Human Milk Oligosaccharides in Cord Blood Are Altered in Gestational Diabetes and Stimulate Feto-Placental Angiogenesis In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Human Milk Oligosaccharide Standards
2.3. HMO Isolation and Analysis by HPLC
2.4. Pooled HMO Preparations and Control Compounds Used in Functional Assays
2.5. Endotoxin Removal
2.6. Feto-Placental Endothelial Cells
2.7. Proliferation
2.8. Immunocytochemistry Staining for Phalloidin
2.9. The 2D In Vitro Network Formation Assay
2.10. The 3D Spheroid Sprouting Assay
2.11. Fibrin Angiogenesis Assay
2.12. Statistical Analysis
3. Results
3.1. HMOs in Cord Blood Are Altered in GDM
3.1.1. Cohort Characteristics
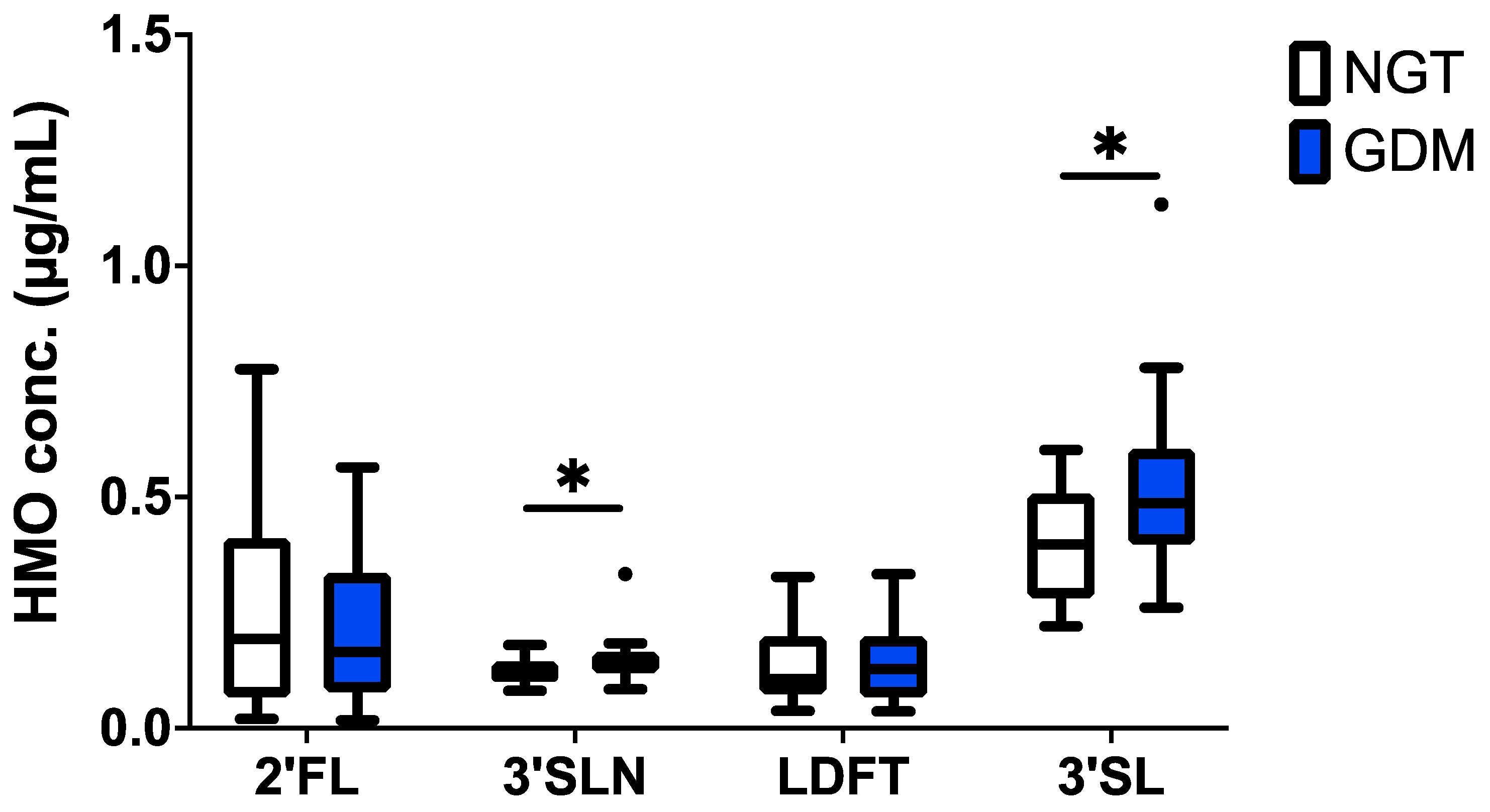
3.1.2. The 3′SL and 3′SLN Increased in GDM Cord Blood
3.1.3. Maternal Factors Are Associated with 3′SL in Cord Blood
3.2. HMOs Affect In Vitro Angiogenesis of fpECs
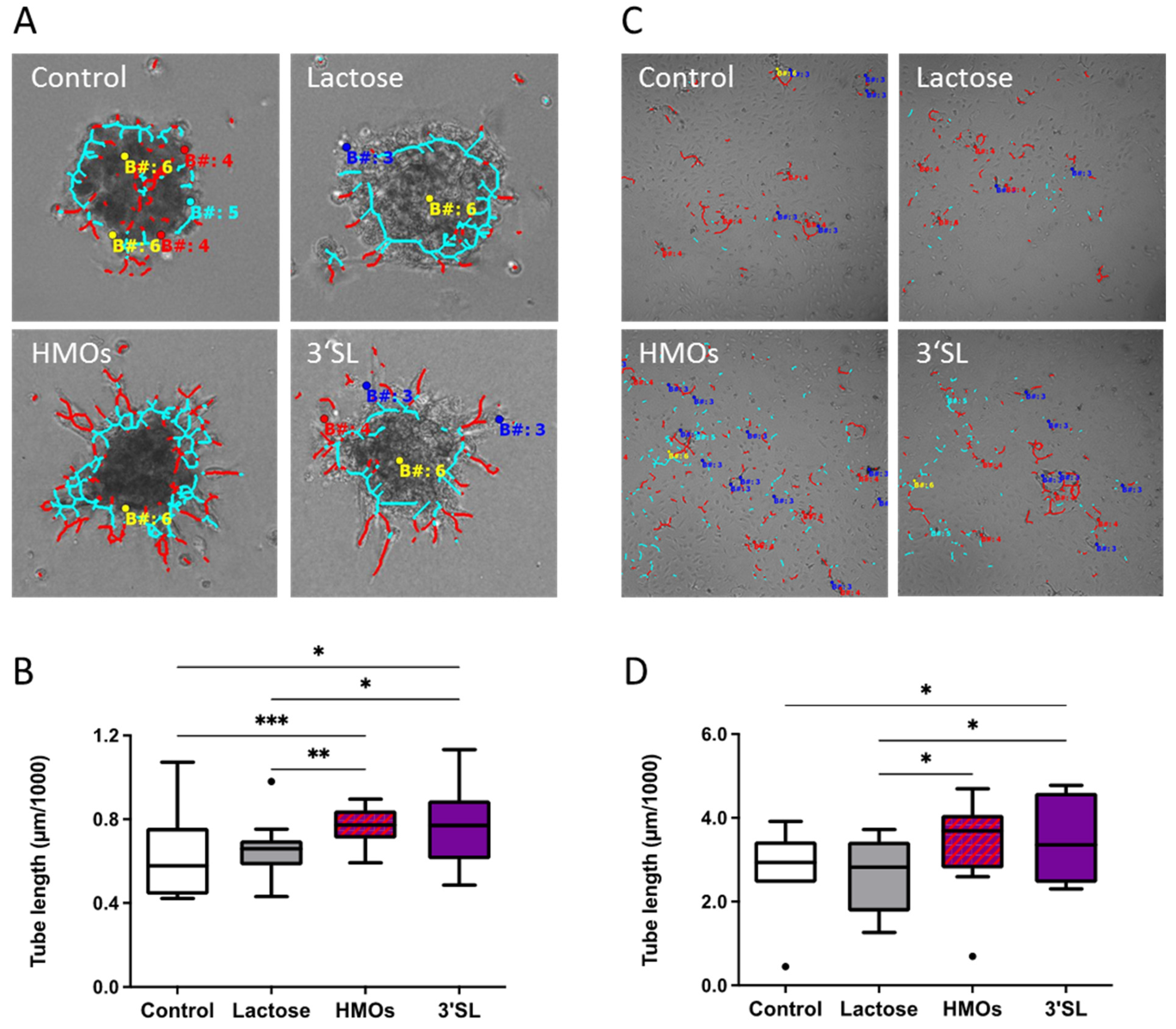
3.2.1. HMOs Stimulate Network Formation of fpECs
3.2.2. HMOs Increase Proliferation of fpECs
3.2.3. HMOs Alter Cytoskeleton Organization in fpECs
3.2.4. HMOs Stimulate In Vitro Angiogenesis of fpECs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HMO | Human milk oligosaccharide |
| 2′FL | 2′-Fucosyllactose |
| LDFT | lacto-difucotetraose |
| 3′SL | 3′-sialyllactose |
| 3′SLN | 3′-sialyllactosamine |
| 6′SLN | 6′-sialyllactosamine |
| LNT | lacto-N-tetraose |
| LNnT | lacto-N-neo-tetraose |
| LNFP | lacto-N-fucopentaose |
| LNDFH1 | lacto-N-difucohexaose 1 |
| LNH | lacto-N-hexaose |
| LST | sialyl-lacto-N-tetraose |
| DSLNT | disialyl-lacto-N-tetraose |
| fpEC | feto-placental endothelial cells |
| GDM | gestational diabetes mellitus |
| oGTT | oral glucose tolerance test |
References
- Bode, L.; Jantscher-Krenn, E. Structure-function relationships of human milk oligosaccharides. Adv. Nutr. 2012, 3, 383S–391S. [Google Scholar] [CrossRef]
- Stahl, B.; Thurl, S.; Henker, J.; Siegel, M.; Finke, B.; Sawatzki, G. Detection of four human milk groups with respect to Lewis-blood-group-dependent oligosaccharides by serologic and chromatographic analysis. Adv. Exp. Med. Biol. 2001, 501, 299–306. [Google Scholar]
- Mank, M.; Hauner, H.; Heck, A.J.R.; Stahl, B. Targeted LC-ESI-MS(2) characterization of human milk oligosaccharide diversity at 6 to 16 weeks post-partum reveals clear staging effects and distinctive milk groups. Anal. Bioanal. Chem. 2020, 412, 6887–6907. [Google Scholar] [CrossRef]
- Kunz, C.; Meyer, C.; Collado, M.C.; Geiger, L.; Garcia-Mantrana, I.; Bertua-Rios, B.; Martinez-Costa, C.; Borsch, C.; Rudloff, S. Influence of Gestational Age, Secretor, and Lewis Blood Group Status on the Oligosaccharide Content of Human Milk. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 789–798. [Google Scholar] [CrossRef]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.L.; Sims, C.R.; Abraham, A.; Bode, L.; Andres, A. Human Milk Oligosaccharide Concentrations and Infant Intakes Are Associated with Maternal Overweight and Obesity and Predict Infant Growth. Nutrients 2021, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martinez-Costa, C.; et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci. Rep. 2019, 9, 11767. [Google Scholar] [CrossRef] [PubMed]
- Isganaitis, E.; Venditti, S.; Matthews, T.J.; Lerin, C.; Demerath, E.W.; Fields, D.A. Maternal obesity and the human milk metabolome: Associations with infant body composition and postnatal weight gain. Am. J. Clin. Nutr. 2019, 110, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kurakevich, E.; Hennet, T.; Hausmann, M.; Rogler, G.; Borsig, L. Milk oligosaccharide sialyl(alpha2,3)lactose activates intestinal CD11c+ cells through TLR4. Proc. Natl. Acad. Sci. USA 2013, 110, 17444–17449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiwegger, T.; Stahl, B.; Schmitt, J.; Boehm, G.; Gerstmayr, M.; Pichler, J.; Dehlink, E.; Loibichler, C.; Urbanek, R.; Szepfalusi, Z. Human milk--derived oligosaccharides and plant-derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr. Res. 2004, 56, 536–540. [Google Scholar] [CrossRef] [Green Version]
- Bode, L.; Kunz, C.; Muhly-Reinholz, M.; Mayer, K.; Seeger, W.; Rudloff, S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb. Haemost. 2004, 92, 1402–1410. [Google Scholar] [CrossRef]
- Hong, P.; Ninonuevo, M.R.; Lee, B.; Lebrilla, C.; Bode, L. Human milk oligosaccharides reduce HIV-1-gp120 binding to dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN). Br. J. Nutr. 2009, 101, 482–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naarding, M.A.; Ludwig, I.S.; Groot, F.; Berkhout, B.; Geijtenbeek, T.B.; Pollakis, G.; Paxton, W.A. Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes. J. Clin. Investig. 2005, 115, 3256–3264. [Google Scholar] [CrossRef] [PubMed]
- Noll, A.J.; Gourdine, J.P.; Yu, Y.; Lasanajak, Y.; Smith, D.F.; Cummings, R.D. Galectins are human milk glycan receptors. Glycobiology 2016, 26, 655–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef] [Green Version]
- Bode, L.; Rudloff, S.; Kunz, C.; Strobel, S.; Klein, N. Human milk oligosaccharides reduce platelet-neutrophil complex formation leading to a decrease in neutrophil beta 2 integrin expression. J. Leukoc. Biol. 2004, 76, 820–826. [Google Scholar] [CrossRef]
- Rudloff, S.; Stefan, C.; Pohlentz, G.; Kunz, C. Detection of ligands for selectins in the oligosaccharide fraction of human milk. Eur. J. Nutr. 2002, 41, 85–92. [Google Scholar] [CrossRef]
- Schumacher, G.; Bendas, G.; Stahl, B.; Beermann, C. Human milk oligosaccharides affect P-selectin binding capacities: In vitro investigation. Nutrition 2006, 22, 620–627. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The human milk oligosaccharide 2’-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Kuntz, S.; Kunz, C.; Rudloff, S. Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. Br. J. Nutr. 2009, 101, 1306–1315. [Google Scholar] [CrossRef] [Green Version]
- Jantscher-Krenn, E.; Aigner, J.; Reiter, B.; Kofeler, H.; Csapo, B.; Desoye, G.; Bode, L.; van Poppel, M.N.M. Evidence of human milk oligosaccharides in maternal circulation already during pregnancy: A pilot study. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E347–E357. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; Treichler, C.; Brandl, W.; Schonbacher, L.; Kofeler, H.; van Poppel, M.N.M. The association of human milk oligosaccharides with glucose metabolism in overweight and obese pregnant women. Am. J. Clin. Nutr. 2019, 110, 1335–1343. [Google Scholar] [CrossRef]
- Zopf, D.; Ginsburg, V.; Hallgren, P.; Jonsson, A.; Lindberg, B.; Lundblad, A. Determination of Leb-active oligosaccharides in urine of pregnant and lactating women by radioimmunoassay. Eur. J. Biochem. 1979, 93, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, P.; Lindberg, B.; Lundblad, A. Quantitation of some urinary oligosaccharides during pregnancy and lactation. J. Biol. Chem. 1977, 252, 1034–1040. [Google Scholar] [CrossRef]
- Pausan, M.R.; Kolovetsiou-Kreiner, V.; Richter, G.L.; Madl, T.; Giselbrecht, E.; Obermayer-Pietsch, B.; Weiss, E.C.; Jantscher-Krenn, E.; Moissl-Eichinger, C. Human Milk Oligosaccharides Modulate the Risk for Preterm Birth in a Microbiome-Dependent and -Independent Manner. mSystems 2020, 5, e00334-20. [Google Scholar] [CrossRef]
- Catalano, P.M.; Ehrenberg, H.M. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006, 113, 1126–1133. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef]
- Biri, A.; Onan, A.; Devrim, E.; Babacan, F.; Kavutcu, M.; Durak, I. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta 2006, 27, 327–332. [Google Scholar] [CrossRef]
- Dabelea, D.; Hanson, R.L.; Lindsay, R.S.; Pettitt, D.J.; Imperatore, G.; Gabir, M.M.; Roumain, J.; Bennett, P.H.; Knowler, W.C. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: A study of discordant sibships. Diabetes 2000, 49, 2208–2211. [Google Scholar] [CrossRef] [Green Version]
- Vambergue, A.; Fajardy, I. Consequences of gestational and pregestational diabetes on placental function and birth weight. World J. Diabetes 2011, 2, 196–203. [Google Scholar] [CrossRef]
- Yessoufou, A.; Moutairou, K. Maternal diabetes in pregnancy: Early and long-term outcomes on the offspring and the concept of “metabolic memory”. Exp. Diabetes Res. 2011, 2011, 218598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, A.; Lawlor, D.A. Long-term health outcomes in offspring born to women with diabetes in pregnancy. Curr. Diabetes Rep. 2014, 14, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschmugl, B.; Brandl, W.; Csapo, B.; van Poppel, M.; Kofeler, H.; Desoye, G.; Wadsack, C.; Jantscher-Krenn, E. Evidence of Human Milk Oligosaccharides in Cord Blood and Maternal-to-Fetal Transport across the Placenta. Nutrients 2019, 11, 2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leach, L. Placental vascular dysfunction in diabetic pregnancies: Intimations of fetal cardiovascular disease? Microcirculation 2011, 18, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, T.M.; Sorensen, F.B.; Klebe, J.G.; Jackson, M.R. Growth and maturation of villi in placentae from well-controlled diabetic women. Placenta 1994, 15, 57–65. [Google Scholar] [CrossRef]
- Lao, T.T.; Lee, C.P.; Wong, W.M. Placental weight to birthweight ratio is increased in mild gestational glucose intolerance. Placenta 1997, 18, 227–230. [Google Scholar] [CrossRef]
- Jirkovska, M.; Kubinova, L.; Janacek, J.; Moravcova, M.; Krejci, V.; Karen, P. Topological properties and spatial organization of villous capillaries in normal and diabetic placentas. J. Vasc. Res. 2002, 39, 268–278. [Google Scholar] [CrossRef]
- Lassance, L.; Miedl, H.; Absenger, M.; Diaz-Perez, F.; Lang, U.; Desoye, G.; Hiden, U. Hyperinsulinemia stimulates angiogenesis of human fetoplacental endothelial cells: A possible role of insulin in placental hypervascularization in diabetes mellitus. J. Clin. Endocrinol. Metab. 2013, 98, E1438–E1447. [Google Scholar] [CrossRef] [Green Version]
- Sobrevia, L.; Abarzua, F.; Nien, J.K.; Salomon, C.; Westermeier, F.; Puebla, C.; Cifuentes, F.; Guzman-Gutierrez, E.; Leiva, A.; Casanello, P. Review: Differential placental macrovascular and microvascular endothelial dysfunction in gestational diabetes. Placenta 2011, 32 (Suppl. 2), S159–S164. [Google Scholar] [CrossRef]
- Gauster, M.; Desoye, G.; Totsch, M.; Hiden, U. The placenta and gestational diabetes mellitus. Curr. Diabetes Rep. 2012, 12, 16–23. [Google Scholar] [CrossRef]
- Desoye, G.; Wells, J.C.K. Pregnancies in Diabetes and Obesity: The Capacity-Load Model of Placental Adaptation. Diabetes 2021, 70, 823–830. [Google Scholar] [CrossRef]
- Jantscher-Krenn, E.; Zherebtsov, M.; Nissan, C.; Goth, K.; Guner, Y.S.; Naidu, N.; Choudhury, B.; Grishin, A.V.; Ford, H.R.; Bode, L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012, 61, 1417–1425. [Google Scholar] [CrossRef]
- Jantscher-Krenn, E.; Marx, C.; Bode, L. Human milk oligosaccharides are differentially metabolised in neonatal rats. Br. J. Nutr. 2013, 110, 640–650. [Google Scholar] [CrossRef] [Green Version]
- Gnoth, M.J.; Kunz, C.; Rudloff, S. Endotoxin-reduced milk oligosaccharide fractions suitable for cell biological studies. Eur. J. Med. Res. 2000, 5, 468–472. [Google Scholar]
- Lang, I.; Schweizer, A.; Hiden, U.; Ghaffari-Tabrizi, N.; Hagendorfer, G.; Bilban, M.; Pabst, M.A.; Korgun, E.T.; Dohr, G.; Desoye, G. Human fetal placental endothelial cells have a mature arterial and a juvenile venous phenotype with adipogenic and osteogenic differentiation potential. Differentiation 2008, 76, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J. Vis. Exp. 2011, 51, 2720. [Google Scholar] [CrossRef]
- di Blasio, L.; Bussolino, F.; Primo, L. Three-dimensional in vitro assay of endothelial cell invasion and capillary tube morphogenesis. Methods Mol. Biol. 2015, 1214, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Koolwijk, P.; van Erck, M.G.; de Vree, W.J.; Vermeer, M.A.; Weich, H.A.; Hanemaaijer, R.; van Hinsbergh, V.W. Cooperative effect of TNFalpha, bFGF, and VEGF on the formation of tubular structures of human microvascular endothelial cells in a fibrin matrix. Role of urokinase activity. J. Cell Biol. 1996, 132, 1177–1188. [Google Scholar] [CrossRef]
- Leopold, B.; Strutz, J.; Weiss, E.; Gindlhuber, J.; Birner-Gruenberger, R.; Hackl, H.; Appel, H.M.; Cvitic, S.; Hiden, U. Outgrowth, proliferation, viability, angiogenesis and phenotype of primary human endothelial cells in different purchasable endothelial culture media: Feed wisely. Histochem. Cell Biol. 2019, 152, 377–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Ruiz, M.; Fulton, D.; Sowa, G.; Languino, L.R.; Fujio, Y.; Walsh, K.; Sessa, W.C. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ. Res. 2000, 86, 892–896. [Google Scholar] [CrossRef]
- Manhardt, C.T.; Punch, P.R.; Dougher, C.W.L.; Lau, J.T.Y. Extrinsic sialylation is dynamically regulated by systemic triggers in vivo. J. Biol. Chem. 2017, 292, 13514–13520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cvitic, S.; Longtine, M.S.; Hackl, H.; Wagner, K.; Nelson, M.D.; Desoye, G.; Hiden, U. The human placental sexome differs between trophoblast epithelium and villous vessel endothelium. PLoS ONE 2013, 8, e79233. [Google Scholar] [CrossRef]
- Blois, S.M.; Conrad, M.L.; Freitag, N.; Barrientos, G. Galectins in angiogenesis: Consequences for gestation. J. Reprod. Immunol. 2015, 108, 33–41. [Google Scholar] [CrossRef]
- Murad, S. Toll-like receptor 4 in inflammation and angiogenesis: A double-edged sword. Front. Immunol. 2014, 5, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mura, M.; Swain, R.K.; Zhuang, X.; Vorschmitt, H.; Reynolds, G.; Durant, S.; Beesley, J.F.; Herbert, J.M.; Sheldon, H.; Andre, M.; et al. Identification and angiogenic role of the novel tumor endothelial marker CLEC14A. Oncogene 2012, 31, 293–305. [Google Scholar] [CrossRef] [Green Version]
- van Nieuw Amerongen, G.P.; Koolwijk, P.; Versteilen, A.; van Hinsbergh, V.W. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Simons, M.; Alitalo, K.; Annex, B.H.; Augustin, H.G.; Beam, C.; Berk, B.C.; Byzova, T.; Carmeliet, P.; Chilian, W.; Cooke, J.P.; et al. State-of-the-Art Methods for Evaluation of Angiogenesis and Tissue Vascularization: A Scientific Statement from the American Heart Association. Circ. Res. 2015, 116, e99–e132. [Google Scholar] [CrossRef] [Green Version]
- Bae, B.; Kim, H.; Park, H.; Koh, Y.J.; Bae, S.J.; Ha, K.T. Anti-Angiogenic Property of Free Human Oligosaccharides. Biomolecules 2021, 11, 775. [Google Scholar] [CrossRef]
- Farhadihosseinabadi, B.; Gholipourmalekabadi, M.; Salimi, M.; Abdollahifar, M.A.; Bagheri, M.; Samadikuchaksaraei, A.; Ghanbarian, H.; Mozafari, M.; Kazemi, B.; Niknejad, H. The in vivo effect of Lacto-N-neotetraose (LNnT) on the expression of type 2 immune response involved genes in the wound healing process. Sci. Rep. 2020, 10, 997. [Google Scholar] [CrossRef]
- Farhadihosseinabadi, B.; Salimi, M.; Kazemi, B.; Samadikuchaksaraei, A.; Ghanbarian, H.; Mozafari, M.; Niknejad, H. Inducing type 2 immune response, induction of angiogenesis, and anti-bacterial and anti-inflammatory properties make Lacto-n-Neotetraose (LNnT) a therapeutic choice to accelerate the wound healing process. Med. Hypotheses 2020, 134, 109389. [Google Scholar] [CrossRef]
- Halloran, M.M.; Carley, W.W.; Polverini, P.J.; Haskell, C.J.; Phan, S.; Anderson, B.J.; Woods, J.M.; Campbell, P.L.; Volin, M.V.; Backer, A.E.; et al. Ley/H: An endothelial-selective, cytokine-inducible, angiogenic mediator. J. Immunol. 2000, 164, 4868–4877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.; Amin, M.A.; Zha, Y.; Harlow, L.A.; Koch, A.E. Mechanism by which H-2g, a glucose analog of blood group H antigen, mediates angiogenesis. Blood 2005, 105, 2343–2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdijk, O.; van Neerven, R.J.J.; Meijer, B.; Savelkoul, H.F.J.; Brugman, S. Induction of human tolerogenic dendritic cells by 3’-sialyllactose via TLR4 is explained by LPS contamination. Glycobiology 2018, 28, 126–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Teraoka, M.; Nishi, N.; Nakakita, S.; Nakamura, T.; Hirashima, M.; Kamitori, S. X-ray structures of human galectin-9 C-terminal domain in complexes with a biantennary oligosaccharide and sialyllactose. J. Biol. Chem. 2010, 285, 36969–36976. [Google Scholar] [CrossRef] [Green Version]
- Chung, T.W.; Kim, E.Y.; Kim, S.J.; Choi, H.J.; Jang, S.B.; Kim, K.J.; Ha, S.H.; Abekura, F.; Kwak, C.H.; Kim, C.H.; et al. Sialyllactose suppresses angiogenesis by inhibiting VEGFR-2 activation, and tumor progression. Oncotarget 2017, 8, 58152–58162. [Google Scholar] [CrossRef] [Green Version]
- Chung, T.W.; Kim, E.Y.; Choi, H.J.; Han, C.W.; Jang, S.B.; Kim, K.J.; Jin, L.; Koh, Y.J.; Ha, K.T. 6’-Sialylgalactose inhibits vascular endothelial growth factor receptor 2-mediated angiogenesis. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Cvitic, S.; Desoye, G.; Hiden, U. Glucose, insulin, and oxygen interplay in placental hypervascularisation in diabetes mellitus. BioMed Res. Int. 2014, 2014, 145846. [Google Scholar] [CrossRef]
- Ensenauer, R.; Brandlhuber, L.; Burgmann, M.; Sobotzki, C.; Zwafink, C.; Anzill, S.; Holdt, L.; Teupser, D.; Hasbargen, U.; Netz, H.; et al. Obese Nondiabetic Pregnancies and High Maternal Glycated Hemoglobin at Delivery as an Indicator of Offspring and Maternal Postpartum Risks: The Prospective PEACHES Mother-Child Cohort. Clin. Chem. 2015, 61, 1381–1390. [Google Scholar] [CrossRef] [Green Version]



| Maternal and Infant Characteristics | NGT Pregnancies n = 25 | GDM Pregnancies n = 26 | p | ||
|---|---|---|---|---|---|
| Maternal age (years) | 28.5 | 4.5 | 33.3 | 5.5 | 0.001 |
| Height (cm) | 167.0 | 6.8 | 164.8 | 4.4 | 0.16 |
| Weight pre-pregnancy (kg) | 81.1 | 18.7 | 79.3 | 17.1 | 0.71 |
| BMI pre-pregnancy (kg/m2) | 28.9 | 5.8 | 29.2 | 6.2 | 0.88 |
| BMI category pre-pregnancy (n, %) | |||||
| Normal weight (BMI < 25) | 7 | 28% | 8 | 30% | 0.54 |
| Overweight (BMI 25–30) | 7 | 28% | 4 | 15% | |
| Obese (BMI > 30) | 11 | 44% | 14 | 54% | |
| BMI at delivery (kg/m2) | 33.5 | 5.5 | 31.7 | 5.5 | 0.31 |
| oGTT 0 h (mg/dL) | 84 | 4.5 | 98 | 10.9 | <0.0001 |
| oGTT 1 h (mg/dL) | 131 | 26.6 | 167 | 41.8 | 0.0011 |
| oGTT 2 h (mg/dL) | 104 | 18.5 | 132 | 23.2 | <0.0001 |
| Gestational age at birth (days) | 274.2 | 7.5 | 275.8 | 7.1 | 0.46 |
| Infant weight (g) | 3482 | 529 | 3414 | 400 | 0.61 |
| Infant length (cm) | 51.0 | 2.1 | 50.0 | 4.2 | 0.31 |
| Ponderal index (kg/m3) | 26.2 | 2.3 | 28.5 | 10.3 | 0.28 |
| Placental weight (g) | 662 | 107 | 589 | 118 | 0.027 |
| Infant sex (n male; %) | 16 | 64% | 15 | 59% | 0.78 |
| Delivery mode (n primary C-section, %) | 18 | 72% | 16 | 62% | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoch, D.; Brandl, W.; Strutz, J.; Köfeler, H.C.; van Poppel, M.N.M.; Bode, L.; Hiden, U.; Desoye, G.; Jantscher-Krenn, E. Human Milk Oligosaccharides in Cord Blood Are Altered in Gestational Diabetes and Stimulate Feto-Placental Angiogenesis In Vitro. Nutrients 2021, 13, 4257. https://doi.org/10.3390/nu13124257
Hoch D, Brandl W, Strutz J, Köfeler HC, van Poppel MNM, Bode L, Hiden U, Desoye G, Jantscher-Krenn E. Human Milk Oligosaccharides in Cord Blood Are Altered in Gestational Diabetes and Stimulate Feto-Placental Angiogenesis In Vitro. Nutrients. 2021; 13(12):4257. https://doi.org/10.3390/nu13124257
Chicago/Turabian StyleHoch, Denise, Waltraud Brandl, Jasmin Strutz, Harald C. Köfeler, Mireille N. M. van Poppel, Lars Bode, Ursula Hiden, Gernot Desoye, and Evelyn Jantscher-Krenn. 2021. "Human Milk Oligosaccharides in Cord Blood Are Altered in Gestational Diabetes and Stimulate Feto-Placental Angiogenesis In Vitro" Nutrients 13, no. 12: 4257. https://doi.org/10.3390/nu13124257
APA StyleHoch, D., Brandl, W., Strutz, J., Köfeler, H. C., van Poppel, M. N. M., Bode, L., Hiden, U., Desoye, G., & Jantscher-Krenn, E. (2021). Human Milk Oligosaccharides in Cord Blood Are Altered in Gestational Diabetes and Stimulate Feto-Placental Angiogenesis In Vitro. Nutrients, 13(12), 4257. https://doi.org/10.3390/nu13124257










