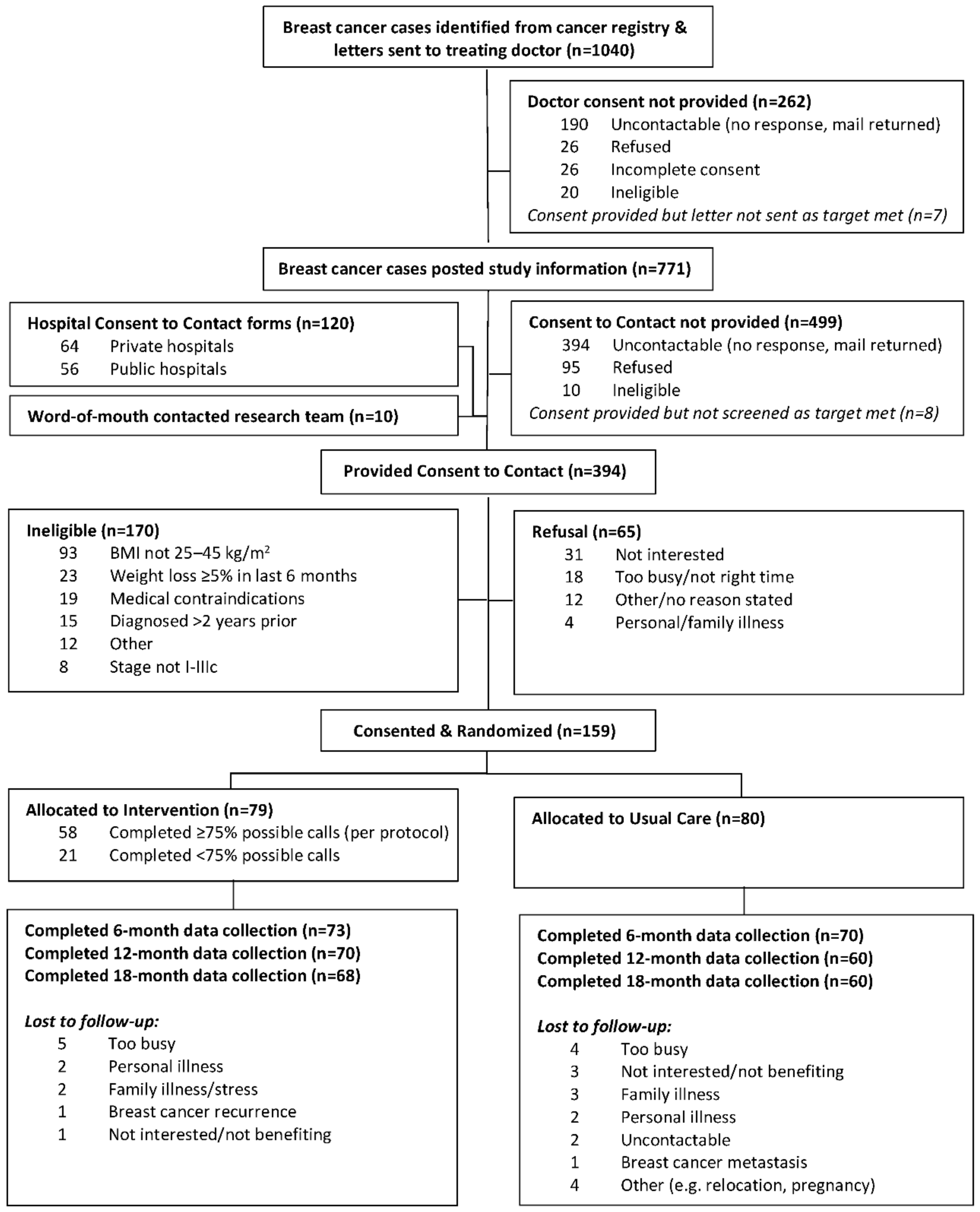
2.1. Participants and Recruitment
2.2. Usual Care
2.3. Weight Loss Intervention
2.4. Data Collection
2.4.1. Primary Outcome
2.4.2. Secondary Outcomes
2.4.3. Adverse Events
2.5. Sample Size
2.6. Data Analysis
3. Results
3.1. Weight and Body Composition
3.2. Metabolic Syndrome
3.3. Patient-Reported Outcomes
3.4. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demark-Wahnefried, W.; Schmitz, K.H.; Alfano, C.M.; Bail, J.R.; Goodwin, P.J.; Thomson, C.A.; Bradley, D.W.; Courneya, K.S.; Befort, C.A.; Denlinger, C.S.; et al. Weight management and physical activity throughout the cancer care continuum. CA Cancer J. Clin. 2018, 68, 64–89. [Google Scholar] [CrossRef] [PubMed]
- Ligibel, J.A.; Alfano, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Burger, R.A.; Chlebowski, R.T.; Fabian, C.J.; Gucalp, A.; Hershman, D.L.; Hudson, M.M.; et al. American society of clinical oncology position statement on obesity and cancer. J. Clin. Oncol. 2014, 32, 3568–3574. [Google Scholar] [CrossRef] [PubMed]
- Lahart, I.M.; Metsios, G.S.; Nevill, A.M.; Carmichael, A.R. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015, 54, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.M.; Al-Homaidh, A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med. Oncol. 2011, 28, 753–765. [Google Scholar] [CrossRef]
- Juvet, L.K.; Thune, I.; Elvsaas, I.K.O.; Fors, E.A.; Lundgren, S.; Bertheussen, G.; Leivseth, G.; Oldervoll, L.M. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: A meta-analysis. Breast 2017, 33, 166–177. [Google Scholar] [CrossRef]
- McNeely, M.L.; Campbell, K.L.; Rowe, B.H.; Klassen, T.P.; Mackey, J.R.; Courneya, K.S. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. CMAJ 2006, 175, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Meneses-Echavez, J.F.; Gonzalez-Jimenez, E.; Ramirez-Velez, R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: A systematic review and meta-analysis. BMC Cancer 2015, 15, 77. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.S.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef] [Green Version]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; The Third Expert Report; World Cancer Research Fund/American Institute for Cancer Research: London, UK, 2018. [Google Scholar]
- Chlebowski, R.T.; Reeves, M.M. Weight Loss Randomized Intervention Trials in Female Cancer Survivors. J. Clin. Oncol. 2016, 34, 4238–4248. [Google Scholar] [CrossRef] [Green Version]
- Reeves, M.M.; Terranova, C.O.; Eakin, E.G.; Demark-Wahnefried, W. Weight loss intervention trials in women with breast cancer: A systematic review. Obes. Rev. 2014, 15, 749–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligibel, J.A.; Barry, W.T.; Alfano, C.; Hershman, D.L.; Irwin, M.; Neuhouser, M.; Thomson, C.A.; Delahanty, L.; Frank, E.; Spears, P.; et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): Study design. NPJ Breast Cancer 2017, 3, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasanisi, P.; Villarini, A.; Gargano, G.; Bruno, E.; Raimondi, M.; Bellegotti, M.; Curtosi, P.; Berrino, F. A randomized controlled trial of diet, physical activity and breast cancer recurrences—The Diana-5 study. Eur. J. Cancer 2012, 48, S283–S284. [Google Scholar] [CrossRef]
- Rack, B.; Andergassen, U.; Neugebauer, J.; Salmen, J.; Hepp, P.; Sommer, H.; Lichtenegger, W.; Friese, K.; Beckmann, M.W.; Hauner, D.; et al. The German SUCCESS C study—The first European lifestyle study on breast cancer. Breast Care 2010, 5, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Aleixo, G.F.P.; Williams, G.R.; Nyrop, K.A.; Muss, H.B.; Shachar, S.S. Muscle composition and outcomes in patients with breast cancer: Meta-analysis and systematic review. Breast Cancer Res. Treat. 2019, 177, 569–579. [Google Scholar] [CrossRef]
- Patnaik, J.L.; Byers, T.; Diguiseppi, C.; Denberg, T.D.; Dabelea, D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J. Natl. Cancer Inst. 2011, 103, 1101–1111. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, K.H.; Speck, R.M.; Rye, S.A.; DiSipio, T.; Hayes, S.C. Prevalence of breast cancer treatment sequelae over 6 years of follow-up: The Pulling Through Study. Cancer 2012, 118, S2217–S2225. [Google Scholar] [CrossRef] [Green Version]
- Beckwee, D.; Leysen, L.; Meuwis, K.; Adriaenssens, N. Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: A systematic review and meta-analysis. Support. Care Cancer 2017, 25, 1673–1686. [Google Scholar] [CrossRef]
- Caan, B.J.; Emond, J.A.; Su, H.I.; Patterson, R.E.; Flatt, S.W.; Gold, E.B.; Newman, V.A.; Rock, C.L.; Thomson, C.A.; Pierce, J.P. Effect of postdiagnosis weight change on hot flash status among early-stage breast cancer survivors. J. Clin. Oncol. 2012, 30, 1492–1497. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.E.; Chang-Claude, J.; Seibold, P.; Vrieling, A.; Heinz, J.; Flesch-Janys, D.; Steindorf, K. Determinants of long-term fatigue in breast cancer survivors: Results of a prospective patient cohort study. Psychooncology 2015, 24, 40–46. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Campbell, K.L.; Hayes, S.C. Weight management and its role in breast cancer rehabilitation. Cancer 2012, 118, S2277–S2287. [Google Scholar] [CrossRef] [Green Version]
- Imayama, I.; Alfano, C.M.; Neuhouser, M.L.; George, S.M.; Wilder Smith, A.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L.; Wang, C.Y.; Duggan, C.; et al. Weight, inflammation, cancer-related symptoms and health related quality of life among breast cancer survivors. Breast Cancer Res. Treat. 2013, 140, 159–176. [Google Scholar] [CrossRef] [Green Version]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Buchanan, T.A.; Spicer, D.V.; Tripathy, D.; Bernstein, L.; Mortimer, J.E. Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 875–883. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef]
- Berrino, F.; Villarini, A.; Traina, A.; Bonanni, B.; Panico, S.; Mano, M.P.; Mercandino, A.; Galasso, R.; Barbero, M.; Simeoni, M.; et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res. Treat. 2014, 147, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Calip, G.S.; Malone, K.E.; Gralow, J.R.; Stergachis, A.; Hubbard, R.A.; Boudreau, D.M. Metabolic syndrome and outcomes following early-stage breast cancer. Breast Cancer Res. Treat. 2014, 148, 363–377. [Google Scholar] [CrossRef]
- Reeves, M.M.; Terranova, C.O.; Erickson, J.M.; Job, J.R.; Brookes, D.S.; McCarthy, N.; Hickman, I.J.; Lawler, S.P.; Fjeldsoe, B.S.; Healy, G.N.; et al. Living well after breast cancer randomized controlled trial protocol: Evaluating a telephone-delivered weight loss intervention versus usual care in women following treatment for breast cancer. BMC Cancer 2016, 16, 830. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef] [Green Version]
- Reeves, M.M.; Winkler, E.A.H.; McCarthy, N.; Lawler, S.P.; Terranova, C.O.; Hayes, S.C.; Janda, M.; Demark-Wahnefried, W.; Eakin, E.G. The Living Well after Breast Cancer™ Pilot Trial: A weight loss intervention for women following treatment for breast cancer. Asia Pac. J. Clin. Oncol. 2017, 13, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Spark, L.C.; Fjeldsoe, B.S.; Eakin, E.G.; Reeves, M.M. Efficacy of a Text Message-Delivered Extended Contact Intervention on Maintenance of Weight Loss, Physical Activity, and Dietary Behavior Change. JMIR mHealth uHealth 2015, 3, e88. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine of the National Academies. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). Available online: http://www.nap.edu/openbook.php?record_id=10490&page=R1 (accessed on 2 November 2021).
- Bandura, A. Social Foundations of Thought and Action: A Social Cognitive Theory; Prentice Hall: Englewood Cliffs, NJ, USA, 1986. [Google Scholar]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Wijndaele, K.; Healy, G.N.; Dunstan, D.W.; Barnett, A.G.; Salmon, J.; Shaw, J.E.; Zimmet, P.Z.; Owen, N. Increased cardiometabolic risk is associated with increased TV viewing time. Med. Sci. Sports Exerc. 2010, 42, 1511–1518. [Google Scholar] [CrossRef] [Green Version]
- Ekelund, U.; Griffin, S.J.; Wareham, N.J. Physical activity and metabolic risk in individuals with a family history of type 2 diabetes. Diabetes Care 2007, 30, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Healy, G.N.; Winkler, E.A.H.; Owen, N.; Anuradha, S.; Dunstan, D.W. Replacing sitting time with standing or stepping: Associations with cardio-metabolic risk biomarkers. Eur. Heart J. 2015, 36, 2643–2649. [Google Scholar] [CrossRef] [Green Version]
- Hays, R.D.; Bjorner, J.B.; Revicki, D.A.; Spritzer, K.L.; Cella, D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual. Life Res. 2009, 18, 873–880. [Google Scholar] [CrossRef] [Green Version]
- Yellen, S.B.; Cella, D.F.; Webster, K.; Blendowski, C.; Kaplan, E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J. Pain Symptom Manag. 1997, 13, 63–74. [Google Scholar] [CrossRef]
- Swenson, K.K.; Nissen, M.J.; Henly, S.J.; Maybon, L.; Pupkes, J.; Zwicky, K.; Tsai, M.L.; Shapiro, A.C. Identification of tools to measure changes in musculoskeletal symptoms and physical functioning in women with breast cancer receiving aromatase inhibitors. Oncol. Nurs. Forum 2013, 40, 549–557. [Google Scholar] [CrossRef] [Green Version]
- Greene, J.G. Constructing a standard climacteric scale. Maturitas 1998, 29, 25–31. [Google Scholar] [CrossRef]
- Thewes, B.; Zachariae, R.; Christensen, S.; Nielsen, T.; Butow, P. The Concerns About Recurrence Questionnaire: Validation of a brief measure of fear of cancer recurrence amongst Danish and Australian breast cancer survivors. J. Cancer Surviv. 2015, 9, 68–79. [Google Scholar] [CrossRef]
- Hormes, J.M.; Lytle, L.A.; Gross, C.R.; Ahmed, R.L.; Troxel, A.B.; Schmitz, K.H. The body image and relationships scale: Development and validation of a measure of body image in female breast cancer survivors. J. Clin. Oncol. 2008, 26, 1269–1274. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Segal, R.J.; Vallis, M.; Ligibel, J.A.; Pond, G.R.; Robidoux, A.; Blackburn, G.L.; Findlay, B.; Gralow, J.R.; Mukherjee, S.; et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: The LISA trial. J. Clin. Oncol. 2014, 32, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Flatt, S.W.; Byers, T.E.; Colditz, G.A.; Demark-Wahnefried, W.; Ganz, P.A.; Wolin, K.Y.; Elias, A.; Krontiras, H.; Liu, J.; et al. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial: A behavioral weight loss intervention in overweight or obese breast cancer survivors. J. Clin. Oncol. 2015, 33, 3169–3176. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Marinac, C.R.; Sears, D.D.; Kerr, J.; Hartman, S.J.; Cadmus-Bertram, L.; Villaseñor, A.; Flatt, S.W.; Godbole, S.; Li, H.; et al. The Effects of Metformin and Weight Loss on Biomarkers Associated with Breast Cancer Outcomes. J. Natl. Cancer Inst. 2018, 110, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, M.; Cartmel, B.; Loftfield, E.; Sanft, T.; Chagpar, A.B.; Zhou, Y.; Playdon, M.; Li, F.; Irwin, M.L. Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: The Lifestyle, Exercise, and Nutrition (LEAN) study. J. Clin. Oncol. 2016, 34, 669–676. [Google Scholar] [CrossRef]
- Nuri, R.; Kordi, M.R.; Moghaddasi, M.; Rahnama, N.; Damirchi, A.; Rahmani-Nia, F.; Emami, H. Effect of combination exercise training on metabolic syndrome parameters in postmenopausal women with breast cancer. J. Cancer Res. Ther. 2012, 8, 238–242. [Google Scholar] [CrossRef]
- Thomas, G.A.; Alvarez-Reeves, M.; Lu, L.; Yu, H.; Irwin, M.L. Effect of exercise on metabolic syndrome variables in breast cancer survivors. Int. J. Endocrinol. 2013, 2013, 168797. [Google Scholar] [CrossRef] [Green Version]
- Guinan, E.; Hussey, J.; Broderick, J.M.; Lithander, F.E.; O’Donnell, D.; Kennedy, M.J.; Connolly, E.M. The effect of aerobic exercise on metabolic and inflammatory markers in breast cancer survivors—A pilot study. Support. Care Cancer 2013, 21, 1983–1992. [Google Scholar] [CrossRef]
- Thomson, C.A.; Stopeck, A.T.; Bea, J.W.; Cussler, E.; Nardi, E.; Frey, G.; Thompson, P.A. Changes in body weight and metabolic indexes in overweight breast cancer survivors enrolled in a randomized trial of low-fat vs. reduced carbohydrate diets. Nutr. Cancer 2010, 62, 1142–1152. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef]
- Garcia, M.; Bihuniak, J.D.; Shook, J.; Kenny, A.; Kerstetter, J.; Huedo-Medina, T.B. The Effect of the Traditional Mediterranean-Style Diet on Metabolic Risk Factors: A Meta-Analysis. Nutrients 2016, 8, 168. [Google Scholar] [CrossRef] [Green Version]
- Heymsfield, S.B.; Gonzalez, M.C.; Shen, W.; Redman, L.; Thomas, D. Weight loss composition is one-fourth fat-free mass: A critical review and critique of this widely cited rule. Obes. Rev. 2014, 15, 310–321. [Google Scholar] [CrossRef]
- Buffart, L.M.; Kalter, J.; Sweegers, M.G.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; May, A.M.; Galvao, D.A.; Chinapaw, M.J.; et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 2017, 52, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, A.; Hortobagyi, T.; Beurskens, R.; Granacher, U. Effects of Supervised vs. Unsupervised Training Programs on Balance and Muscle Strength in Older Adults: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 2341–2361. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Troxel, A.B.; Dean, L.T.; DeMichele, A.; Brown, J.C.; Sturgeon, K.; Zhang, Z.; Evangelisti, M.; Spinelli, B.; Kallan, M.J.; et al. Effect of Home-Based Exercise and Weight Loss Programs on Breast Cancer-Related Lymphedema Outcomes Among Overweight Breast Cancer Survivors: The WISER Survivor Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1605–1613. [Google Scholar] [CrossRef]
- Kwan, M.L.; Roh, J.M.; Laurent, C.A.; Lee, J.; Tang, L.; Hershman, D.; Kushi, L.H.; Yao, S. Patterns and reasons for switching classes of hormonal therapy among women with early-stage breast cancer. Cancer Causes Control 2017, 28, 557–562. [Google Scholar] [CrossRef]
- Brier, M.J.; Chambless, D.L.; Chen, J.; Mao, J.J. Ageing perceptions and non-adherence to aromatase inhibitors among breast cancer survivors. Eur. J. Cancer 2018, 91, 145–152. [Google Scholar] [CrossRef]
- Pineda-Moncusi, M.; Servitja, S.; Tusquets, I.; Diez-Perez, A.; Rial, A.; Cos, M.L.; Campodarve, I.; Rodriguez-Morera, J.; Garcia-Giralt, N.; Nogues, X. Assessment of early therapy discontinuation and health-related quality of life in breast cancer patients treated with aromatase inhibitors: B-ABLE cohort study. Breast Cancer Res. Treat. 2019, 177, 53–60. [Google Scholar] [CrossRef]
- Irwin, M.L.; Cartmel, B.; Gross, C.P.; Ercolano, E.; Li, F.; Yao, X.; Fiellin, M.; Capozza, S.; Rothbard, M.; Zhou, Y.; et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J. Clin. Oncol. 2015, 33, 1104–1111. [Google Scholar] [CrossRef]
- Nyrop, K.A.; Callahan, L.F.; Cleveland, R.J.; Arbeeva, L.L.; Hackney, B.S.; Muss, H.B. Randomized Controlled Trial of a Home-Based Walking Program to Reduce Moderate to Severe Aromatase Inhibitor-Associated Arthralgia in Breast Cancer Survivors. Oncologist 2017, 22, 1238–1249. [Google Scholar] [CrossRef] [Green Version]
- Befort, C.A.; Klemp, J.R.; Austin, H.L.; Perri, M.G.; Schmitz, K.H.; Sullivan, D.K.; Fabian, C.J. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Res. Treat. 2012, 132, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Janni, W.; Rack, B.; Friedl, T.; Müller, V.; Lorenz, R.; Rezai, M.; Tesch, H.; Heinrich, G.; Andergassen, U.; Harbeck, N.; et al. Lifestyle Intervention and Effect on Disease-free Survival in Early Breast Cancer Pts: Interim Analysis from the Randomized SUCCESS C Study. Cancer Res. 2019, 79, GS5-03. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Australian Cancer Incidence and Mortality (ACIM) Books: Breast Cancer; Australian Institute of Health and Welfare: Canberra, ACT, Australia, 2015. [Google Scholar]
| Characteristic | Usual Care (n = 80) | Intervention (n = 79) |
|---|---|---|
| Mean (SD) | ||
| Age (years) | 54.9 (9.3) | 55.9 (9.1) |
| BMI (kg/m2) | 31.3 (5.2) | 31.4 (4.9) |
| Months since diagnosis | 10.8 (5.3) | 10.7 (4.8) |
| Months since treatment completion | 4.9 (4.6) | 5.2 (4.7) |
| n (%) | ||
| Menopausal status at diagnosis | ||
| Premenopausal | 31 (39%) | 28 (35%) |
| Perimenopausal a | 15 (19%) | 6 (8%) |
| Postmenopausal a | 34 (42%) | 45 (57%) |
| Breast cancer stage b | ||
| Stage 1 | 46 (58%) | 40 (51%) |
| Stage 2 | 24 (30%) | 30 (38%) |
| Stage 3 | 9 (11%) | 9 (11%) |
| Estrogen receptor status b | ||
| Positive | 72 (91%) | 67 (85%) |
| Negative | 7 (9%) | 12 (15%) |
| HER2 b | ||
| Positive | 9 (11%) | 11 (14%) |
| Negative | 68 (86%) | 68 (86%) |
| Equivocal | 2 (3%) | 0 (0%) |
| Chemotherapy treatment | 51 (64%) | 48 (61%) |
| Radiotherapy treatment | 63 (79%) | 63 (80%) |
| Endocrine treatment | ||
| None | 35 (44%) | 32 (41%) |
| SERM | 19 (24%) | 22 (28%) |
| Aromatase inhibitor | 26 (32%) | 24 (30%) |
| GnRH agonist | 0 (0%) | 1 (1%) |
| Metabolic syndrome present | 37 (48%) | 37 (47%) |
| Charlson Comorbidity Index c | ||
| 0 | 51 (64%) | 47 (60%) |
| 1 | 18 (22%) | 13 (16%) |
| ≥2 a | 11 (14%) | 19 (24%) |
| Married or stable union | 56 (70.0%) | 54 (68.4%) |
| Caucasian | 78 (97.5%) | 78 (98.7%) |
| Employment status | ||
| Paid work | 44 (55%) | 50 (63%) |
| Retired, home duties, unable to work, other | 36 (45%) | 29 (37%) |
| n (%) | ||
| Highest education level | ||
| High school or less | 32 (40%) | 32 (41%) |
| Technical/trade/diploma | 21 (26%) | 16 (20%) |
| University or higher | 27 (34%) | 31 (39%) |
| Gross household income (AUD) d | ||
| <$82,056 per year | 34 (42%) | 37 (47%) |
| ≥$82,056 per year | 38 (48%) | 33 (42%) |
| Not reported/not known | 8 (10%) | 9 (11%) |
| Outcome | Timepoint | Intervention | Usual Care | Intervention Effect (Intervention—Usual Care) | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean Change (95% CI) | n | Mean Change (95% CI) | Mean Difference (95% CI) | p | d a | ||
| Weight (% of baseline value) | Baseline M (SD) | 79 | 83.9 (14.2) | 80 | 83.6 (13.6) | |||
| 6 months | 73 | −4.61 (−5.77, −3.44) | 70 | −0.52 (−1.70, 0.67) | −4.09 (−5.75, −2.43) | <0.001 | −0.30 | |
| 12 months b | 70 | −5.06 (−6.46, −3.66) | 60 | −0.58 (−2.02, 0.85) | −4.48 (−6.48, −2.47) | <0.001 | −0.32 | |
| 18 months | 68 | −3.69 (−5.23, −2.16) | 60 | −0.62 (−2.22, 0.97) | −3.07 (−5.28, −0.86) | 0.007 | −0.22 | |
| Weight (kg) | Baseline M (SD) | 79 | 83.9 (14.2) | 80 | 83.6 (13.6) | |||
| 6 months | 73 | −3.74 (−4.71, −2.76) | 70 | −0.43 (−1.42, 0.56) | −3.31 (−4.70, −1.92) | <0.001 | −0.24 | |
| 12 months b | 70 | −4.12 (−5.28, −2.96) | 60 | −0.52 (−1.71, 0.67) | −3.60 (−5.26, −1.94) | <0.001 | −0.26 | |
| 18 months | 68 | −3.03 (−4.34, −1.73) | 60 | −0.56 (−1.91, 0.80) | −2.48 (−4.36, −0.59) | 0.010 | −0.18 | |
| Total fat mass (kg) | Baseline M (SD) | 73 | 38.8 (10.4) | 70 | 37.5 (10.2) | |||
| 6 months | 67 | −3.13 (−3.97, −2.30) | 62 | 0.13 (−0.73, 1.00) | −3.26 (−4.47, −2.06) | <0.001 | −0.32 | |
| 12 months b | 64 | −3.27 (−4.26, −2.29) | 54 | 0.05 (−0.98, 1.08) | −3.32 (−4.75, −1.90) | <0.001 | −0.32 | |
| 18 months | 63 | −2.11 (−3.19, −1.03) | 54 | −0.29 (−1.43, 0.85) | −1.82 (−3.39, −0.25) | 0.023 | −0.18 | |
| Total lean mass (kg) | Baseline M (SD) | 73 | 42.8 (5.0) | 70 | 43.6 (5.2) | |||
| 6 months | 67 | −0.96 (−1.28, −0.63) | 62 | −0.24 (−0.57, 0.09) | −0.71 (−1.18, −0.25) | 0.002 | −0.14 | |
| 12 months b | 64 | −1.07 (−1.46, −0.68) | 54 | −0.52 (−0.93, −0.10) | −0.55 (−1.12, 0.02) | 0.059 | −0.11 | |
| 18 months | 63 | −1.20 (−1.63, −0.77) | 54 | −0.14 (−0.59, 0.32) | −1.06 (−1.68, −0.43) | <0.001 | −0.21 | |
| Metabolic syndrome risk score | Baseline M (SD) | 78 | 0.65 (0.60) | 77 | 0.63 (0.59) | |||
| 6 months | 69 | −0.19 (−0.27, −0.11) | 65 | 0.03 (−0.05, 0.12) | −0.22 (−0.34, −0.10) | <0.001 | −0.37 | |
| 12 months b | 67 | −0.18 (−0.27, −0.08) | 56 | 0.01 (−0.09, 0.11) | −0.19 (−0.32, −0.05) | 0.006 | −0.32 | |
| 18 months | 66 | −0.15 (−0.24, −0.06) | 57 | 0.01 (−0.08, 0.11) | −0.16 (−0.29, −0.03) | 0.014 | −0.27 | |
| Waist circumference (cm) | Baseline M (SD) | 79 | 106.7 (11.7) | 80 | 104.9 (10.4) | |||
| 6 months | 73 | −3.47 (−4.95, −1.99) | 70 | −0.64 (−2.14, 0.87) | −2.83 (−4.94, −0.71) | 0.009 | −0.26 | |
| 12 months b | 70 | −5.50 (−7.11, −3.89) | 60 | −2.30 (−3.98, −0.62) | −3.20 (−5.53, −0.87) | 0.007 | −0.29 | |
| 18 months | 68 | −5.29 (−6.81, −3.78) | 60 | −2.50 (−4.08, −0.91) | −2.80 (−4.99, −0.61) | 0.012 | −0.25 | |
| Triglycerides (mmol/L) c | Baseline M (SD) | 78 | 1.4 (0.7) | 78 | 1.5 (0.9) | |||
| 6 months | 71 | −0.03 (−0.12, 0.05) | 67 | 0.08 (−0.01, 0.18) | −0.11 (−0.24, 0.01) | 0.081 | −0.14 | |
| 12 months b | 67 | −0.08 (−0.18, 0.02) | 57 | 0.04 (−0.08, 0.16) | −0.12 (−0.28, 0.03) | 0.125 | −0.15 | |
| 18 months | 66 | −0.11 (−0.20, −0.02) | 59 | −0.01 (−0.11, 0.09) | −0.10 (−0.24, 0.03) | 0.124 | −0.13 | |
| HDL-cholesterol (mmol/L) | Baseline M (SD) | 78 | 1.4 (0.3) | 78 | 1.4 (0.4) | |||
| 6 months | 71 | 0.02 (−0.02, 0.07) | 67 | −0.02 (−0.06, 0.03) | 0.04 (−0.02, 0.10) | 0.182 | 0.13 | |
| 12 months b | 67 | 0.05 (0.00, 0.09) | 57 | −0.01 (−0.06, 0.04) | 0.06 (−0.01, 0.12) | 0.110 | 0.17 | |
| 18 months | 66 | 0.06 (0.01, 0.11) | 59 | 0.00 (−0.04, 0.05) | 0.06 (−0.01, 0.12) | 0.097 | 0.17 | |
| Systolic blood pressure (mmHg) | Baseline M (SD) | 79 | 125.3 (12.2) | 79 | 123.4 (11.3) | |||
| 6 months | 71 | −1.69 (−4.21, 0.83) | 68 | 3.44 (0.86, 6.02) | −5.13 (−8.73, −1.52) | 0.005 | −0.44 | |
| 12 months b | 70 | 1.05 (−1.70, 3.80) | 59 | 2.20 (−0.77, 5.17) | −1.15 (−5.20, 2.90) | 0.577 | −0.10 | |
| 18 months | 68 | 3.07 (−0.37, 6.52) | 59 | 5.54 (1.88, 9.19) | −2.46 (−7.48, 2.56) | 0.336 | −0.21 | |
| Diastolic blood pressure (mmHg) | Baseline M (SD) | 79 | 78.7 (9.4) | 79 | 77.9 (7.3) | |||
| 6 months | 71 | −0.35 (−2.03, 1.32) | 68 | 2.40 (0.69, 4.12) | −2.76 (−5.15, −0.36) | 0.024 | −0.33 | |
| 12 months b | 70 | 0.60 (−1.07, 2.27) | 59 | 1.51 (−0.29, 3.30) | −0.90 (−3.36, 1.55) | 0.470 | −0.11 | |
| 18 months | 68 | 1.06 (−0.80, 2.92) | 59 | 3.39 (1.41, 5.36) | −2.33 (−5.04, 0.39) | 0.093 | −0.28 | |
| Fasting plasma glucose (mmol/L) | Baseline M (SD) | 78 | 5.5 (1.2) | 78 | 5.6 (1.1) | |||
| 6 months | 71 | −0.34 (−0.49, −0.19) | 67 | −0.21 (−0.36, −0.05) | −0.13 (−0.35, 0.08) | 0.230 | −0.11 | |
| 12 months b | 67 | −0.17 (−0.32, −0.03) | 57 | 0.06 (−0.10, 0.21) | −0.23 (−0.44, −0.02) | 0.032 | −0.20 | |
| 18 months | 66 | −0.12 (−0.29, 0.04) | 59 | −0.10 (−0.27, 0.08) | −0.02 (−0.26, 0.22) | 0.844 | −0.02 | |
| Outcome | Timepoint | Intervention | Usual Care | Intervention Effect (Intervention—Usual Care) | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean Change (95% CI) | n | Mean Change (95% CI) | Mean Difference (95% CI) | p | d a | ||
| QOL Physical Health component (T score) b | Baseline M (SD) | 78 | 44.8 (6.9) | 77 | 45.9 (6.6) | |||
| 6 months | 69 | 2.43 (1.31, 3.56) | 65 | 0.46 (−0.70, 1.62) | 1.98 (0.36, 3.59) | 0.017 | 0.29 | |
| 12 months c | 65 | 3.16 (1.82, 4.50) | 58 | 0.50 (−0.90, 1.91) | 2.66 (0.71, 4.60) | 0.007 | 0.39 | |
| 18 months | 65 | 1.56 (0.13, 2.99) | 57 | 0.38 (−1.13, 1.89) | 1.18 (−0.90, 3.26) | 0.266 | 0.18 | |
| QOL Mental Health component (T score) b | Baseline M (SD) | 78 | 46.1 (7.2) | 77 | 45.5 (6.3) | |||
| 6 months | 69 | 1.34 (0.10, 2.57) | 65 | −0.17 (−1.44, 1.10) | 1.51 (−0.27, 3.28) | 0.097 | 0.22 | |
| 12 months c | 65 | 1.98 (0.68, 3.28) | 58 | 0.21 (−1.15, 1.58) | 1.77 (−0.12, 3.66) | 0.067 | 0.26 | |
| 18 months | 65 | −0.36 (−1.98, 1.26) | 57 | 0.17 (−1.54, 1.88) | −0.53 (−2.89, 1.83) | 0.659 | −0.08 | |
| Fatigue b | Baseline M (SD) | 77 | 35.5 (9.7) | 77 | 37.6 (9.5) | |||
| 6 months | 68 | 3.21 (1.57, 4.86) | 65 | 0.75 (−0.94, 2.43) | 2.47 (0.11, 4.83) | 0.040 | 0.26 | |
| 12 months c | 64 | 4.29 (2.57, 6.01) | 58 | 2.26 (0.46, 4.05) | 2.03 (−0.46, 4.53) | 0.110 | 0.21 | |
| 18 months | 63 | 2.63 (0.81, 4.46) | 57 | 1.99 (0.09, 3.89) | 0.65 (−2.00, 3.29) | 0.632 | 0.07 | |
| Musculoskeletal Pain | Baseline M (SD) | 63 | 1.5 (1.1) | 59 | 1.6 (1.0) | |||
| 6 months | 56 | −0.21 (−0.44, 0.02) | 50 | 0.40 (0.16, 0.64) | −0.61 (−0.94, −0.28) | <0.001 | −0.58 | |
| 12 months c | 52 | −0.19 (−0.42, 0.04) | 46 | 0.32 (0.08, 0.56) | −0.51 (−0.84, −0.18) | 0.003 | −0.49 | |
| 18 months | 53 | −0.07 (−0.32, 0.18) | 44 | 0.26 (−0.01, 0.52) | −0.33 (−0.69, 0.04) | 0.079 | −0.31 | |
| Menopausal Symptoms—Psychological subscale | Baseline M (SD) | 75 | 10.0 (6.2) | 76 | 9.6 (5.7) | |||
| 6 months | 66 | −1.40 (−2.51, −0.30) | 65 | −0.14 (−1.25, 0.98) | −1.27 (−2.84, 0.30) | 0.113 | −0.21 | |
| 12 months c | 62 | −1.90 (−3.20, −0.60) | 58 | −0.62 (−1.96, 0.71) | −1.28 (−3.14, 0.59) | 0.179 | −0.22 | |
| 18 months | 61 | −1.24 (−2.54, 0.06) | 56 | −0.22 (−1.56, 1.12) | −1.02 (−2.88, 0.85) | 0.286 | −0.17 | |
| Menopausal Symptoms—Somatic subscale | Baseline M (SD) | 76 | 5.5 (4.3) | 76 | 5.1 (4.0) | |||
| 6 months | 67 | −0.67 (−1.31, −0.04) | 65 | 0.58 (−0.07, 1.22) | −1.25 (−2.15, −0.34) | 0.007 | −0.30 | |
| 12 months c | 62 | −0.77 (−1.53, −0.01) | 58 | −0.07 (−0.85, 0.71) | −0.70 (−1.79, 0.39) | 0.206 | −0.17 | |
| 18 months | 63 | −0.67 (−1.47, 0.13) | 56 | 0.27 (−0.56, 1.10) | −0.94 (−2.10, 0.21) | 0.108 | −0.23 | |
| Menopausal Symptoms—Vasomotor subscale | Baseline M (SD) | 76 | 2.6 (2.2) | 76 | 2.4 (2.1) | |||
| 6 months | 67 | 0.24 (−0.13, 0.62) | 65 | 0.49 (0.11, 0.87) | −0.25 (−0.78, 0.29) | 0.367 | −0.12 | |
| 12 months c | 63 | 0.06 (−0.36, 0.48) | 58 | 0.42 (−0.02, 0.85) | −0.35 (−0.96, 0.25) | 0.250 | −0.17 | |
| 18 months | 63 | −0.22 (−0.65, 0.21) | 56 | 0.32 (−0.13, 0.77) | −0.54 (−1.16, 0.08) | 0.089 | −0.25 | |
| Fear of Cancer Recurrence | Baseline M (SD) | 77 | 14.5 (9.6) | 76 | 15.5 (9.7) | |||
| 6 months | 68 | −2.20 (−3.74, −0.65) | 64 | −1.08 (−2.67, 0.51) | −1.12 (−3.34, 1.10) | 0.321 | −0.12 | |
| 12 months c | 64 | −2.24 (−3.83, −0.65) | 57 | −3.35 (−5.02, −1.67) | 1.11 (−1.21, 3.42) | 0.348 | 0.12 | |
| 18 months | 64 | −2.45 (−4.24, −0.65) | 55 | −1.99 (−3.91, −0.07) | −0.46 (−3.08, 2.17) | 0.734 | −0.05 | |
| Body Image—Total score | Baseline M (SD) | 78 | 2.8 (0.6) | 77 | 2.7 (0.6) | |||
| 6 months | 69 | −0.35 (−0.46, −0.24) | 65 | −0.14 (−0.25, −0.03) | −0.21 (−0.37, −0.05) | 0.010 | −0.36 | |
| 12 months c | 65 | −0.43 (−0.54, −0.32) | 58 | −0.25 (−0.36, −0.13) | −0.18 (−0.35, −0.02) | 0.030 | −0.31 | |
| 18 months | 65 | −0.30 (−0.42, −0.17) | 57 | −0.21 (−0.35, −0.08) | −0.08 (−0.27, 0.10) | 0.380 | −0.14 | |
| Intervention (n = 11 Participants) | No. of Events | Usual Care (n = 10 Participants) | No. of Events | |
|---|---|---|---|---|
| Life-threatening (n = 4) a | Stage IV breast cancer (bone metastasis) | 1 | Heart episode during surgery | 1 |
| Stage IV breast cancer (i.e., bone metastasis, site unknown) | 2 | |||
| Severe/undesirable (n = 21) b | Musculoskeletal events requiring hospitalization or surgery | 6 c | Musculoskeletal events requiring hospitalization or surgery | 1 |
| Genitourinary events requiring hospitalization or surgery | 4 | Gastrointestinal events requiring hospitalization or surgery | 1 | |
| Other events requiring hospitalization or surgery | 1 | Genitourinary events requiring hospitalization or surgery | 2 | |
| Local breast cancer recurrence | 1 | Respiratory events requiring hospitalization or surgery | 3 | |
| Other events requiring hospitalization or surgery | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reeves, M.M.; Terranova, C.O.; Winkler, E.A.H.; McCarthy, N.; Hickman, I.J.; Ware, R.S.; Lawler, S.P.; Eakin, E.G.; Demark-Wahnefried, W. Effect of a Remotely Delivered Weight Loss Intervention in Early-Stage Breast Cancer: Randomized Controlled Trial. Nutrients 2021, 13, 4091. https://doi.org/10.3390/nu13114091
Reeves MM, Terranova CO, Winkler EAH, McCarthy N, Hickman IJ, Ware RS, Lawler SP, Eakin EG, Demark-Wahnefried W. Effect of a Remotely Delivered Weight Loss Intervention in Early-Stage Breast Cancer: Randomized Controlled Trial. Nutrients. 2021; 13(11):4091. https://doi.org/10.3390/nu13114091
Chicago/Turabian StyleReeves, Marina M., Caroline O. Terranova, Elisabeth A. H. Winkler, Nicole McCarthy, Ingrid J. Hickman, Robert S. Ware, Sheleigh P. Lawler, Elizabeth G. Eakin, and Wendy Demark-Wahnefried. 2021. "Effect of a Remotely Delivered Weight Loss Intervention in Early-Stage Breast Cancer: Randomized Controlled Trial" Nutrients 13, no. 11: 4091. https://doi.org/10.3390/nu13114091
APA StyleReeves, M. M., Terranova, C. O., Winkler, E. A. H., McCarthy, N., Hickman, I. J., Ware, R. S., Lawler, S. P., Eakin, E. G., & Demark-Wahnefried, W. (2021). Effect of a Remotely Delivered Weight Loss Intervention in Early-Stage Breast Cancer: Randomized Controlled Trial. Nutrients, 13(11), 4091. https://doi.org/10.3390/nu13114091







