The Effect of a Single Bout of Exercise on Vitamin B2 Status Is Not Different between High- and Low-Fit Females
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Study Subjects
2.3. Study Design
2.4. Blood Sampling and Hemolysate Preparation
2.5. Protein Content Determination
2.6. Erythrocyte Glutathione Reductase Activity Coefficient (EGRAC) Assay
2.7. Statistical Analyses
3. Results
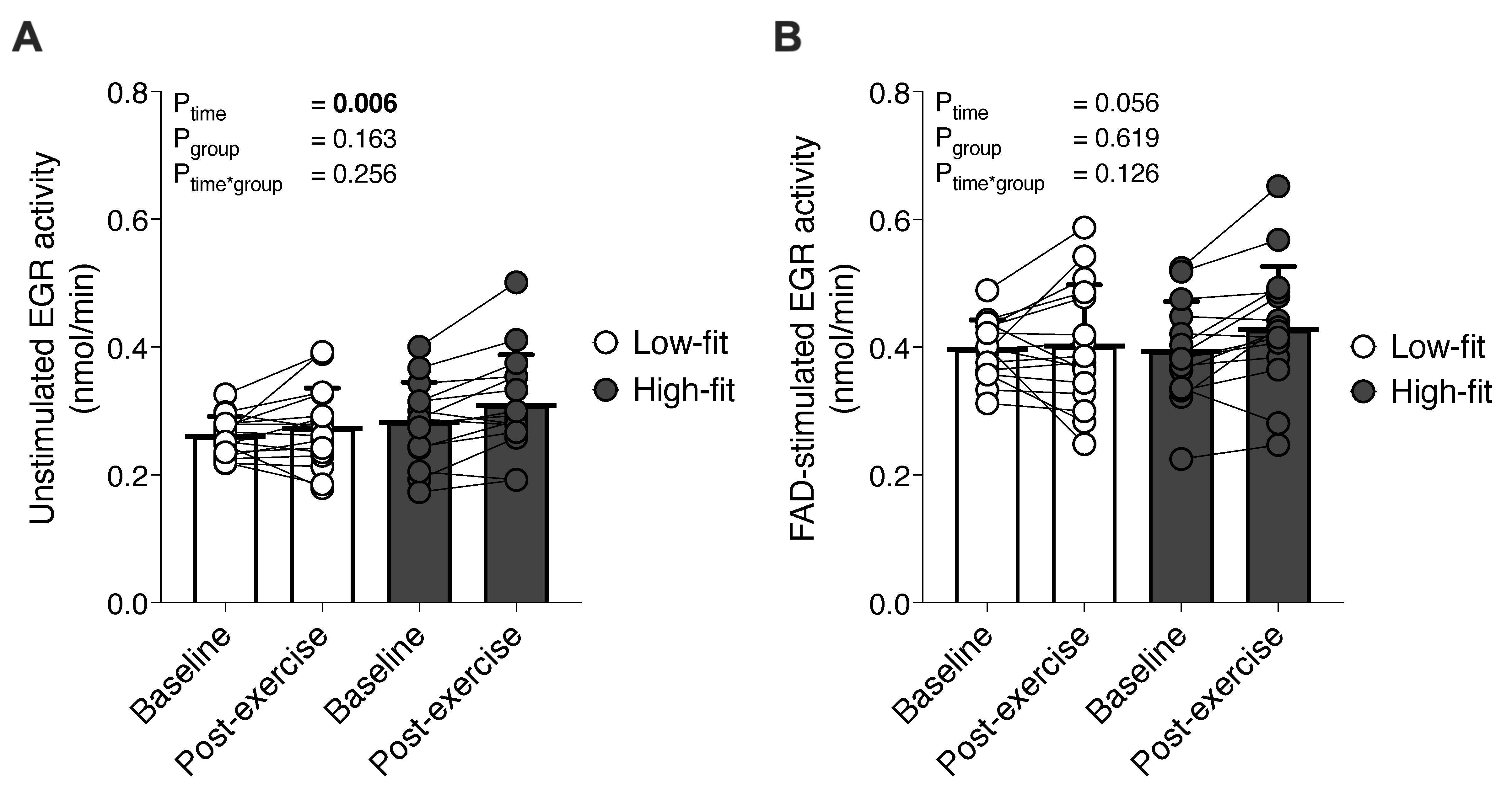
3.1. EGR Enzymatic Activities Were Increased after a Single Bout of Exercise, but Were Not Significantly Different in High-Fit Compared to Low-Fit Females
3.2. The EGRAC Response to a Single Bout of Exercise Is Not Different between High-Fit and Low-Fit Females and Not Related to the EGR Response
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, J.; Liu, Z.; Chuang, C.-C.; Yang, W.; Zuo, L. Redox mechanism of reactive oxygen species in exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K. Oxidants and antioxidants in exercise. J. Appl. Physiol. 1995, 79, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid. Redox Signal. 2011, 15, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 2006, 163, 94–112. [Google Scholar] [CrossRef]
- Lienhart, W.-D.; Gudipati, V.; Macheroux, P. The human flavoproteome. Arch. Biochem. Biophys. 2013, 535, 150–162. [Google Scholar] [CrossRef] [Green Version]
- Joosten, V.; van Berkel, W.J. Flavoenzymes. Curr. Opin. Chem. Biol. 2007, 11, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Schulz, G.E.; Schirmer, R.H.; Sachsenheimer, W.; Pai, E.F. The structure of the flavoenzyme glutathione reductase. Nat. Cell Biol. 1978, 273, 120–124. [Google Scholar] [CrossRef]
- Woolf, K.; Manore, M.M. B-vitamins and exercise: Does exercise alter requirements? Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 453–484. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Dietary reference values for riboflavin. EFSA J. 2017, 15, e04919. [Google Scholar] [CrossRef] [Green Version]
- Bayoumi, R.A.; Rosalki, S.B. Evaluation of methods of coenzyme activation of erythrocyte enzymes for detection of deficiency of vitamins B1, B2, and B6. Clin. Chem. 1976, 22, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Sauberlich, H.E.; Judd, J.H.; Nichoalds, G.E.; Broquist, H.P.; Darby, W.J. Application of the erythrocyte glutathione reductase assay in evaluating riboflavin nutritional status in a high school student population. Am. J. Clin. Nutr. 1972, 25, 756–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoey, L.; McNulty, H.; Strain, J. Studies of biomarker responses to intervention with riboflavin: A systematic review. Am. J. Clin. Nutr. 2009, 89, 1960S–1980S. [Google Scholar] [CrossRef] [PubMed]
- Belko, A.Z.; Meredith, M.P.; Kalkwarf, H.J.; Obarzanek, E.; Weinberg, S.; Roach, R.; McKeon, G.; Roe, D.A. Effects of exercise on riboflavin requirements: Biological validation in weight reducing women. Am. J. Clin. Nutr. 1985, 41, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Belko, A.Z.; Obarzanek, E.; Kalkwarf, H.J.; Rotter, M.A.; Bogusz, S.; Miller, D.; Haas, J.D.; Roe, D.A. Effects of exercise on riboflavin requirements of young women. Am. J. Clin. Nutr. 1983, 37, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Belko, A.Z.; Obarzanek, E.; Roach, R.; Rotter, M.; Urban, G.; Weinberg, S.; Roe, D.A. Effects of aerobic exercise and weight loss on riboflavin requirements of moderately obese, marginally deficient young women. Am. J. Clin. Nutr. 1984, 40, 553–561. [Google Scholar] [CrossRef]
- Winters, L.R.T.; Yoon, J.S.; Kalkwarf, H.J.; Davies, J.C.; Berkowitz, M.G.; Haas, J.; Roe, D.A. Riboflavin requirements and exercise adaptation in older women. Am. J. Clin. Nutr. 1992, 56, 526–532. [Google Scholar] [CrossRef]
- Soares, M.J.; Satyanarayana, K.; Bamjlt, M.S.; Jacob, C.M.; Ramana, Y.V.; Rao, S.S. The effect of exercise on the riboflavin status of adult men. Br. J. Nutr. 1993, 69, 541–551. [Google Scholar] [CrossRef]
- Ohno, H.; Yahata, T.; Sato, Y.; Yamamura, K.; Taniguchi, N. Physical training and fasting erythrocyte activities of free radical scavenging enzyme systems in sedentary men. Graefe’s Arch. Clin. Exp. Ophthalmol. 1988, 57, 173–176. [Google Scholar] [CrossRef]
- Fogelholm, M. Micronutrient status in females during a 24-week fitness-type exercise program. Ann. Nutr. Metab. 1992, 36, 209–218. [Google Scholar] [CrossRef]
- Evelo, C.; Palmen, N.G.M.; Artur, Y.; Janssen, G.M.E. Changes in blood glutathione concentrations, and in erythrocyte glutathione reductase and glutathione S-transferase activity after running training and after participation in contests. Graefe’s Arch. Clin. Exp. Ophthalmol. 1992, 64, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Weight, L.M.; Noakes, T.D.; Labadarios, D.; Graves, J.; Jacobs, P.; Berman, P.A. Vitamin and mineral status of trained athletes including the effects of supplementation. Am. J. Clin. Nutr. 1988, 47, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Guilland, J.C.; Penaranda, T.; Gallet, C.; Boggio, V.; Fuchs, F.; Klepping, J. Vitamin status of young athletes including the effects of supplementation. Med. Sci. Sports Exerc. 1989, 21, 441–449. [Google Scholar] [CrossRef]
- Keith, R.E.; Alt, L.A. Riboflavin status of female athletes consuming normal diets. Nutr. Res. 1991, 11, 727–734. [Google Scholar] [CrossRef]
- Malara, M.; Hübner-Wozniak, E.; Lewandowska, I. Assessment of intake and nutritional status of vitamin B1, B2, and B6 in men and women with different physical activity levels. Biol. Sport 2013, 30, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, A.; Boilard, F.; Breton, M.-F.; Bessette, H.; Roberge, A.G. The effects of a riboflavin supplementation on the nutritional status and performance of elite swimmers. Nutr. Res. 1984, 4, 201–208. [Google Scholar] [CrossRef]
- Rokitzki, L.; Sagredos, A.; Keck, E.; Sauer, B.; Keul, J. Assessment of vitamin B2 status in performance athletes of various types of sports. J. Nutr. Sci. Vitaminol. 1994, 40, 11–22. [Google Scholar] [CrossRef]
- Frank, T.; Kühl, M.; Makowski, B.; Bitsch, R.; Jahreis, G.; Hübscher, J. Does a 100-km walking affect indicators of vitamin status? Int. J. Vitam. Nutr. Res. 2000, 70, 238–250. [Google Scholar] [CrossRef]
- Ohno, H.; Sato, Y.; Yamashita, K.; Doi, R.; Arai, K.; Kondo, T.; Taniguchi, N. The effect of brief physical exercise on free radical scavenging enzyme systems in human red blood cells. Can. J. Physiol. Pharmacol. 1986, 64, 1263–1265. [Google Scholar] [CrossRef]
- Tauler, P.; Aguiló, A.; Guix, P.; Jiménez, F.; Villa, G.; Tur, J.; Cordova, A.; Pons, A. Pre-exercise antioxidant enzyme activities determine the antioxidant enzyme erythrocyte response to exercise. J. Sports Sci. 2005, 23, 5–13. [Google Scholar] [CrossRef]
- Lagerwaard, B.; Keijer, J.; McCully, K.K.; De Boer, V.C.J.; Nieuwenhuizen, A.G. In vivo assessment of muscle mitochondrial function in healthy, young males in relation to parameters of aerobic fitness. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 119, 1799–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagerwaard, B.; Janssen, J.J.E.; Cuijpers, I.; Keijer, J.; de Boer, V.C.J.; Nieuwenhuizen, A.G. Muscle mitochondrial capacity in high-and low-fitness females using near-infrared spectroscopy. Physiol. Rep. 2021, 9, e14838. [Google Scholar] [CrossRef] [PubMed]
- Streppel, M.T.; De Vries, J.H.M.; Meijboom, S.; Beekman, M.; De Craen, A.J.M.; Slagboom, P.E.; Feskens, E.J.M. Relative validity of the food frequency questionnaire used to assess dietary intake in the Leiden Longevity Study. Nutr. J. 2013, 12, 75. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.H.E.; Bradley, A.; Mushtaq, S.; Williams, E.A.; Powers, H.J. Effects of methodological variation on assessment of riboflavin status using the erythrocyte glutathione reductase activation coefficient assay. Br. J. Nutr. 2008, 102, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox Signal 2013, 18, 1208–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suboticanec, K.; Stavljenić, A.; Schalch, W.; Buzina, R. Effects of pyridoxine and riboflavin supplementation on physical fitness in young adolescents. Int. J. Vitam. Nutr. Res. 1990, 60, 81–88. [Google Scholar]
- Boisvert, W.A.; Castaneda, C.; Mendoza, I.; Langeloh, G.; Solomons, N.W.; Gershoff, S.N.; Russell, R.M. Prevalence of riboflavin deficiency among Guatemalan elderly people and its relationship to milk intake. Am. J. Clin. Nutr. 1993, 58, 85–90. [Google Scholar] [CrossRef]
- Conley, K.E. Mitochondria to motion: Optimizing oxidative phosphorylation to improve exercise performance. J. Exp. Biol. 2016, 219, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Lanza, I.R.; Nair, K.S. Muscle mitochondrial changes with aging and exercise. Am. J. Clin. Nutr. 2009, 89, 467S–471S. [Google Scholar] [CrossRef] [Green Version]
- Gollnick, P.D.; Armstrong, R.B.; Saubert, C.W.; Piehl, K.; Saltin, B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J. Appl. Physiol. 1972, 33, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Molé, P.A.; Oscai, L.B.; Holloszy, J.O. Adaptation of muscle to exercise. J. Clin. Investig. 1971, 50, 2323–2330. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [Green Version]
- Fogelholm, M.; Ruokonen, I.; Laakso, J.T.; Vuorimaa, T.; Himberg, J.-J. Lack of association between indices of vitamin B1, B2, and B6, status and exercise-induced blood lactate in young adults. Int. J. Sport Nutr. 1993, 3, 165–176. [Google Scholar] [CrossRef]
- Haralambie, G. Vitamin B2 status in athletes and the influence of Riboflavin administration on neuromuscular irritability. Ann. Nutr. Metab. 1976, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Powers, H.J.; Hill, M.H.; Mushtaq, S.; Dainty, J.R.; Majsak-Newman, G.; Williams, A.E. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM). Am. J. Clin. Nutr. 2011, 93, 1274–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jungert, A.; McNulty, H.; Hoey, L.; Ward, M.; Strain, J.J.; Hughes, C.F.; McAnena, L.; Neuhäuser-Berthold, M.; Pentieva, K. Riboflavin is an important determinant of vitamin B-6 status in healthy adults. J. Nutr. 2020, 150, 2699–2706. [Google Scholar] [CrossRef]
- Aljaadi, A.M.; How, R.E.; Loh, S.P.; Hunt, E.S.; Karakochuk, C.D.; Barr, I.S.; McAnena, L.; Ward, M.; McNulty, H.; Khor, G.L.; et al. suboptimal biochemical riboflavin status is associated with lower hemoglobin and higher rates of anemia in a sample of Canadian and Malaysian women of reproductive age. J. Nutr. 2019, 149, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Mataix, J.; Aranda, P.; Sánchez, C.; Montellano, M.A.; Planells, E.; Llopis, J. Assessment of thiamin (vitamin B1) and riboflavin (vitamin B2) status in an adult Mediterranean population. Br. J. Nutr. 2003, 90, 661–666. [Google Scholar] [CrossRef] [Green Version]
- Preziosi, P.; Galan, P.; Deheeger, M.; Yacoub, N.; Drewnowski, A.; Hercberg, S. Breakfast type, daily nutrient intakes and vitamin and mineral status of French children, adolescents and adults. J. Am. Coll. Nutr. 1999, 18, 171–178. [Google Scholar] [CrossRef]
- Löwik, M.R.; Schrijver, J.; Odink, J.; Berg, H.V.D.; Wedel, M.; Hermus, R.J. Nutrition and aging: Nutritional status of “apparently healthy” elderly (Dutch nutrition surveillance system). J. Am. Coll. Nutr. 1990, 9, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, K.F.; Van Staveren, W.A. The Dutch national food consumption survey: Design, methods and first results. Food Policy 1991, 16, 257–260. [Google Scholar] [CrossRef]
- Health Council of the Netherlands. Voedingsnormen voor Vitamines en Mineralen voor Volwassenen. 2018. Available online: https://www.gezondheidsraad.nl/documenten/adviezen/2018/09/18/gezondheidsraad-herziet-voedingsnormen-voor-volwassenen (accessed on 28 September 2021).
- Glatzle, D.; Körner, W.F.; Christeller, S.; Wiss, O. Method for the detection of a biochemical riboflavin deficiency. Stimulation of NADPH2-dependent glutathione reductase from human erythrocytes by FAD in vitro. Investigations on the vitamin B2 status in healthly people and geriatric patients. J. Int. Vitaminol. 1970, 40, 166–183. [Google Scholar]

| Low-Fit (N = 16) | High-Fit (N = 15) | |
|---|---|---|
| Age (y) | 24.0 (21.3–25.5) | 21.8 (21.6–23.7) |
| Weight (kg) | 59.2 ± 7.2 | 61.2 ± 7.0 |
| Height (m) | 1.63 ± 0.08 | 1.68 ± 0.05 * |
| Fat mass (% of weight) | 28.9 ± 3.9 | 25.1 ± 4.4 * |
| O2peak (mL/kg/min) | 35.1 (32.2–35.7) | 50.4 (49.0–54.0) **** |
| Baecke total score | 7.3 ± 1.0 | 9.5 ± 0.8 **** |
| Hemoglobin (mmol/L) | 8.4 ± 0.6 | 8.5 ± 0.6 |
| Use of birth control pill | 6/16 | 7/15 |
| If not; 17β-estradiol (pmol/L) | 470.9 (337.2–590.1) | 217.4 (109.1–895.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janssen, J.J.E.; Lagerwaard, B.; Nieuwenhuizen, A.G.; Timmers, S.; de Boer, V.C.J.; Keijer, J. The Effect of a Single Bout of Exercise on Vitamin B2 Status Is Not Different between High- and Low-Fit Females. Nutrients 2021, 13, 4097. https://doi.org/10.3390/nu13114097
Janssen JJE, Lagerwaard B, Nieuwenhuizen AG, Timmers S, de Boer VCJ, Keijer J. The Effect of a Single Bout of Exercise on Vitamin B2 Status Is Not Different between High- and Low-Fit Females. Nutrients. 2021; 13(11):4097. https://doi.org/10.3390/nu13114097
Chicago/Turabian StyleJanssen, Joëlle J. E., Bart Lagerwaard, Arie G. Nieuwenhuizen, Silvie Timmers, Vincent C. J. de Boer, and Jaap Keijer. 2021. "The Effect of a Single Bout of Exercise on Vitamin B2 Status Is Not Different between High- and Low-Fit Females" Nutrients 13, no. 11: 4097. https://doi.org/10.3390/nu13114097
APA StyleJanssen, J. J. E., Lagerwaard, B., Nieuwenhuizen, A. G., Timmers, S., de Boer, V. C. J., & Keijer, J. (2021). The Effect of a Single Bout of Exercise on Vitamin B2 Status Is Not Different between High- and Low-Fit Females. Nutrients, 13(11), 4097. https://doi.org/10.3390/nu13114097






