Chickpea-Derived Prebiotic Substances Trigger Biofilm Formation by Bacillus subtilis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.1.1. Chickpea Milk Preparation
2.1.2. Preparation of the Prebiotic Stock Suspensions
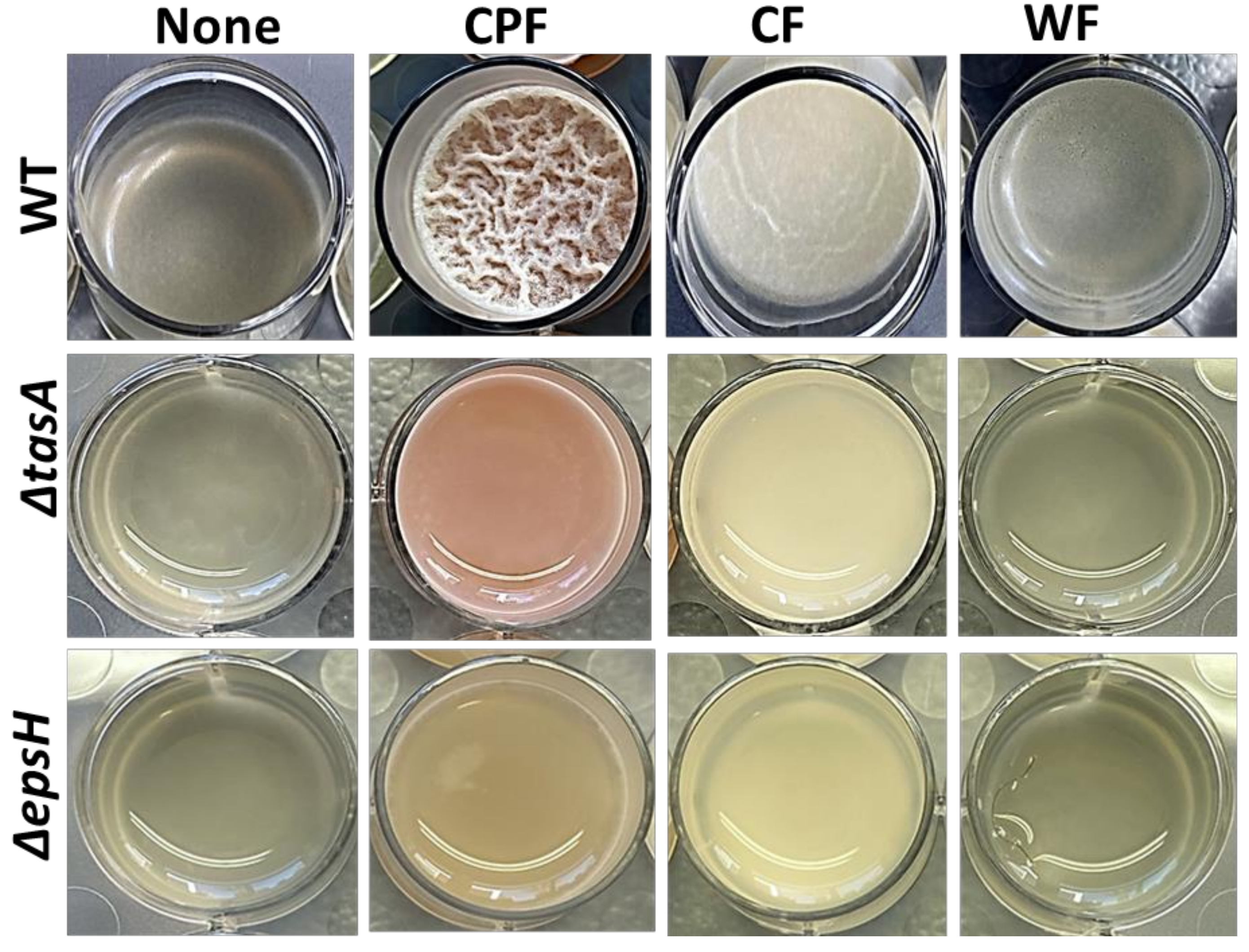
2.2. Macroscopic Assessment of Biofilm Formation in the Prebiotic Enriched Media
2.3. Growth Curve Analysis of B. subtilis in the Presence of Different Prebiotic Fibers
2.4. Determining the Effect of Prebiotic Supplementation on the Expression of Biofilm Matrix Operon, tapA, Using β-Galactosidase Assay
2.5. Visualizing the Impact of Prebiotics on Biofilm Formation Using Scanning Electron Microscopy
2.6. Analysis of Survival of B. subtilis Cells Transitioning through In Vitro System
2.7. Determining the Effect of CPF on the Generation of Pulcherrimin by B. subtilis
2.8. Statistical Analysis
3. Results
3.1. Chickpea Fibers Enhance Pellicle Formation and Pulcherrimin Production by Bacillus subtilis
3.2. Microscopic Characterization of the CPF-Triggered Biofilm Formation
3.3. Regulation of Matrix Gene Expression in the Presence of Prebiotic Substances
3.4. Adherence of B. subtilis to CPF Fibers Governs the Survivability of B. subtilis during In Vitro Digestion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Slavin, J.L. Position of the American Dietetic Association: Health Implications of Dietary Fiber. J. Am. Diet. Assoc. 2008, 108, 1716–1731. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary Fiber and Fiber-Rich By-Products of Food Processing: Characterisation, Technological Functionality, and Commercial Applications: A Review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Manthey, F.A.; Hareland, G.A.; Huseby, D.J. Soluble and Insoluble Dietary Fiber Content and Composition in Oat. Cereal Chem. 1999, 76, 417–420. [Google Scholar] [CrossRef]
- Salminen, S.; Bouley, C.; Boutron-Ruault, M.C.; Cummings, J.H.; Franck, A.; Gibson, G.R.; Isolauri, E.; Moreau, M.C.; Roberfroid, M.; Rowland, I. Functional Food Science and Gastrointestinal Physiology and Function. Br. J. Nutr. 1998, 80 (Suppl. S1), S147–S171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szlufman, C.; Shemesh, M. Role of Probiotic Bacilli in Developing Symbiotic Food: Challenges and Opportunities. Front. Microbiol. 2021, 12, 811. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of Gut Bacteria on Human Health and Diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Kopp-Hoolihan, L. Prophylactic and Therapeutic Uses of Probiotics: A Review. J. Am. Diet. Assoc. 2001, 101, 229–241. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Prebiotics, and probiotics: Are they functional foods? Am. J. Clin. Nutr. 2000, 71 (Suppl. S6), 1682S–1690S. [Google Scholar] [CrossRef]
- Ohigashi, S.; Sudo, K.; Kobayashi, D.; Takahashi, O.; Takahashi, T.; Asahara, T.; Nomoto, K.; Onodera, H. Changes of the Intestinal Microbiota, Short Chain Fatty Acids, and Fecal PH in Patients with Colorectal Cancer. Dig. Dis. Sci. 2013, 58, 1717–1726. [Google Scholar] [CrossRef]
- Pasvolsky, R.; Zakin, V.; Ostrova, I.; Shemesh, M. Butyric acid released during milk lipolysis triggers biofilm formation of Bacillus species. Int. J. Food Microbiol. 2014, 181, 19–27. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gupta, K.; Sharma, A.; Das, M.; Ansari, I.A.; Dwivedi, P.D. Health Risks and Benefits of Chickpea (Cicer Arietinum) Consumption. J. Agric. Food. Chem. 2017, 65, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Rachwa-Rosiak, D.; Nebesny, E.; Budryn, G. Chickpeas—composition, nutritional value, health benefits, application to bread and snacks: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1137–1145. [Google Scholar] [CrossRef]
- Kokare, C.R.; Chakraborty, S.; Khopade, A.N.; Mahadik, K.R. Biofilm: Importance and Applications. Indian J. Biotechnol. 2009, 8, 159–168. [Google Scholar]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I.; Alarcon Bacterial, E.I. Bacterial Biofilm Formation on Implantable Devices and Approaches to Its Treatment and Prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, C.; Rajasekharan, S.K.; Reifen, R.; Shemesh, M. Mitigating Milk-Associated Bacteria through Inducing Zinc Ions Antibiofilm Activity. Foods 2020, 9, 1094. [Google Scholar] [CrossRef]
- Yahav, S.; Berkovich, Z.; Ostrov, I.; Reifen, R.; Shemesh, M. Encapsulation of Beneficial Probiotic Bacteria in Extracellular Matrix from Biofilm-Forming Bacillus subtilis. Artif. Cells. Nanomed. Biotechnol. 2018, 46 (Suppl. S2), 974–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimelman, H.; Shemesh, M. Probiotic Bifunctionality of Bacillus Subtilis-Rescuing Lactic Acid Bacteria from Desiccation and Antagonizing Pathogenic Staphylococcus aureus. Microorganisms 2019, 7, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasekharan, S.K.; Paz-Aviram, T.; Galili, S.; Berkovich, Z.; Reifen, R.; Shemesh, M. Biofilm Formation onto Starch Fibres by Bacillus subtilis Governs Its Successful Adaptation to Chickpea Milk. Microb. Biotechnol. 2021, 14, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, M.; Chai, Y. A combination of glycerol and manganese promotes biofilm formation in Bacillus subtilis via the histidine kinase KinD signaling. J. Bacteriol. 2013, 195, 2747–2754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnaouteli, S.; Matoz-Fernandez, D.A.; Porter, M.; Kalamara, M.; Abbott, J.; MacPhee, C.E.; Davidson, F.A.; Stanley-Wall, N.R. Pulcherrimin Formation Controls Growth Arrest of the Bacillus subtilis Biofilm. Proc. Natl. Acad. Sci. USA 2019, 116, 13553–13562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thibodeau, S.A.; Fang, R.; Joung, J.K. High-throughput β-galactosidase assay for bacterial cell-based reporter systems. BioTechniques 2004, 36, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Oknin, H.; Steinberg, D.; Shemesh, M. Magnesium Ions Mitigate Biofilm Formation of Bacillus Species via Downregulation of Matrix Genes Expression. Front. Microbiol. 2015, 6, 907. [Google Scholar] [CrossRef] [Green Version]
- Pandit, S.; Fazilati, M.; Gaska, K.; Derouiche, A.; Nypelö, T.; Mijakovic, I.; Kádár, R. The Exo-Polysaccharide Component of Extracellular Matrix Is Essential for the Viscoelastic Properties of Bacillus Subtilis Biofilms. Int. J. Mol. Sci. 2020, 21, 6755. [Google Scholar] [CrossRef]
- Rubinstein, S.M.; Kolodkin-Gal, I.; Mcloon, A.; Chai, L.; Kolter, R.; Losick, R.; Weitz, D.A. Osmotic pressure can regulate matrix gene expression in Bacillus subtilis. Mol. Microbiol. 2012, 86, 426–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shemesh, M.; Pasvolsky, R.; Zakin, V. External pH is a cue for the behavioral switch that determines surface motility and biofilm formation of Alicyclobacillus acidoterrestris. J. Food Prot. 2014, 8, 1252–1440. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, M.; Kolter, R.; Losick, R. The biocide chlorine dioxide stimulates biofilm formation in Bacillus subtilis by activation of the histidine kinase KinC. J. Bacteriol. 2010, 92, 6352–6356. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoah, Y.S.; Rajasekharan, S.K.; Reifen, R.; Shemesh, M. Chickpea-Derived Prebiotic Substances Trigger Biofilm Formation by Bacillus subtilis. Nutrients 2021, 13, 4228. https://doi.org/10.3390/nu13124228
Amoah YS, Rajasekharan SK, Reifen R, Shemesh M. Chickpea-Derived Prebiotic Substances Trigger Biofilm Formation by Bacillus subtilis. Nutrients. 2021; 13(12):4228. https://doi.org/10.3390/nu13124228
Chicago/Turabian StyleAmoah, Yaa Serwaah, Satish Kumar Rajasekharan, Ram Reifen, and Moshe Shemesh. 2021. "Chickpea-Derived Prebiotic Substances Trigger Biofilm Formation by Bacillus subtilis" Nutrients 13, no. 12: 4228. https://doi.org/10.3390/nu13124228
APA StyleAmoah, Y. S., Rajasekharan, S. K., Reifen, R., & Shemesh, M. (2021). Chickpea-Derived Prebiotic Substances Trigger Biofilm Formation by Bacillus subtilis. Nutrients, 13(12), 4228. https://doi.org/10.3390/nu13124228






