Differential and Synergistic Effects of Low Birth Weight and Western Diet on Skeletal Muscle Vasculature, Mitochondrial Lipid Metabolism and Insulin Signaling in Male Guinea Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Handling
2.3. Analysis of Growth and Food Intake
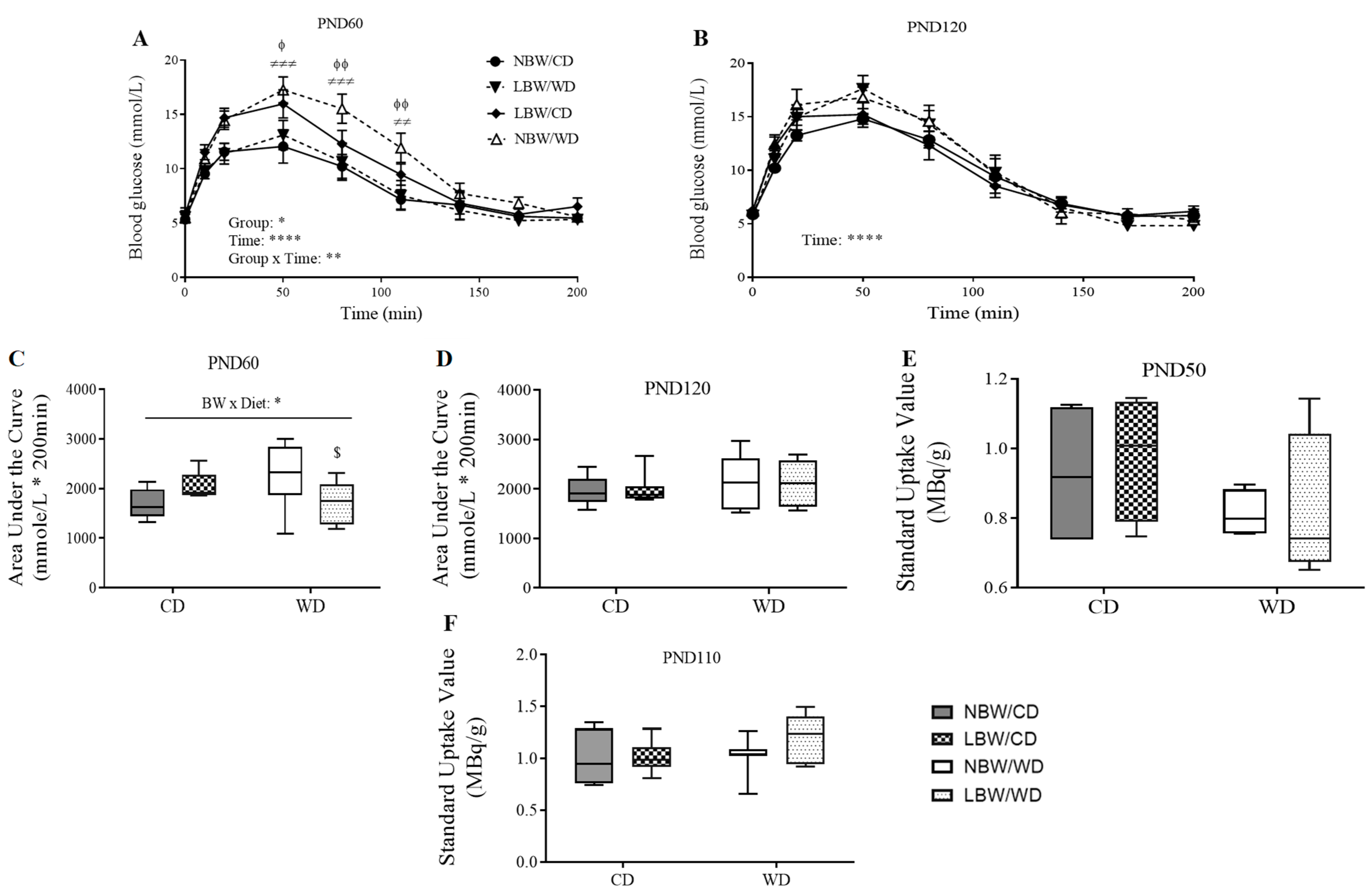
2.4. Assessment of Whole-Body Glucose Tolerance
2.5. Assessment of Skeletal Muscle Glucose Uptake
2.6. Tissue Collection
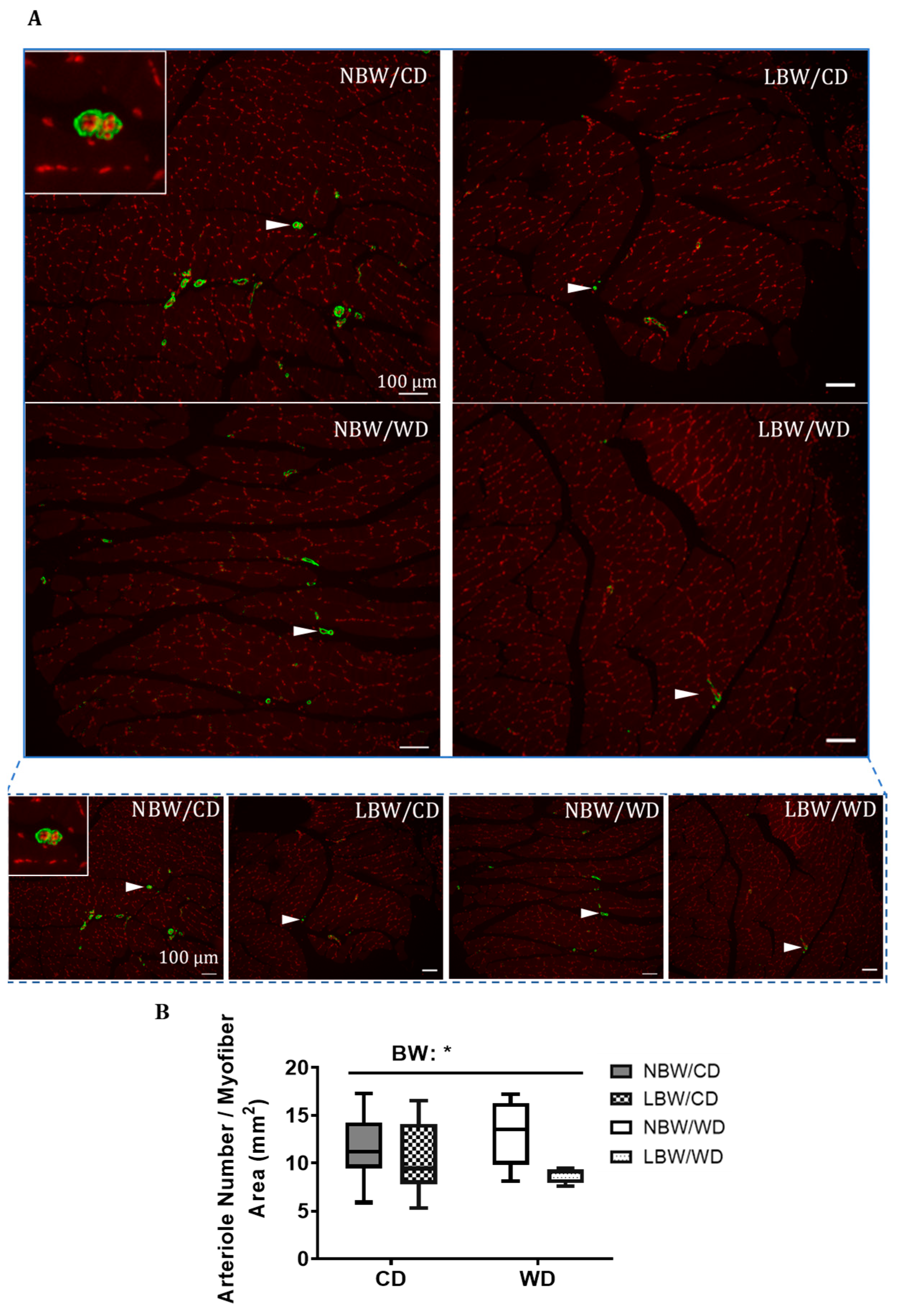
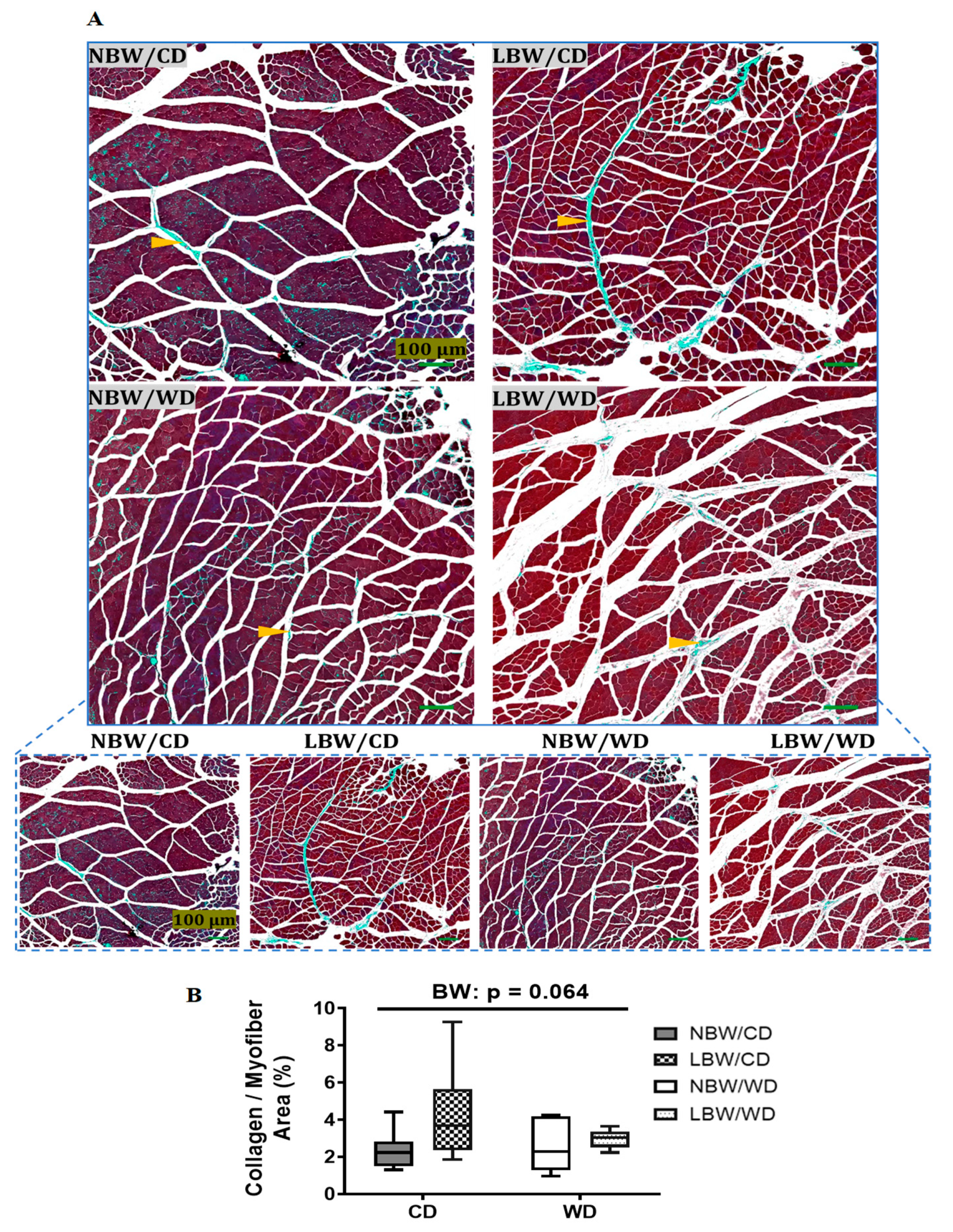
2.7. Determination of Fatty Infiltration, Arteriole Density, and Interstitial Fibrosis in Skeletal Muscle
2.8. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.9. Protein Extraction and Immunoblotting
2.10. Thin Liquid Chromatography and Gas Chromatography
2.11. Extraction and LC-MS/MS Method for the Analysis of Acylcarnitines and Amino Acids
2.12. Statistical Analyses
3. Results
3.1. Characteristics at Birth and Growth Performance during the Lactation Period
3.2. Post-Weaning Growth Performance, Energy Intake, and Body Composition
3.3. Muscle Arteriole Density, Interstitial Fibrosis, and Fatty Infiltration
3.4. Wholebody and Muscle-Specific Glucose Homeostasis
3.5. Skeletal Muscle Lipid Classes and Fatty Acid Profile
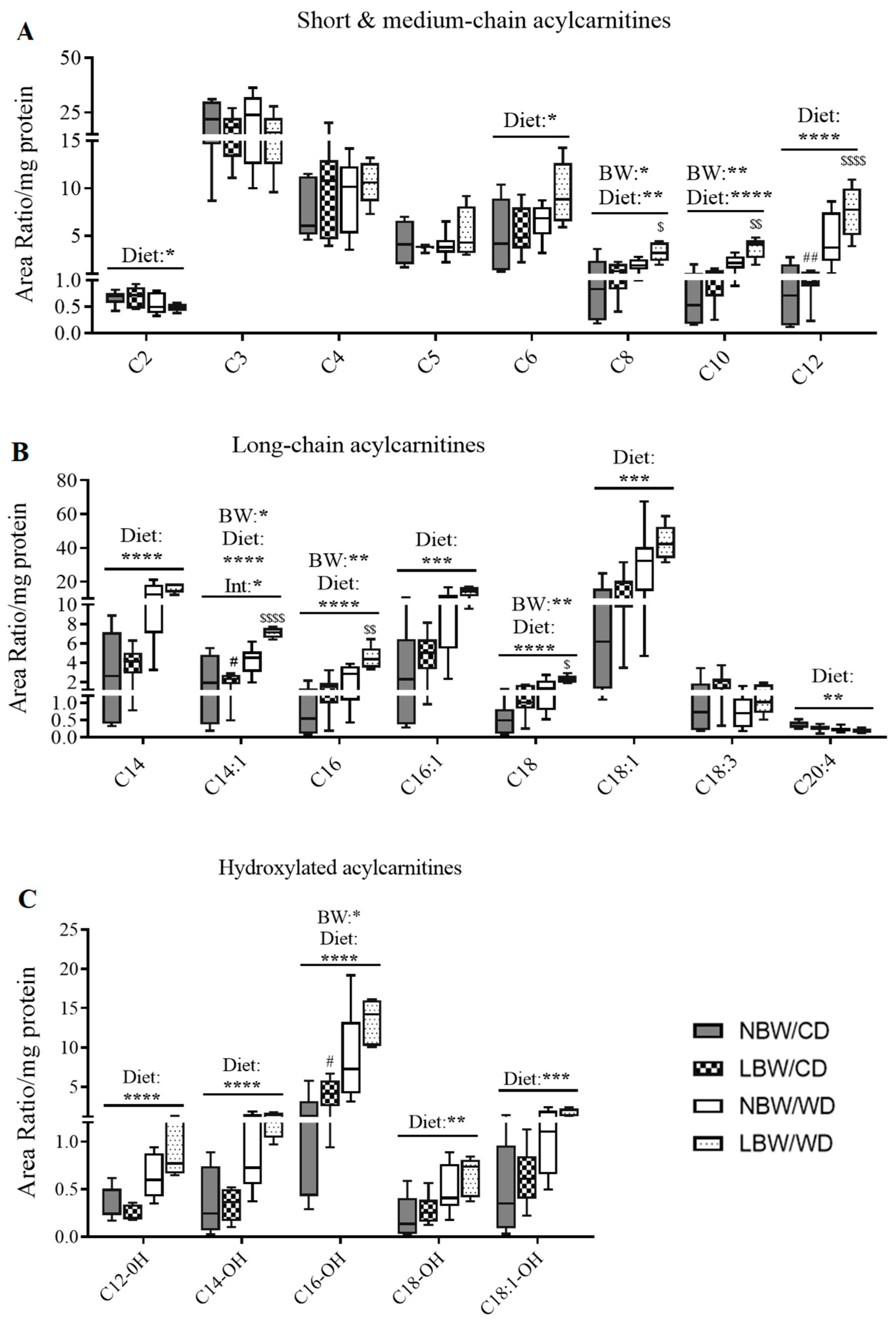
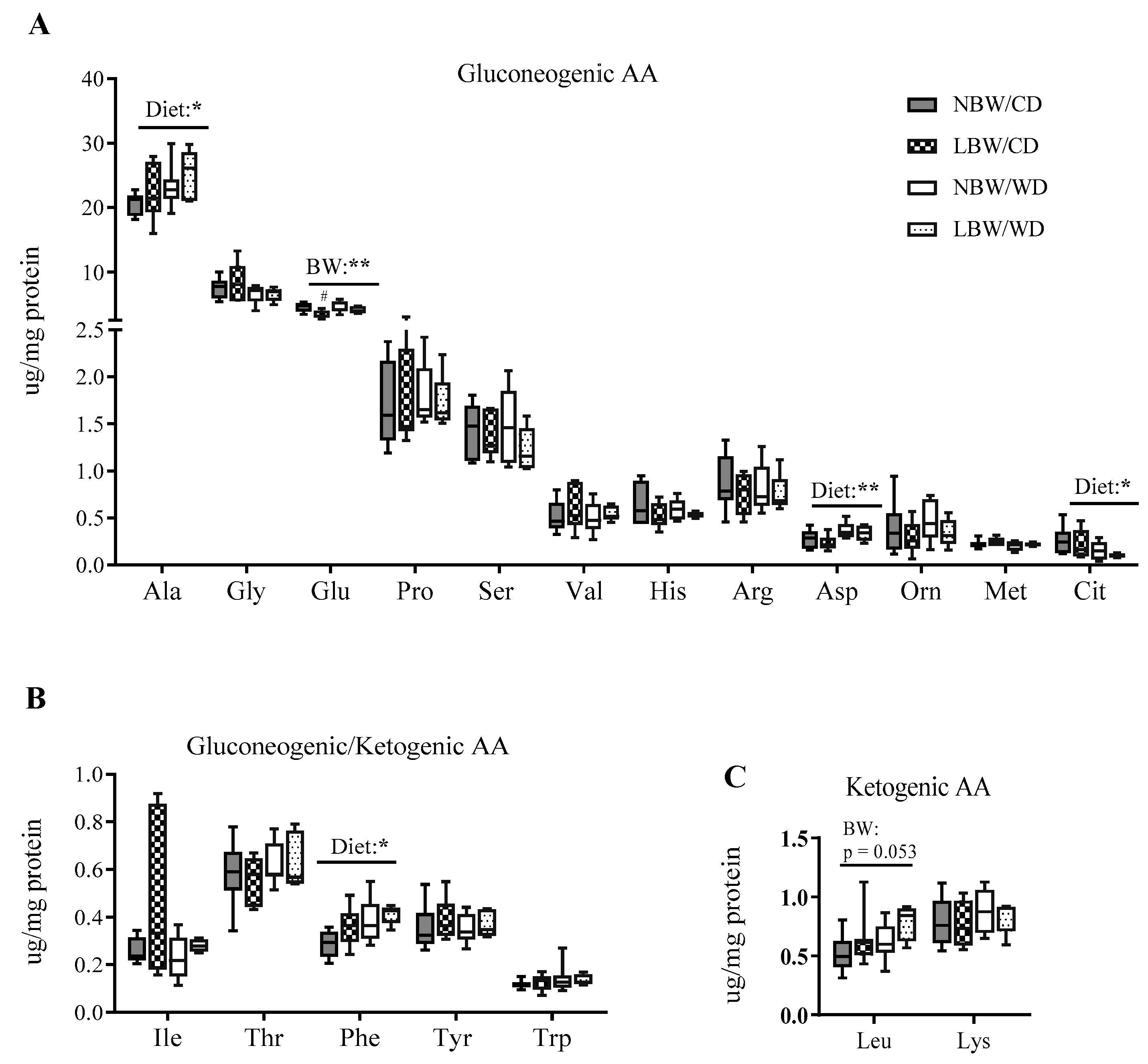
3.6. Markers of Mitochondrial Overload and Altered Amino Acid Profile in Muscle of WD-fed and LBW Offspring
3.7. Principle Components Analysis (PCA) of Muscle Acylcarnitines and Amino Acids
3.8. Skeletal Muscle Insulin Signaling Signature
3.9. Expression of Genes Involved in Mitochondrial β-Oxidation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, J.A.; Lee, J.H.; Lim, S.Y.; Ha, H.S.; Kwon, H.S.; Park, Y.M.; Lee, W.C.; Kang, M.I.; Yim, H.W.; Yoon, K.H.; et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J. Diabetes Investig. 2013, 4, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Standl, E. Aetiology and consequences of the metabolic syndrome. Eur. Heart J. Suppl. 2005, 7, D10–D13. [Google Scholar] [CrossRef][Green Version]
- Brøns, C.; Jensen, C.B.; Storgaard, H.; Alibegovic, A.; Jacobsen, S.; Nilsson, E.; Astrup, A.; Quistorff, B.; Vaag, A. Mitochondrial function in skeletal muscle is normal and unrelated to insulin action in young men born with low birth weight. J. Clin. Endocrinol. Metab. 2008, 93, 3885–3892. [Google Scholar] [CrossRef]
- Szendroedi, J.; Schmid, A.I.; Chmelik, M.; Toth, C.; Brehm, A.; Krssak, M.; Nowotny, P.; Wolzt, M.; Waldhausl, W.; Roden, M. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med. 2007, 4, 0858–0867. [Google Scholar] [CrossRef]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.B.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Aguer, C.; McCoin, C.S.; Knotts, T.A.; Thrush, A.B.; Ono-Moore, K.; McPherson, R.; Dent, R.; Hwang, D.H.; Adams, S.H.; Harper, M.E. Acylcarnitines: Potential implications for skeletal muscle insulin resistance. FASEB J. 2015, 29, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.; Krssak, M.; Bernroider, E.; Anderwald, C.; Brehm, A.; Meyerspeer, M.; Nowotny, P.; Roth, E.; Waldhäusl, W.; Roden, M. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 2002, 51, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Flati, V.; Caliaro, F.; Speca, S.; Corsetti, G.; Cardile, A.; Nisoli, E.; Bottinelli, R.; D’Antona, G. Essential amino acids improve insulin activation of akt/mtor signaling in soleus muscle of aged rats. Int. J. Immunopathol. Pharmacol. 2010, 23, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E.; Brambilla, E.; Luzi, L.; Landaker, E.J.; Kahn, C.R. Bidirectional modulation of insulin action by amino acids. J. Clin. Investig. 1998, 101, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Serné, E.H.; De Jongh, R.T.; Eringa, E.C.; IJzerman, R.G.; Stehouwer, C.D.A. Microvascular dysfunction: A potential pathophysiological role in the metabolic syndrome. Hypertension 2007, 50, 204–211. [Google Scholar] [CrossRef]
- Manrique, C.; Lastra, G.; Sowers, J.R. New insights into insulin action and resistance in the vasculature. Ann. N. Y. Acad. Sci. 2014, 1311, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Vasileska, A.; Rechkoska, G. Global and Regional Food Consumption Patterns and Trends. Procedia-Soc. Behav. Sci. 2012, 44, 363–369. [Google Scholar] [CrossRef]
- Sami, W.; Ansari, T.; Butt, N.S.; Rashid, M.; Hamid, A. Effect of Diet Counseling on Type 2 Diabetes Mellitus. Int. J. Sci. Technol. Res. 2017, 4, 112–118. [Google Scholar]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Fazakerley, D.J.; Krycer, J.R.; Kearney, A.L.; Hocking, S.L.; James, D.E. Muscle and adipose tissue insulin resistance: Malady without mechanism? J. Lipid Res. 2019, 60, 1720–1732. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.S.; Kang, L.; Wasserman, D.H. The extracellular matrix and insulin resistance. Trends Endocrinol. Metab. 2015, 26, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef]
- Barker, D.J.P.; Martyn, C.N.; Osmond, C.; Hales, C.N.; Fall, C.H.D. Growth in utero and serum cholesterol concentrations in adult life. BMJ 1993, 307, 1524–1527. [Google Scholar] [CrossRef]
- Ozanne, S.E.; Hales, C.N. Early programming of glucose-insulin metabolism. Trends Endocrinol. Metab. 2002, 13, 368–373. [Google Scholar] [CrossRef]
- Hofman, P.L.; Cutfield, W.S.; Robinson, E.M.; Bergman, R.N.; Menon, R.K.; Sperling, M.A.; Gluckman, P.D. Insulin Resistance in Short Children with Intrauterine Growth Retardation 1. J. Clin. Endocrinol. Metab. 1997, 82, 402–406. [Google Scholar] [CrossRef]
- Jaquet, D.; Gaboriau, A.; Czernichow, P.; Levy-Marchal, C. Insulin Resistance Early in Adulthood in Subjects Born with Intrauterine Growth Retardation 1. J. Clin. Endocrinol. Metab. 2000, 85, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, J.P.E.; Albertsson-, K.; Kwan, E.Y.W.; Lam, B.C.C.; Low, L.C.K. The Timing of Early Postnatal Catch-Up Growth in Normal, Full-Term Infants Born Short for Gestational Age. Horm. Res. 1997, 48, 17–24. [Google Scholar] [CrossRef]
- Yates, D.T.; Macko, A.R.; Nearing, M.; Chen, X.; Rhoads, R.P.; Limesand, S.W. Developmental Programming in Response to Intrauterine Growth Restriction Impairs Myoblast Function and Skeletal Muscle Metabolism. J. Pregnancy 2012, 2012, 631038. [Google Scholar] [CrossRef]
- Stange, K.; Miersch, C.; Sponder, G.; Röntgen, M. Low birth weight influences the postnatal abundance and characteristics of satellite cell subpopulations in pigs. Sci. Rep. 2020, 10, 6149. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.I.; Wesolowski, S.R.; Gilje, E.A.; Baker, P.R.; Reisz, J.A.; D’Alessandro, A.; Hay, W.W.; Rozance, P.J.; Brown, L.D. Skeletal muscle amino acid uptake is lower and alanine production is greater in late gestation intrauterine growth-restricted fetal sheep hindlimb. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2019, 317, R615–R629. [Google Scholar] [CrossRef] [PubMed]
- Blesson, C.S.; Sathishkumar, K.; Chinnathambi, V.; Yallampalli, C. Gestational protein restriction impairs insulin regulated glucose transport mechanisms in gastrocnemius muscles of adult male offspring. Endocrinology 2014, 155, 3036–3046. [Google Scholar] [CrossRef]
- Abd El-Wahed, M.A.; El-Farghali, O.G.; ElAbd, H.S.A.; El-Desouky, E.D.; Hassan, S.M. Metabolic derangements in IUGR neonates detected at birth using UPLC-MS. Egypt. J. Med. Hum. Genet. 2017, 18, 281–287. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Swann, J.R. Metabolic phenotyping of malnutrition during the first 1000 days of life. Eur. J. Nutr. 2019, 58, 909–930. [Google Scholar] [CrossRef]
- Beauchamp, B.; Thrush, A.B.; Quizi, J.; Antoun, G.; McIntosh, N.; Al-Dirbashi, O.Y.; Patti, M.E.; Harper, M.E. Undernutrition during pregnancy in mice leads to dysfunctional cardiac muscle respiration in adult offspring. Biosci. Rep. 2015, 35, e00200. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Mai, M.; Tönjes, A.; Kovacs, P.; Stumvoll, M.; Fiedler, G.M.; Leichtle, A.B. Serum levels of acylcarnitines are altered in prediabetic conditions. PLoS ONE 2013, 8, e82459. [Google Scholar] [CrossRef] [PubMed]
- Germani, D.; Puglianiello, A.; Cianfarani, S. Uteroplacental insufficiency down regulates insulin receptor and affects expression of key enzymes of long-chain fatty acid (LCFA) metabolism in skeletal muscle at birth. Cardiovasc. Diabetol. 2008, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhang, J.; Wei, H.; Zhang, H.; Guan, Y.; Wang, X.; Tong, X. Reduction of the PI3K/Akt related signaling activities in skeletal muscle tissues involves insulin resistance in intrauterine growth restriction rats with catch-up growth. PLoS ONE 2019, 14, e0216665. [Google Scholar] [CrossRef]
- Longo, M.; Refuerzo, J.S.; Mann, L.; Leon, M.; Moussa, H.N.; Sibai, B.M.; Blackwell, S.C. Adverse effect of high-fat diet on metabolic programming in offspring born to a murine model of maternal hypertension. Am. J. Hypertens. 2016, 29, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Castorani, V.; Polidori, N.; Giannini, C.; Blasetti, A.; Chiarelli, F. Insulin resistance and type 2 diabetes in children. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Kinzler, W.L.; Vintzileos, A.M. Fetal growth restriction: A modern approach. Curr. Opin. Obs. Gynecol. 2008, 20, 125–131. [Google Scholar] [CrossRef]
- Henriksen, T.; Clausen, T. The fetal origins hypothesis: Placental insufficiency and inheritance versus maternal malnutrition in well-nourished populations. Acta Obstet. Gynecol. Scand. 2002, 81, 112–114. [Google Scholar] [CrossRef]
- UNICEF-WHO Low Birthweight Estimates: Levels and Trends 2000–2015. Available online: https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(18)30565-5/fulltext (accessed on 20 November 2021).
- Colella, M.; Frérot, A.; Novais, A.R.B.; Baud, O. Neonatal and Long-Term Consequences of Fetal Growth Restriction. Curr. Pediatr. Rev. 2018, 14, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Grundy, D. Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J. Physiol. 2015, 593, 2547–2549. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The arrive guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, 1769–1777. [Google Scholar] [CrossRef]
- Turner, A.J.; Trudinger, B.J. A modification of the uterine artery restriction technique in the guinea pig fetus produces asymmetrical ultrasound growth. Placenta 2009, 30, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, T.A.; Rehn, A.E.; Dieni, S.; Duncan, J.R.; Wlodek, M.E.; Owens, J.A.; Rees, S.M. Cardiovascular and renal disease in the adolescent guinea pig after chronic placental insufficiency. Am. J. Obstet. Gynecol. 2004, 191, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Sarr, O.; Blake, A.; Thompson, J.A.; Zhao, L.; Rabicki, K.; Walsh, J.C.; Welch, I.; Regnault, T.R.H. The differential effects of low birth weight and Western diet consumption upon early life hepatic fibrosis development in guinea pig. J Physiol. 2016, 6, 1753–1772. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, A. The western-style diet: A major risk factor for impaired kidney function and chronic kidney disease. Am. J. Physiol.-Ren. Physiol. 2011, 301, F919–F931. [Google Scholar] [CrossRef]
- Kind, K.L.; Clifton, P.M.; Grant, P.A.; Owens, P.C.; Sohlstrom, A.; Roberts, C.T.; Robinson, J.S.; Owens, J.A. Effect of maternal feed restriction during pregnancy on glucose tolerance in the adult guinea pig. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R140–R152. [Google Scholar] [CrossRef]
- Gomez-Pinilla, P.J.; Gomez, M.F.; Hedlund, P.; Sward, K.; Hellstrand, P.; Camello, P.J.; Pozo, M.J.; Andersson, K.E. Effect of melatonin on age associated changes in Guinea pig bladder function. J. Urol. 2007, 177, 1558–1561. [Google Scholar] [CrossRef] [PubMed]
- Ayala, J.E.; Samuel, V.T.; Morton, G.J.; Obici, S.; Croniger, C.M.; Shulman, G.I.; Wasserman, D.H.; McGuinness, O.P. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. DMM Dis. Model. Mech. 2010, 3, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Sadato, N.; Tsuchida, T.; Nakaumra, S.; Waki, A.; Uematsu, H.; Takahashi, N.; Hayashi, N.; Yonekura, Y.; Ishii, Y. Non-invasive estimation of the net influx constant using the standardized uptake value for quantification of FDG uptake of tumours. Eur. J. Nucl. Med. 1998, 25, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C. Anatomy of SUV. Nucl. Med. Biol. 2000, 27, 643–646. [Google Scholar] [CrossRef]
- Sarr, O.; Thompson, J.A.; Zhao, L.; Lee, T.Y.; Regnault, T.R.H. Low birth weight male guinea pig offspring display increased visceral adiposity in early adulthood. PLoS ONE 2014, 9, e98433. [Google Scholar] [CrossRef]
- Peter, J.B.; Barnard, R.J.; Edgerton, V.R.; Gillespie, C.A.; Stempel, K.E. Metabolic Profiles of Three Fiber Types of Skeletal Muscle in Guinea Pigs and Rabbits. Biochemistry 1972, 11, 2627–2633. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lojpur, T.; Easton, Z.; Raez-Villanueva, S.; Laviolette, S.; Holloway, A.C.; Hardy, D.B. Δ9-Tetrahydrocannabinol leads to endoplasmic reticulum stress and mitochondrial dysfunction in human BeWo trophoblasts. Reprod. Toxicol. 2019, 87, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Sander, H.; Wallace, S.; Plouse, R.; Tiwari, S.; Gomes, A.V. Ponceau S waste: Ponceau S staining for total protein normalization. Anal. Biochem. 2019, 575, 44–53. [Google Scholar] [CrossRef]
- Klaiman, J.M.; Price, E.R.; Guglielmo, C.G. Fatty acid composition of pectoralis muscle membrane, intramuscular fat stores and adipose tissue of migrant and wintering white-throated sparrows (Zonotrichia albicollis). J. Exp. Biol. 2009, 212, 3865–3872. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Hudson, E.D.; Helleur, R.J.; Parrish, C.C. Thin-layer chromatography-pyrolysis-gas chromatography-mass spectrometry: A multidimensional approach to marine lipid class and molecular species analysis. J. Chromatogr. Sci. 2001, 39, 146–152. [Google Scholar] [CrossRef][Green Version]
- Lepage, N.; Aucoin, S. Measurement of plasma/serum acylcarnitines using tandem mass spectrometry. Methods Mol. Biol. 2010, 603, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Allison, B.J.; Castillo-Melendez, M.; Jenkin, G.; Polglase, G.R.; Miller, S.L. Neonatal morbidities of fetal growth restriction: Pathophysiology and impact. Front. Endocrinol. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Alisjahbana, B.; Rivami, D.S.; Octavia, L.; Susilawati, N.; Pangaribuan, M.; Alisjahbana, A.; Diana, A. Intrauterine growth retardation (IUGR) as determinant and environment as modulator of infant mortality and morbidity: The Tanjungsari Cohort Study in Indonesia. Asia Pac. J. Clin. Nutr. 2019, 28, S17–S31. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Twinn, D.S.; Ozanne, S.E. Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiol. Behav. 2006, 88, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P.; Hales, C.N.; Fall, C.H.D.; Osmond, C.; Phipps, K.; Clark, P.M.S. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): Relation to reduced fetal growth. Diabetologia 1993, 36, 62–67. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J.; Clark, P.M.; Cox, L.J.; Fall, C.; Osmond, C.; Winter, P.D. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991, 303, 1019–1022. [Google Scholar] [CrossRef]
- Hagiwara, N. Genetic Dissection of the Physiological Role of Skeletal Muscle in Metabolic Syndrome. New J. Sci. 2014, 2014, 635146. [Google Scholar] [CrossRef]
- Drewnowski, A. Nutrient density: Addressing the challenge of obesity. Br. J. Nutr. 2018, 120, S8–S14. [Google Scholar] [CrossRef]
- Hosseini, Z.; Whiting, S.J.; Vatanparast, H. Current evidence on the association of the metabolic syndrome and dietary patterns in a global perspective. Nutr. Res. Rev. 2016, 29, 152–162. [Google Scholar] [CrossRef]
- Mahdy, M.A.A. Skeletal muscle fibrosis: An overview. Cell Tissue Res. 2019, 375, 575–588. [Google Scholar] [CrossRef]
- Wallis, M.G.; Wheatley, C.M.; Rattigan, S.; Barrett, E.J.; Clark, A.D.H.; Clark, M.G. Insulin-mediated hemodynamic changes are impaired in muscle of Zucker obese rats. Diabetes 2002, 51, 3492–3498. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.S.; Lantier, L.; Hasenour, C.M.; James, F.D.; Bracy, D.P.; Wasserman, D.H. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes 2013, 62, 572–580. [Google Scholar] [CrossRef]
- Clough, G.F.; Norman, M. The Microcirculation: A Target for Developmental Priming. Microcirculation 2011, 18, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, J.F.; Ashton, C.J.; Stickland, N.C. Fetal programming of fat and collagen in porcine skeletal muscles. J. Anat. 2005, 207, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Levitt, N.S.; Lambert, E.V.; Woods, D.; Hales, C.N.; Andrew, R.; Seckl, J.R. Impaired glucose tolerance and elavated blood pressure in low birth rate weight, nonobese, young South African adults: Early programming of cortisol axis. J. Clin. Endocrinol. Metab. 2000, 85, 4611–4618. [Google Scholar] [CrossRef] [PubMed]
- Ozanne, S.E.; Jensen, C.B.; Tingey, K.J.; Storgaard, H.; Madsbad, S.; Vaag, A.A. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia 2005, 48, 547–552. [Google Scholar] [CrossRef]
- Jensen, C.B.; Storgaard, H.; Madsbad, S.; Richter, E.A.; Vaag, A.A. Altered skeletal muscle fiber composition and size precede whole-body insulin resistance in young men with low birth weight. J. Clin. Endocrinol. Metab. 2007, 92, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- McAuley, K.; Mann, J. Nutritional determinants of insulin resistance. J. Lipid Res. 2006, 47, 1668–1676. [Google Scholar] [CrossRef]
- Ikeda, Y.; Olsen, G.S.; Ziv, E.; Hansen, L.L.; Busch, A.K.; Hansen, B.F.; Shafrir, E.; Mosthaf-seedorf, L. Cellular Mechanism of Nutritionally Induced Insulin Resistance in Psammomys Obesus: Overexpression of Protein Kinase C in Skeletal Muscle Precedes the Onset of Hyperinsulinemia and. Diabetes 2001, 50, 584–592. [Google Scholar] [CrossRef]
- Muniyappa, R.; Iantorno, M.; Quon, M.J. An Integrated View of Insulin Resistance and Endothelial Dysfunction. Endocrinol. Metab. Clin. North Am. 2008, 37, 685–711. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.K. Skeletal muscle as a therapeutic target for delaying type 1 diabetic complications. World J. Diabetes 2015, 6, 1323. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Gavin, M.G.; Quinn, W.J.; Luongo, T.S.; Gelfer, R.G.; Baur, J.A.; Titchenell, P.M. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol. Metab. 2019, 28, 1–13. [Google Scholar] [CrossRef]
- Szendroedi, J.; Yoshimura, T.; Phielix, E.; Koliaki, C.; Marcucci, M.; Zhang, D.; Jelenik, T.; Müller, J.; Herder, C.; Nowotny, P.; et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 9597–9602. [Google Scholar] [CrossRef]
- Bakker, W.; Sipkema, P.; Stehouwer, C.D.A.; Serne, E.H.; Smulders, Y.M.; Van Hinsbergh, V.W.M.; Eringa, E.C. Protein kinase C θ activation induces insulin-mediated constriction of muscle resistance arteries. Diabetes 2008, 57, 706–713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, L.; Sampat, K.; Hu, N.; Zakari, J.; Yuspa, S.H. Protein kinase C negatively regulates Akt activity and modifies UVC-induced apoptosis in mouse keratinocytes. J. Biol. Chem. 2006, 281, 3237–3243. [Google Scholar] [CrossRef]
- Werner, E.D.; Lee, J.; Hansen, L.; Yuan, M.; Shoelson, S.E. Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. J. Biol. Chem. 2004, 279, 35298–35305. [Google Scholar] [CrossRef]
- Aguirre, V.; Werner, E.D.; Giraud, J.; Lee, Y.H.; Shoelson, S.E.; White, M.F. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 2002, 277, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Bloch-Damti, A.; Potashnik, R.; Gual, P.; Le Marchand-Brustel, Y.; Tanti, J.F.; Rudich, A.; Bashan, N. Differential effects of IRS1 phosphorylated on Ser307 or Ser632 in the induction of insulin resistance by oxidative stress. Diabetologia 2006, 49, 2463–2473. [Google Scholar] [CrossRef] [PubMed]
- Yaspelkis, B.B.; Kvasha, I.A.; Figueroa, T.Y. High-fat feeding increases insulin receptor and IRS-1 coimmunoprecipitation with SOCS-3, IKKα/β phosphorylation and decreases PI-3 kinase activity in muscle. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Bruce, C.R.; Hoy, A.J.; Turner, N.; Watt, M.J.; Allen, T.L.; Carpenter, K.; Cooney, G.J.; Febbraio, M.A.; Kraegen, E.W. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes 2009, 58, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H. Insulin resistance: Definition and consequences. Exp. Clin. Endocrinol. Diabetes 2001, 109, S135–S148. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, Y.; Cline, G.W.; Zhang, D.; Zong, H.; Wang, Y.; Bergeron, R.; Kim, J.K.; Cushman, S.W.; Cooney, G.J.; et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 2002, 277, 50230–50236. [Google Scholar] [CrossRef] [PubMed]
- Zierath, J.R.; Krook, A.; Wallberg-Henriksson, H. Insulin action and insulin resistance in human skeletal muscle. Diabetologia 2000, 43, 821–835. [Google Scholar] [CrossRef]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- Lee, J.S.; Pinnamaneni, S.K.; Su, J.E.; In, H.C.; Jae, H.P.; Chang, K.K.; Sinclair, A.J.; Febbraio, M.A.; Watt, M.J. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: Role of intramuscular accumulation of lipid metabolites. J. Appl. Physiol. 2006, 100, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Li, L.; Fan, J.; Sun, X.; Yin, Y. N-6:n-3 PUFA ratio is involved in regulating lipid metabolism and inflammation in pigs. Br. J. Nutr. 2014, 111, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, O.P.; Dahl, D.B.; Brechtel, K.; Machann, J.; Haap, M.; Maier, T.; Loviscach, M.; Stumvoll, M.; Claussen, C.D.; Schick, F.; et al. Effects of Intravenous and Dietary Lipid Challenge on Intramyocellular Lipid Content and the Relation with Insulin Sensitivity in Humans. Diabetes 2001, 50, 2579–2584. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Lebed, B.; Schatz, M.; Homko, C.; Lemieux, S. Effects of Acute Changes of Plasma Free Fatty Acids on Intramyocellular Fat Content and Insulin Resistance in Healthy Subjects. Diabetes 2001, 50, 1612–1617. [Google Scholar] [CrossRef]
- Simard, G.; Darmaun, D.; Agnoux, A.M.; Antignac, J.; Simard, G.; Poupeau, G.; Darmaun, D.; Parnet, P. Time window-dependent effect of perinatal maternal protein restriction on insulin sensitivity and energy substrate oxidation in adult male offspring. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 307, 184–197. [Google Scholar] [CrossRef]
- Koves, T.R.; Li, P.; An, J.; Akimoto, T.; Slentz, D.; Ilkayeva, O.; Dohm, G.L.; Yan, Z.; Newgard, C.B.; Muoio, D.M. Peroxisome Proliferator-activated Receptor-γ Co-activator 1α-mediated Metabolic Remodeling of Skeletal Myocytes Mimics Exercise Training and Reverses Lipid-induced Mitochondrial Inefficiency. J. Biol. Chem. 2005, 280, 33588–33598. [Google Scholar] [CrossRef]
- Wong, K.E.; Mikus, C.R.; Slentz, D.H.; Seiler, S.E.; Debalsi, K.L.; Ilkayeva, O.R.; Crain, K.I.; Kinter, M.T.; Kien, C.L.; Stevens, R.D.; et al. Muscle-specific overexpression of PGC-1α does not augment metabolic improvements in response to exercise and caloric restriction. Diabetes 2015, 64, 1532–1543. [Google Scholar] [CrossRef]
- Schulz, H. Beta oxidation of fatty acids. Biochim. Biophys. Acta-Lipids Lipid Metab. 1991, 1081, 109–120. [Google Scholar] [CrossRef]
- Lane, R.H.; Maclennan, N.K.; Daood, M.J.; Hsu, J.L.; Janke, S.M.; Pham, T.D.; Puri, A.R.; Watchko, J.F. IUGR alters postnatal rat skeletal muscle peroxisome proliferator-activated receptor-γ coactivator-1 gene expression in a fiber specific manner. Pediatr. Res. 2003, 53, 994–1000. [Google Scholar] [CrossRef]
- Kewalramani, G.; Bilan, P.J.; Klip, A. Muscle insulin resistance: Assault by lipids, cytokines and local macrophages. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Dziewulska, A.; Dobrzyn, P.; Jazurek, M.; Pyrkowska, A.; Ntambi, J.M.; Dobrzyn, A. Monounsaturated fatty acids are required for membrane translocation of protein kinase C-theta induced by lipid overload in skeletal muscle. Mol. Membr. Biol. 2012, 29, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.X.; Li, X.W.; Fu, W.; Zhang, W.Q. Metabolomic Research on Newborn Infants with Intrauterine Growth Restriction. Medicine 2016, 95, e3564. [Google Scholar] [CrossRef]
- Lent-Schochet, D.; McLaughlin, M.; Ramakrishnan, N.; Jialal, I. Exploratory metabolomics of metabolic syndrome: A status report. World J. Diabetes 2019, 10, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Appleton, H.; Rosentrater, K.A. Sweet dreams (Are made of this): A review and perspectives on aspartic acid production. Fermentation 2021, 7, 49. [Google Scholar] [CrossRef]
- Huang, S.S.; Lin, J.Y.; Chen, W.S.; Liu, M.H.; Cheng, C.W.; Cheng, M.L.; Wang, C.H. Phenylalanine- and leucine-defined metabolic types identify high mortality risk in patients with severe infection. Int. J. Infect. Dis. 2019, 85, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Mantha, O.L.; Polakof, S.; Huneau, J.F.; Mariotti, F.; Poupin, N.; Zalko, D.; Fouillet, H. Early changes in tissue amino acid metabolism and nutrient routing in rats fed a high-fat diet: Evidence from natural isotope abundances of nitrogen and carbon in tissue proteins. Br. J. Nutr. 2018, 119, 981–991. [Google Scholar] [CrossRef]
- Bansal, A.; Simmons, R.A. Epigenetics and developmental origins of diabetes: Correlation or causation? Am. J. Physiol.-Endocrinol. Metab. 2018, 315, E15–E28. [Google Scholar] [CrossRef]
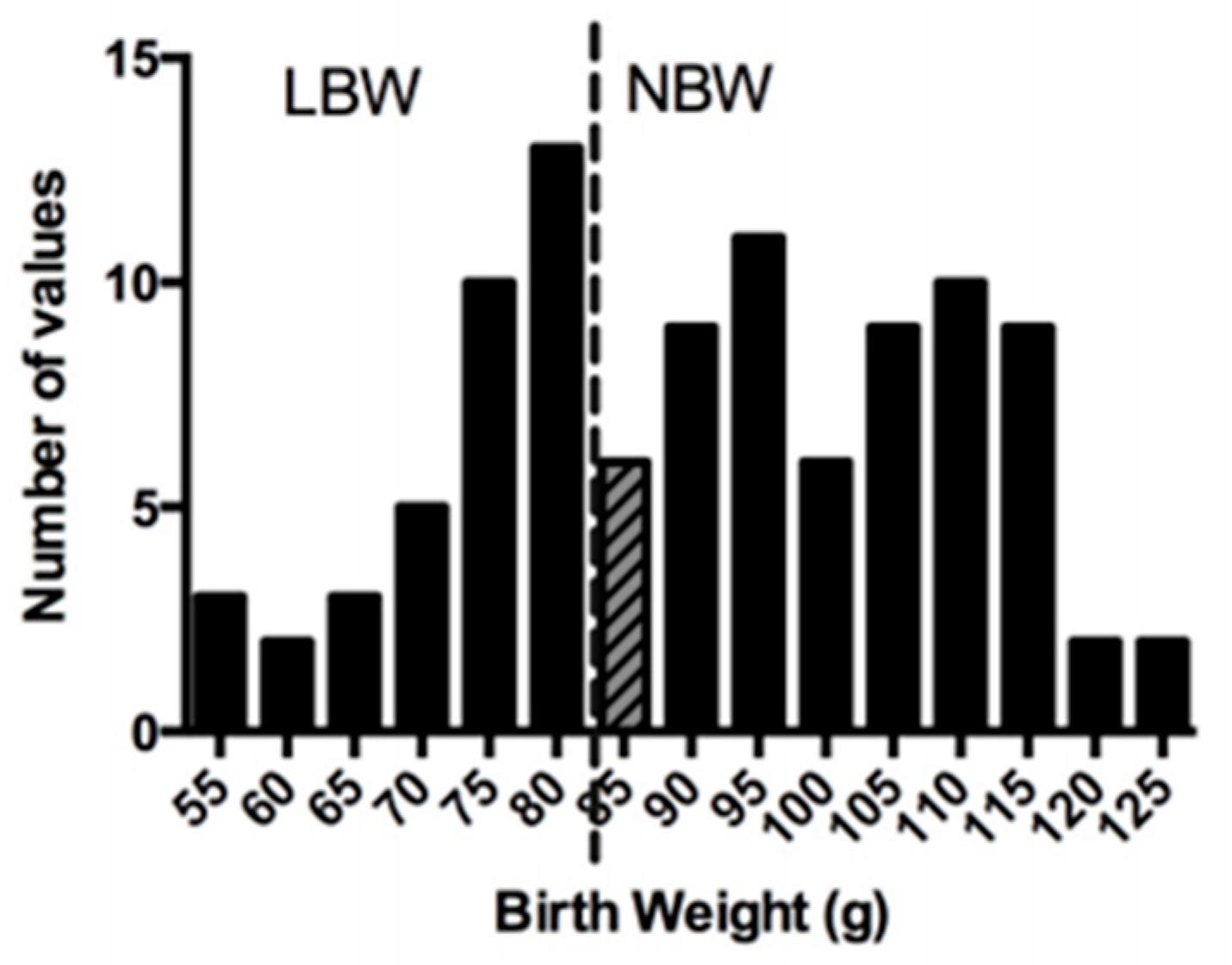





| Bi-Parietal Distance (cm) | Crown-Rump Length (cm) | Abdominal Circumference (cm) | Ponderal Index (g/cm3) | Birth Weight/Birth Length (g/cm) | Absolute Growth Rate (g/day) | %Increase in Body Weight | Fractional Growth Rate | |
|---|---|---|---|---|---|---|---|---|
| NBW | 2.16 ± 0.035 | 15.49 ± 0.32 | 11.56 ± 0.17 | 0.029 ± 0.002 | 6.7 ± 0.21 | 9.50 ± 0.26 | 115.7 ± 3.79 | 0.092 ± 0.002 |
| LBW | 2.04 ± 0.039 | 13.97 ± 0.38 | 10.98 ± 0.24 | 0.029 ± 0.002 | 5.46 ± 0.22 | 8.29 ± 0.24 | 149.6 ± 6.14 | 0.113 ± 0.004 |
| * | ** | p = 0.0544 | ns | *** | ** | **** | **** |
| p Values for Main Effect | ||||
|---|---|---|---|---|
| Factor | Metabolite Description | Birth Weight | Diet | Birth Weight and Diet |
| PC1, medium- & long-chain acylcarnitines, eigenvalue 12.6 (43.40%) | C2 (−0.715) C8 (0.556) C10 (0.797) C12 (0.886) C14 (0.801) C16 (0.929) C18 (0.836) C14:1 (0.766) C16:1 (0.877) C18:1 (0.921) | p = 0.273 | p < 0.0001 | p = 0.469 |
| PC2, hydroxylated acylcarnitines, eigenvalue 3.9 (13.71%) | C4 (0.865) C6 (0.784) C4⋯OH (0.721) C12⋯OH (0.723) C14⋯OH (0.783) C16⋯OH (0.741) C18⋯OH (0.814) C18:1⋯OH (0.764) | p = 0.415 | p = 0.076 | p = 0.569 |
| PC3, poly-unsaturated long-chain acylcarnitines, eigenvalue 3.3 (11.36%) | C18:2 (0.807) C18:3 (0.813) C20:4 (0.716) C22:5 (0.925) | p = 0.775 | p = 0.022 | p = 0.996 |
| PC4, gluconeogenic amino acids, eigenvalue 2.2 (7.53%) | Leu (0.723) Ile (0.603) Val (0.801) Tyr (0.554) Phe (0.673) | p = 0.075 | p = 0.587 | p = 0.459 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunlop, K.; Sarr, O.; Stachura, N.; Zhao, L.; Nygard, K.; Thompson, J.A.; Hadway, J.; Richardson, B.S.; Bureau, Y.; Borradaile, N.; et al. Differential and Synergistic Effects of Low Birth Weight and Western Diet on Skeletal Muscle Vasculature, Mitochondrial Lipid Metabolism and Insulin Signaling in Male Guinea Pigs. Nutrients 2021, 13, 4315. https://doi.org/10.3390/nu13124315
Dunlop K, Sarr O, Stachura N, Zhao L, Nygard K, Thompson JA, Hadway J, Richardson BS, Bureau Y, Borradaile N, et al. Differential and Synergistic Effects of Low Birth Weight and Western Diet on Skeletal Muscle Vasculature, Mitochondrial Lipid Metabolism and Insulin Signaling in Male Guinea Pigs. Nutrients. 2021; 13(12):4315. https://doi.org/10.3390/nu13124315
Chicago/Turabian StyleDunlop, Kristyn, Ousseynou Sarr, Nicole Stachura, Lin Zhao, Karen Nygard, Jennifer A. Thompson, Jennifer Hadway, Bryan S. Richardson, Yves Bureau, Nica Borradaile, and et al. 2021. "Differential and Synergistic Effects of Low Birth Weight and Western Diet on Skeletal Muscle Vasculature, Mitochondrial Lipid Metabolism and Insulin Signaling in Male Guinea Pigs" Nutrients 13, no. 12: 4315. https://doi.org/10.3390/nu13124315
APA StyleDunlop, K., Sarr, O., Stachura, N., Zhao, L., Nygard, K., Thompson, J. A., Hadway, J., Richardson, B. S., Bureau, Y., Borradaile, N., Lee, T.-Y., & Regnault, T. R. H. (2021). Differential and Synergistic Effects of Low Birth Weight and Western Diet on Skeletal Muscle Vasculature, Mitochondrial Lipid Metabolism and Insulin Signaling in Male Guinea Pigs. Nutrients, 13(12), 4315. https://doi.org/10.3390/nu13124315







