Prevalence and Determinants of Vitamin D Deficiency in 9595 Mongolian Schoolchildren: A Cross-Sectional Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Setting
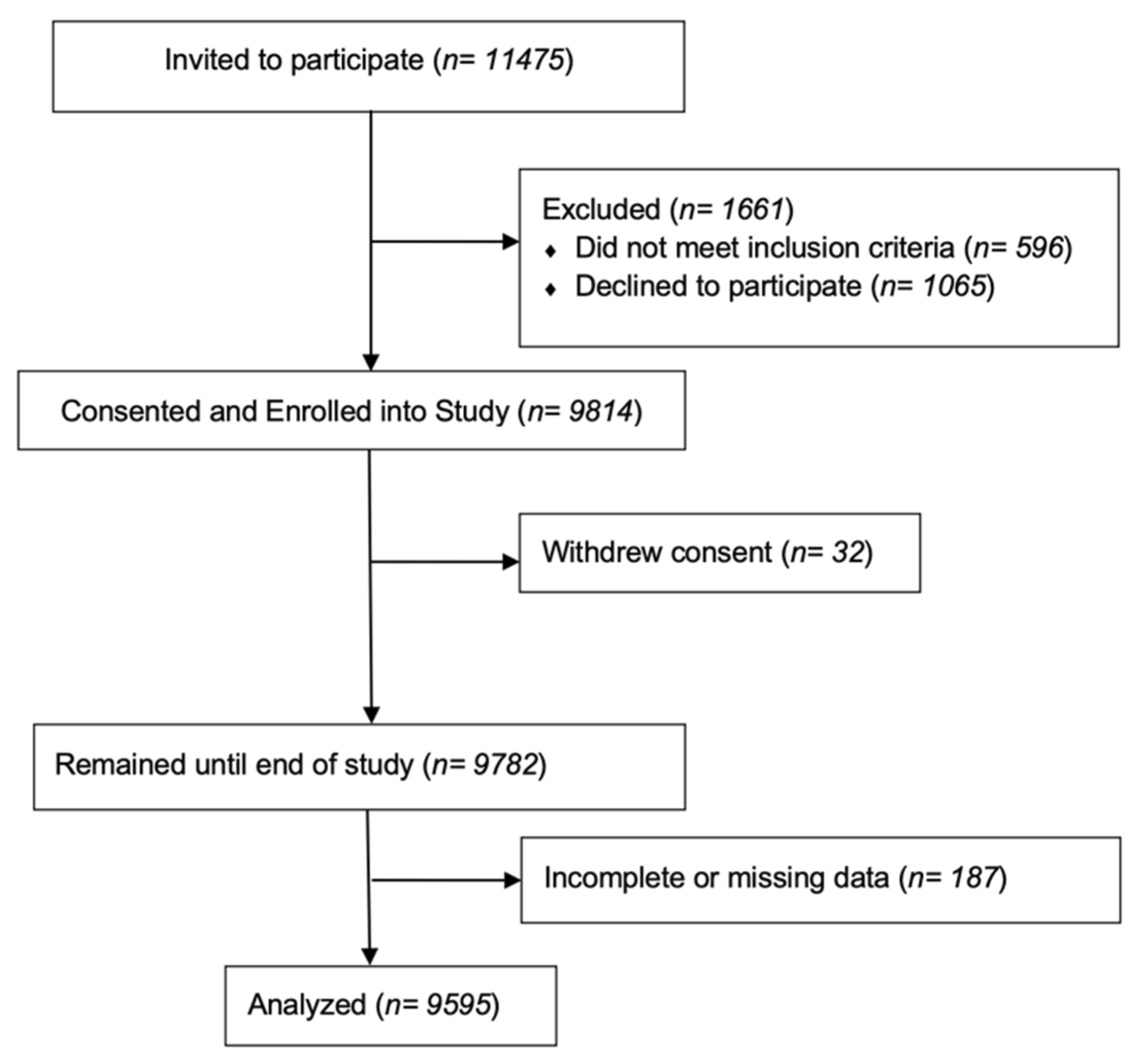
2.2. Sample Size and Eligibility
2.3. Data Collection and Measurement
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Predictors of Vitamin D Deficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cashman, K.D.; van den Heuvel, E.G.; Schoemaker, R.J.W.; Prévéraud, D.P.; Macdonald, H.M.; Arcot, J. 25-Hydroxyvitamin D as a Biomarker of Vitamin D Status and Its Modeling to Inform Strategies for Prevention of Vitamin D Deficiency within the Population. Adv. Nutr. 2017, 8, 947–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bromage, S.; Rich-Edwards, J.W.; Tselmen, D.; Baylin, A.; Houghton, L.A.; Baasanjav, N.; Ganmaa, D. Seasonal Epidemiology of Serum 25-Hydroxyvitamin D Concentrations among Healthy Adults Living in Rural and Urban Areas in Mongolia. Nutrients 2016, 8, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganmaa, D.; Holick, M.F.; Rich-Edwards, J.W.; Frazier, L.A.; Davaalkham, D.; Ninjin, B.; Janes, C.; Hoover, R.N.; Troisi, R. Vitamin D deficiency in reproductive age Mongolian women: A cross sectional study. J. Steroid Biochem. Mol. Biol. 2014, 139, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bromage, S.; Enkhmaa, D.; Baatar, T.; Garmaa, G.; Bradwin, G.; Yondonsambuu, B.; Sengee, T.; Jamts, E.; Suldsuren, N.; McElrath, T.F.; et al. Comparison of seasonal serum 25-hydroxyvitamin D concentrations among pregnant women in Mongolia and Boston. J. Steroid Biochem. Mol. Biol. 2019, 193, 105427. [Google Scholar] [CrossRef]
- Otani, S.; Majbauddin, A.; Qing, Q.; Intekhab, A.; Susa, R.; Fujitani, Y.; Masumoto, T.; Amano, H.; Kurozawa, Y.; Davaalkham, D. Spatial Epidemiology of Vitamin D Status in Mongolia. Environ. Epidemiol. 2019, 3, 298. [Google Scholar]
- Komatsu, F.; Kagawa, Y.; Kawabata, T.; Kaneko, Y.; Purvee, B.; Otgon, J.; Chimedregzen, U. Dietary habits of Mongolian people, and their influence on lifestyle-related diseases and early aging. Curr. Aging Sci. 2008, 1, 84–100. [Google Scholar] [CrossRef]
- Public Health Institute of Mongolia. Nutrition Status of the Population of Mongolia—5th National Nutrition Survey Report; Ministry of Health: Ulaanbaatar, Mongolia, 2017. [Google Scholar]
- Mongolian University of Science and Technology School of Food Engineering and Biotechnology. Flour Fortification Manual for Producers; Mongolian University of Science and Technology School of Food Engineering and Biotechnology: Ulaanbaatar, Mongolia, 2009. [Google Scholar]
- Ganmaa, D.; Khudyakov, P.; Buyanjargal, U.; Baigal, D.; Baatar, M.; Enkhamgalan, N.; Martineau, A.R. Risk factors for active tuberculosis in 938 QuantiFERON-positive schoolchildren in Mongolia: A community-based cross-sectional study. BMC Infect. Dis. 2019, 19, 532. [Google Scholar] [CrossRef] [Green Version]
- Ganmaa, D.; Uyanga, B.; Zhou, X.; Gantsetseg, G.; Delgerekh, B.; Enkhmaa, D.; Khulan, D.; Ariunzaya, S.; Sumiya, E.; Bolortuya, B.; et al. Vitamin D Supplements for Prevention of Tuberculosis Infection and Disease. N. Engl. J. Med. 2020, 383, 359–368. [Google Scholar] [CrossRef]
- Sempos, C.T.; Betz, J.M.; Camara, J.E.; Carter, G.D.; Cavalier, E.; Clarke, M.; Dowling, K.G.; Durazo-Arvizu, R.A.; Hoofnagle, A.N.; Liu, A.; et al. General Steps to Standardize the Laboratory Measurement of Serum Total 25-Hydroxyvitamin D. J. AOAC Int. 2017, 100, 1230–1233. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Important for Prevention of Osteoporosis, Cardiovascular Heart Disease, Type 1 Diabetes, Autoimmune Diseases, and Some Cancers. South. Med. J. 2005, 98, 1024–1026. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Metzger, D.L. Calculator: WHO Anthropometric Z-Scores 0-19y. 2021. Available online: https://apps.cpeg-gcep.net/quickZ_WHO/ (accessed on 10 September 2021).
- Nwosu, B.U.; Kum-Nji, P. Tobacco smoke exposure is an independent predictor of vitamin D deficiency in US children. PLoS ONE 2018, 13, e0205342. [Google Scholar] [CrossRef]
- Byun, E.J.; Heo, J.; Cho, S.H.; Lee, J.D.; Kim, H.S. Suboptimal vitamin D status in Korean adolescents: A nationwide study on its prevalence, risk factors including cotinine-verified smoking status and association with atopic dermatitis and asthma. BMJ Open 2017, 7, e016409. [Google Scholar] [CrossRef]
- Cadranel, J.; Hance, A.J.; Milleron, B.; Paillard, F.; Akoun, G.M.; Garabedian, M. Vitamin D Metabolism in Tuberculosis: Production of 1,25(OH)2D3by Cells Recovered by Bronchoalveolar Lavage and the Role of This Metabolite in Calcium Homeostasis. Am. Rev. Respir. Dis. 1988, 138, 984–989. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, E.; Gonzalez-Rodriguez, L.G.; Ortega Anta, R.M.; López-Sobaler, A.M. Consumption of eggs may prevent vitamin D deficiency in schoolchildren. Nutr. Hosp. 2013, 28, 794–801. [Google Scholar]
- Spiro, A.; Buttriss, J. Vitamin D: An overview of vitamin D status and intake in Europe. Nutr. Bull. 2014, 39, 322–350. [Google Scholar] [CrossRef] [Green Version]
- Ganmaa, D.; Stuart, J.; Sumberzul, N.; Ninjin, B.; Giovannucci, E.; Kleinman, K.; Holick, M.F.; Willett, W.C.; Frazier, L.A.; Rich-Edwards, J.W. Vitamin D supplementation and growth in urban Mongol school children: Results from two randomized clinical trials. PLoS ONE 2017, 12, e0175237. [Google Scholar] [CrossRef] [Green Version]
- Camargo, C.A.; Ganmaa, D.; Frazier, A.L.; Kirchberg, F.F.; Stuart, J.; Kleinman, K.; Sumberzul, N.; Rich-Edwards, J.W. Randomized Trial of Vitamin D Supplementation and Risk of Acute Respiratory Infection in Mongolia. Pediatrics 2012, 130, e561–e567. [Google Scholar] [CrossRef] [Green Version]
- Camargo, C.A., Jr.; Ganmaa, D.; Sidbury, R.; Erdenedelger, K.; Radnaakhand, N.; Khandsuren, B. Randomized trial of vitamin D supplementation for winter-related atopic dermatitis in children. J. Allergy Clin. Immunol. 2014, 134, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Erkhembayar, R.; Dickinson, E.; Badarch, D.; Narula, I.; Warburton, D.; Thomas, G.N.; Ochir, C.; Manaseki-Holland, S. Early policy actions and emergency response to the COVID-19 pandemic in Mongolia: Experiences and challenges. Lancet Glob. Heal. 2020, 8, e1234–e1241. [Google Scholar] [CrossRef]
- Janes, C.R. Failed development and vulnerability to climate change in Central Asia: Implications for food security and health. Asia Pac. J. Public Health 2010, 22, e236S–e245S. [Google Scholar] [CrossRef] [PubMed]
- May, T. Culture and Customs of Mongolia; ABC-CLIO: Santa Barbara, CA, USA, 2008. [Google Scholar]
- Bromage, S.; Ganmaa, D.; Rich-Edwards, J.W.; Rosner, B.; Bater, J.; Fawzi, W.W. Projected effectiveness of mandatory industrial fortification of wheat flour, milk, and edible oil with multiple micronutrients among Mongolian adults. PLoS ONE 2018, 13, e0201230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uush, T.; Fraser, D.R. Situation and Factors Associated with Rickets among Children in Mongolia; Public Health Institute Nutrition Research Center: Ulaanbaatar, Mongolia, 2003. [Google Scholar]
- Lander, R.L.; Enkhjargal, T.; Batjargal, J.; Bailey, K.B.; Diouf, S.; Green, T.J.; Skeaff, C.M.; Gibson, R.S. Multiple micronutrient deficiencies persist during early childhood in Mongolia. Asia Pac. J. Clin. Nutr. 2008, 17, 429–440. [Google Scholar] [CrossRef]
| Total (N = 9592) n (%) | |
|---|---|
| Age, years | |
| Mean (SD) | 9.41 (1.58) |
| Median [Min, Max] | 9.24 [5.84, 14.2] |
| Gender | |
| Female | 4764 (49.7%) |
| Male | 4828 (50.3%) |
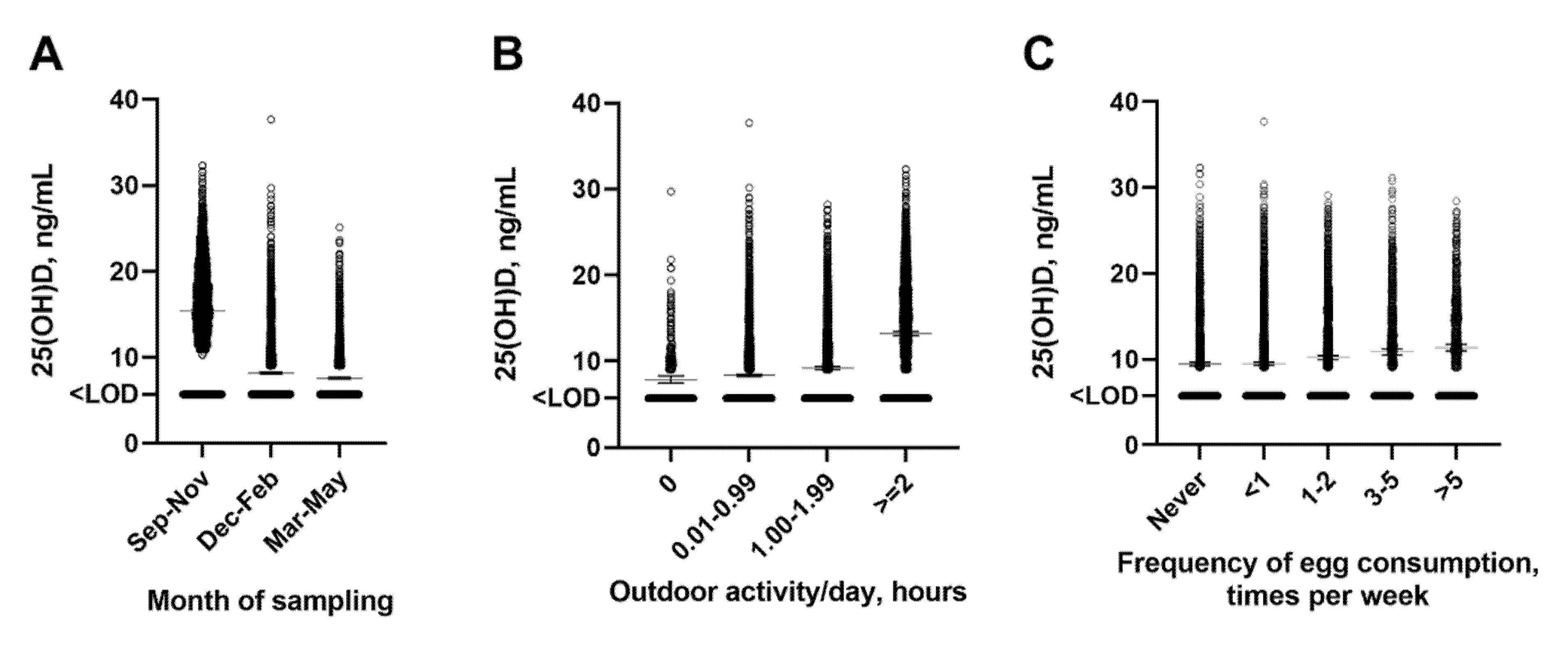
| Month of sampling | |
| Sep–Nov | 2589 (27.0%) |
| Dec–Feb | 4169 (43.5%) |
| March–May | 2834 (29.5%) |
| District | |
| Bayangol | 565 (5.9%) |
| Bayanzurkh | 2319 (24.2%) |
| Chingeltei | 1528 (15.9%) |
| Khan-Uul | 1085 (11.3%) |
| Songino-Khairkhan | 3430 (35.8%) |
| Sukhbaatar | 651 (6.8%) |
| Other | 14 (0.1%) |
| Highest level of parental education 1 | |
| University | 3202 (33.4%) |
| Technical/polytechnic/diploma | 469 (4.9%) |
| Secondary | 5104 (53.2%) |
| Primary | 482 (5.0%) |
| None | 335 (3.5%) |
| Household income quartile (USD) 2,3 | |
| Quartile 4 (3507–7015) | 3168 (33.0%) |
| Quartile 3 (245–3507) | 2111 (22.0%) |
| Quartile 2 (175–245) | 2337 (24.4%) |
| Quartile 1 (0–175) | 1976 (20.6%) |
| Type of accommodation | |
| Centrally Heated | 2219 (23.1%) |
| Not Centrally Heated | 3731 (38.9%) |
| Ger With Fence | 3197 (33.3%) |
| Ger Without Fence | 445 (4.6%) |
| Frequency of egg consumption | |
| Every day or almost every day | 841 (8.8%) |
| 3–5 times per week | 1107 (11.5%) |
| 1–2 times per week | 2250 (23.5%) |
| 1–4 times in past month | 3292 (34.3%) |
| None | 2102 (21.9%) |
| Frequency of liver/internal organs’ consumption | |
| None | 5002 (52.1%) |
| 1–4 times in past month | 3701 (38.6%) |
| 1–2 times per week | 653 (6.8%) |
| 3–5 times per week | 135 (1.4%) |
| Every day or almost every day | 101 (1.1%) |
| Frequency of red meat consumption | |
| Every day or almost every day | 9280 (96.7%) |
| 3–5 times per week | 153 (1.6%) |
| 1–2 times per week | 63 (0.7%) |
| 1–4 times in past month | 42 (0.4%) |
| None | 54 (0.6%) |
| Frequency of fish or seafood consumption | |
| Every day or almost every day | 78 (0.8%) |
| 3–5 times per week | 133 (1.4%) |
| 1–2 times per week | 582 (6.1%) |
| 1–4 times in past month | 2643 (27.6%) |
| None | 6156 (64.2%) |
| TB classification | |
| No TB | 8643 (90.1%) |
| Latent TB | 569 (5.9%) |
| Active TB | 270 (2.8%) |
| Indeterminate | 110 (1.1%) |
| Any smoking inside household | |
| No | 6084 (63.4%) |
| Yes | 3508 (36.6%) |
| Subject actively smoking | |
| No | 9544 (99.5%) |
| Yes | 48 (0.5%) |
| BMI-for-age Z-score | |
| ≤2.00 (underweight) | 114 (1.2%) |
| −2.00 to 2.00 (normal range) | 8938 (93.2%) |
| 2.01 to 3.00 (overweight) | 428 (4.5%) |
| >3.00 (obese) | 97 (1.0%) |
| Frequency of Outdoor Activity | |
| Greater than 2 h | 2628 (27.4%) |
| 1–2 h | 2934 (30.6%) |
| Less than 1 h | 3725 (38.8%) |
| None | 305 (3.2%) |
| Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| Proportion with 25(OH)D <10 ng/mL (%) | Crude OR 1 (95% CI) | Adjusted OR (95% CI) 2 | p | P for Trend | |
| Age (years) 3 | 1.07 (1.04, 1.09) | 1.09 (1.05,1.13) | <0.001 | ||
| 6.00–6.99 | 73/254 (28.7%) | N/A | N/A | ||
| 7.00–7.99 | 1099/1946 (56.5%) | N/A | N/A | ||
| 8.00–8.99 | 1325/2183 (60.7%) | N/A | N/A | ||
| 9.00–9.99 | 987/1658 (59.5%) | N/A | N/A | ||
| 10.00–10.99 | 1045/1625 (64.3%) | N/A | N/A | ||
| 11.00–11.99 | 900/1046 (86.0%) | N/A | N/A | ||
| 12.00–12.99 | 245/488 (50.2%) | N/A | N/A | ||
| 13.00–13.99 | 18/32 (56.3%) | N/A | N/A | ||
| Gender | |||||
| Male | 2792/4828 (57.8%) | Ref | Ref | ||
| Female | 2900/4764 (60.9%) | 1.13 (1.05, 1.23) | 1.23 (1.11,1.35) | <0.001 | |
| Month of sampling | |||||
| Sep–Nov | 452/2589 (17.5%) | Ref | Ref | ||
| Dec–Feb | 3030/4169 (72.7%) | 12.58 (11.14, 14.23) | 5.28 (4.53, 6.15) | <0.001 | |
| March–May | 2210/2834 (78.0%) | 16.74 (14.65, 19.18) | 14.85 (12.46, 17.74) | <0.001 | |
| District | |||||
| Bayangol | 110/565 (19.5%) | Ref | Ref | ||
| Bayanzurkh | 1707/2319 (73.6%) | 11.54 (9.22, 14.55) | 3.61 (2.80, 4.66) | <0.001 | |
| Chingeltei | 1171/1528 (76.6%) | 13.57 (10.71, 17.31) | 3.46 (2.65, 4.55) | <0.001 | |
| Khan-Uul | 266/1085 (24.5%) | 1.34 (1.05, 1.73) | 0.91 (0.7, 1.2) | 0.50 | |
| Songino-Khairkhan | 1963/3430 (57.2%) | 5.53 (4.46, 6.92) | 1.04 (0.8, 1.35) | 0.78 | |
| Sukhbaatar | 469/651 (72.0%) | 10.66 (8.178, 14.01) | 2.78 (2.06, 3.77) | <0.001 | |
| Other | 6/14 (42.9%) | 3.10 (1.00, 9.10) | 0.93(0.27, 3.2) | 0.91 | |
| Highest level of parental education | |||||
| University | 1564/3202 (48.8%) | Ref | Ref | ||
| Technical/polytechnic/diploma | 276/469 (58.8%) | 1.50 (1.23, 1.82) | 1.24 (0.97, 1.58) | 0.09 | <0.001 |
| Secondary | 3288/5104 (64.4%) | 1.90 (1.73, 2.07) | 1.36 (1.21, 1.52) | <0.001 | |
| Primary | 328/482 (68.0%) | 2.23 (1.82, 2.74) | 1.32 (1.04, 1.69) | 0.03 | |
| None | 236/335 (70.4%) | 2.49 (1.96, 3.20) | 1.48 (1.11, 1.99) | 0.01 | |
| Household income quartile | |||||
| Quartile 4 | 1577/3168 (49.8%) | Ref | Ref | ||
| Quartile 3 | 1275/2111 (60.4%) | 1.54 (1.38, 1.72) | N/A | N/A | N/A |
| Quartile 2 | 1502/2337 (64.3%) | 1.81 (1.63, 2.03) | N/A | N/A | |
| Quartile 1 | 1338/1976 (67.7%) | 2.12 (1.88 2. 40) | N/A | N/A | |
| Type of accommodation | |||||
| Centrally Heated | 963/2219 (43.4%) | Ref | Ref | ||
| Not Centrally Heated | 2357/3731 (63.2%) | 2.49 (2.02, 3.09) | N/A | N/A | N/A |
| Ger With Fence | 2080/3197 (65.1%) | 2.42 (2.17, 2.71) | N/A | N/A | |
| Ger Without Fence | 292/445 (65.6%) | 2.24 (2.01, 2.49) | N/A | N/A | |
| Frequency of egg consumption | |||||
| Every day or almost every day | 378/841 (44.9%) | Ref | Ref | ||
| 3–5 times per week | 569/1107 (51.4%) | 1.30 (1.08, 1.55) | 1.40 (1.13, 1.73) | <0.001 | <0.001 |
| 1–2 times per week | 1283/2250 (57.0%) | 1.63 (1.39, 1.91) | 1.54 (1.27, 1.86) | <0.001 | |
| 1–4 times in past month | 2101/3292 (63.8%) | 2.16 (1.85, 2.52) | 1.92 (1.60, 2.31) | <0.001 | |
| None | 1361/2102 (64.7%) | 2.25 (1.91, 2.65) | 1.98 (1.62, 2.41) | <0.001 | |
| Frequency of liver/internal organs consumption | |||||
| None | 2945/5002 (58.9%) | Ref | Ref | ||
| 1–4 times in past month | 2180/3701 (58.9%) | 1.00 (−0.92, 1.09) | N/A | N/A | N/A |
| 1–2 times per week | 408/653 (62.5%) | 1.16−0.98, 1.38) | N/A | N/A | |
| 3–5 times per week | 86/135 (63.7%) | 1.23 (−0.86, 1.76) | N/A | N/A | |
| Every day or almost every day | 73/101 (72.3%) | 1.82 (1.19, 2.87) | N/A | N/A | |
| Frequency of red meat consumption | |||||
| Every day or almost every day | 5511/9280 (59.4%) | Ref | Ref | ||
| 3–5 times per week | 89/153 (58.2%) | 0.95 (0.69, 1.32) | N/A | N/A | N/A |
| 1–2 times per week | 34/63 (54.0%) | 0.80 (0.49, 1.33) | N/A | N/A | |
| 1–4 times in past month | 26/42 (61.9%) | 1.11 (0.60, 2.12) | N/A | N/A | |
| None | 32/54 (59.3%) | 0.99 (0.58, 1.74) | N/A | N/A | |
| Frequency of fish or seafood consumption | |||||
| Every day or almost every day | 27/78 (34.6%) | Ref | Ref | ||
| 3–5 times per week | 58/133 (43.6%) | 1.46 (0.82, 2.63) | N/A | N/A | N/A |
| 1–2 times per week | 299/582 (51.4%) | 2.00 (1.23, 3.31) | N/A | N/A | |
| 1–4 times in past month | 1482/2643 (56.1%) | 2.41 (1.52, 3.92) | N/A | N/A | |
| None | 3826/6156 (62.2%) | 3.10 (1.96, 5.03) | N/A | N/A | |
| TB classification | |||||
| No TB | 5074/8643 (58.7%) | Ref | Ref | ||
| Latent TB | 364/569 (64.0%) | 1.25 (1.05, 1.49) | 0.99 (0.80, 1.23) | 0.93 | |
| Active TB | 194/270 (71.9%) | 1.80 (1.38, 2.36) | 1.40 (1.03, 1.94) | 0.04 | |
| Indeterminate | 60/110 (54.5%) | 0.84 (0.58, 1.23) | 0.75 (0.48, 1.2) | 0.22 | |
| Any smoking inside household | |||||
| No | 3454/6084 (56.8%) | Ref | Ref | ||
| Yes | 2238/3508 (63.8%) | 1.34 (1.23, 1.46) | 1.13 (1.02, 1.25) | 0.02 | |
| Subject actively smoking | |||||
| No | 5667/9544 (59.4%) | Ref | Ref | ||
| Yes | 25/48 (52.1%) | 0.74 (0.42, 1.31) | N/A | N/A | |
| BMI-for-age Z-score | |||||
| <−2.00 | 61/114 (53.5%) | Ref | Ref | ||
| −2.00 to 1.99 | 5332/8938 (59.7%) | 1.28 (0.88, 1.86) | N/A | N/A | N/A |
| 2.00 to 3.00 | 239/428 (55.8%) | 1.10 (0.72, 1.66) | N/A | N/A | |
| >3.00 | 51/97 (52.6%) | 0.96 (0.56, 1.66) | N/A | N/A | |
| Frequency of Daily Outdoor Activity | |||||
| Greater than 2 h | 932/2628 (35.5%) | Ref | Ref | ||
| 1–2 h | 1872/2934 (63.8%) | 3.21 (2.87, 3.58) | 1.50 (1.30, 1.72) | <0.001 | <0.001 |
| Less than 1 h | 2659/3725 (71.4%) | 4.54 (4.08, 5.05) | 1.68 (1.47, 1.93) | <0.001 | |
| None | 229/305 (75.1%) | 5.48 (4.20, 7.23) | 1.72 (1.27, 2.34) | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bater, J.; Bromage, S.; Jambal, T.; Tsendjav, E.; Lkhagvasuren, E.; Jutmann, Y.; Martineau, A.R.; Ganmaa, D. Prevalence and Determinants of Vitamin D Deficiency in 9595 Mongolian Schoolchildren: A Cross-Sectional Study. Nutrients 2021, 13, 4175. https://doi.org/10.3390/nu13114175
Bater J, Bromage S, Jambal T, Tsendjav E, Lkhagvasuren E, Jutmann Y, Martineau AR, Ganmaa D. Prevalence and Determinants of Vitamin D Deficiency in 9595 Mongolian Schoolchildren: A Cross-Sectional Study. Nutrients. 2021; 13(11):4175. https://doi.org/10.3390/nu13114175
Chicago/Turabian StyleBater, Jorick, Sabri Bromage, Tuyatsetseg Jambal, Enkhjargal Tsendjav, Enkhsaikhan Lkhagvasuren, Yanjmaa Jutmann, Adrian R. Martineau, and Davaasambuu Ganmaa. 2021. "Prevalence and Determinants of Vitamin D Deficiency in 9595 Mongolian Schoolchildren: A Cross-Sectional Study" Nutrients 13, no. 11: 4175. https://doi.org/10.3390/nu13114175
APA StyleBater, J., Bromage, S., Jambal, T., Tsendjav, E., Lkhagvasuren, E., Jutmann, Y., Martineau, A. R., & Ganmaa, D. (2021). Prevalence and Determinants of Vitamin D Deficiency in 9595 Mongolian Schoolchildren: A Cross-Sectional Study. Nutrients, 13(11), 4175. https://doi.org/10.3390/nu13114175







