The Use of Mushrooms and Spirulina Algae as Supplements to Prevent Growth Inhibition in a Pre-Clinical Model for an Unbalanced Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
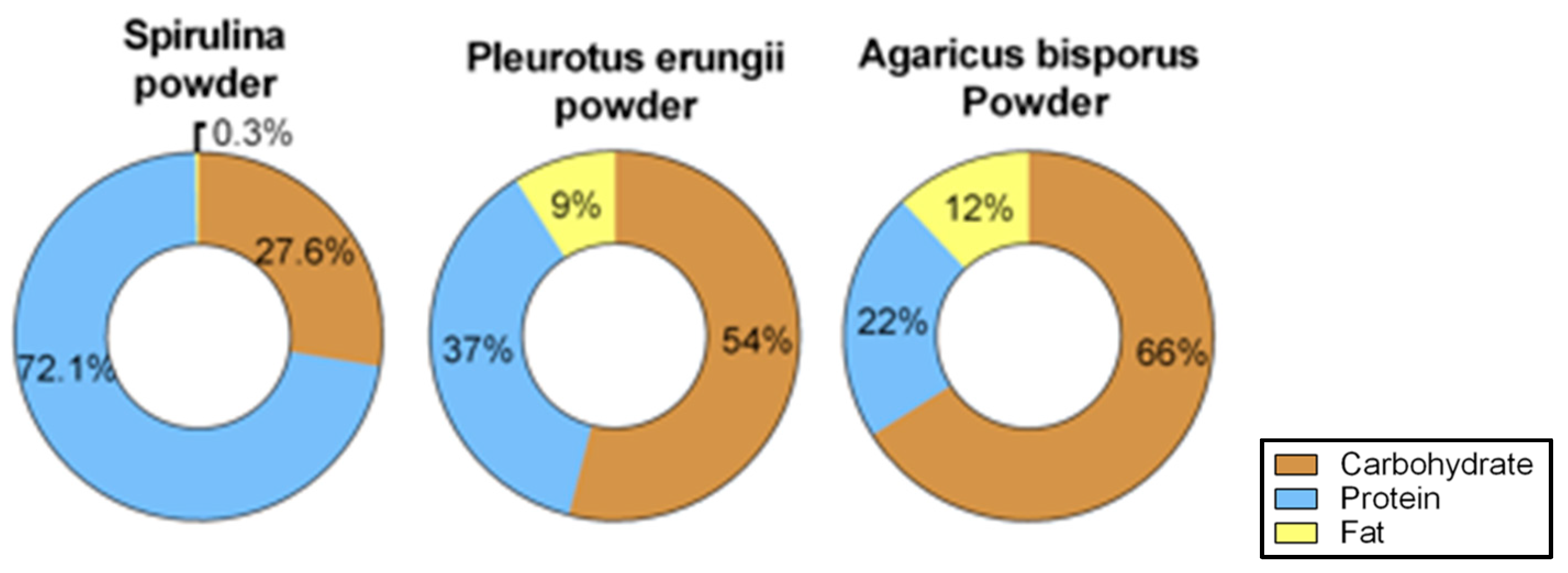
2.2. Diet Preparation and Composition
2.3. Histological Staining of Growth-Plate (GP) Sections
2.4. Imaging and Measurement of GPs
2.5. Bone Microarchitecture
2.6. Three Point Bending for Bone Mechanical Analysis
2.7. Macro Ad Micro-Nutrient Analysis
2.8. Caecum Sample Collection and DNA Extraction
2.9. 16SrRNASequencing
2.10. Bioinformatics Analysis
2.11. Statistical Analysis
3. Results
3.1. The Positive Effect of Nutritional Supplements on Growth Pattern
3.2. The Positive Effect of Nutritional Supplements on Bone Quality
3.3. Diet Composition Analysis of the Supplements
3.3.1. Treatment of Amino Acids and Mineral Deficiencies
3.3.2. Functional Fatty Acids
3.4. Microbiome Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- UNICEF; WHO. The International Bank for Reconstruction and Development/The World Bank. In Levels and Trends in Child Malnutrition: Key Findings of the 2019 Edition of the Joint Child Malnutrition Estimates; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Gödecke, T.; Stein, A.J.; Qaim, M. The global burden of chronic and hidden hunger: Trends and determinants. Glob. Food Sec. 2018, 17, 21–29. [Google Scholar] [CrossRef]
- Lucas, T.; Horton, R. The 21st-century great food transformation. Lancet 2019, 393, 386–387. [Google Scholar] [CrossRef]
- Branca, F.; Demaio, A.; Udomkesmalee, E.; Baker, P.; Aguayo, V.M.; Barquera, S.; Dain, K.; Keir, L.; Lartey, A.; Mugambi, G.; et al. A new nutrition manifesto for a new nutrition reality. Lancet 2020, 395, 8–10. [Google Scholar] [CrossRef]
- Lustig, R.H. Ultraprocessed Food: Addictive, Toxic, and Ready for Regulation. Nutrients 2020, 12, 3401. [Google Scholar] [CrossRef]
- Moubarac, J.-C. Ultra-Processed Foods in Canada: Consumption, Impact on Diet Quality and Policy Implications; University of Montreal: Montreal, QC, Canada, 2017. [Google Scholar] [CrossRef]
- Aslam, M.N.; Jepsen, K.J.; Khoury, B.; Graf, K.H.; Varani, J. Bone structure and function in male C57BL/6 mice: Effects of a high-fat Western-style diet with or without trace minerals. Bone Rep. 2016, 5, 141–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaretsky, J.; Griess-fishheimer, S.; Carmi, A.; Shmul, T.T.; Ofer, L.; Sinai, T.; Penn, S. Ultra-processed food targets bone quality via endochondral ossi fi cation. Bone Res. 2021, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, J.-P.; Theintz, G.; Law, F.; Slosman, D.; Rizzoli, R. Peak bone mass. Osteoporos. Int. 1994, 4, S7–S13. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, C.; Sjögren, K. Effects of the gut microbiota on bone mass. Trends Endocrinol. Metab. 2015, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Veldurthy, V.; Patel, N.; Wei, R.; Ramsey, W.; Isales, C.M.; Lavoie, B.; Chishti, S.; Parameswaran, N.; Britton, R.A.; et al. Understanding the Gut-Bone Signaling Axis; Advances in Experimental Medicine and Biology Series; McCabe, L.R., Parameswaran, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 1033, ISBN 978-3-319-66651-8. [Google Scholar]
- The Council for Responsible Nutrition. Who Takes Dietary Supplements? And Why? The Council for Responsible Nutrition: Washington, DC, USA, 2019. [Google Scholar]
- Kamiński, M.; Kręgielska-Narożna, M.; Bogdański, P. Determination of the Popularity of Dietary Supplements Using Google Search Rankings. Nutrients 2020, 12, 908. [Google Scholar] [CrossRef] [Green Version]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- de Morais, M.G.; da Silva Vaz, B.; de Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. Biomed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [Green Version]
- Hoseini, S.M.; Khosravi-Darani, K.; Mozafari, M.R. Nutritional and Medical Applications of Spirulina Microalgae. Mini-Rev. Med. Chem. 2013, 13, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Könkö, K.; Eurola, M.; Pihlava, J.-M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of Vitamins, Mineral Elements, and Some Phenolic Compounds in Cultivated Mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef]
- Iñiguez, M.; Pérez-Matute, P.; Villanueva-Millán, M.J.; Recio-Fernández, E.; Roncero-Ramos, I.; Pérez-Clavijo, M.; Oteo, J.-A. Agaricus bisporus supplementation reduces high-fat diet-induced body weight gain and fatty liver development. J. Physiol. Biochem. 2018, 74, 635–646. [Google Scholar] [CrossRef]
- Jeong, S.C.; Jeong, Y.T.; Yang, B.K.; Islam, R.; Koyyalamudi, S.R.; Pang, G.; Cho, K.Y.; Song, C.H. White button mushroom (Agaricus bisporus) lowers blood glucose and cholesterol levels in diabetic and hypercholesterolemic rats. Nutr. Res. 2010, 30, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Zacharia, A.J.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.B.K.S.; Bisen, P.S. Spirulina in health care management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, P.G. Committee Report AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1939, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Koren, N.; Simsa-maziel, S.; Shahar, R.; Schwartz, B.; Monsonego-ornan, E. ScienceDirect Exposure to omega-3 fatty acids at early age accelerate bone growth and improve bone quality. J. Nutr. Biochem. 2014, 25, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Simsa-maziel, S.; Zaretsky, J.; Reich, A.; Koren, Y.; Shahar, R.; Monsonego-ornan, E. IL-1RI participates in normal growth plate development and bone modeling. Am. J. Physiol. Endocrinol. Metab. 2013, 305, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idelevich, A.; Kerschnitzki, M.; Shahar, R.; Monsonego-Ornan, E. 1,25(OH)2D3 Alters Growth Plate Maturation and Bone Architecture in Young Rats with Normal Renal Function. PLoS ONE 2011, 6, e20772. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Rozner, R.; Vernikov, J.; Griess-Fishheimer, S.; Travinsky, T.; Penn, S.; Schwartz, B.; Mesilati-Stahy, R.; Argov-Argaman, N.; Shahar, R.; Monsonego-Ornan, E. The Role of Omega-3 Polyunsaturated Fatty Acids from Different Sources in Bone Development. Nutrients 2020, 12, 3494. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Nutritional aspects of bone health. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.H.; van der Meulen, M.C.H. Whole Bone Mechanics and Bone Quality. Clin. Orthop. Relat. Res. 2011, 469, 2139–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maynard, L.A. The atwater system of calculating the caloric value of diets. J. Nutr. 1944, 28, 443–452. [Google Scholar] [CrossRef]
- Le, A.; Ng, A.; Kwan, T.; Cusmano-Ozog, K.; Cowan, T.M. A rapid, sensitive method for quantitative analysis of underivatized amino acids by liquid chromatography–tandem mass spectrometry (LC–MS/MS). J. Chromatogr. B 2014, 944, 166–174. [Google Scholar] [CrossRef]
- Subcommittee on Laboratory Animal Nutrition; Committee on Animal Nutrition; Board on Agriculture; National Research Council. Nutrient Requirements of Laboratory Animals, 4th ed.; National Academies Press: Washington, DC, USA, 1995; ISBN 978-0-309-05126-2. [Google Scholar]
- Gonzalez, M.H.; Souza, G.B.; Oliveira, R.V.; Forato, L.A.; Nóbrega, J.A.; Nogueira, A.R.A. Microwave-assisted digestion procedures for biological samples with diluted nitric acid: Identification of reaction products. Talanta 2009, 79, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Argov-Argaman, N.; Anggrek, J.; Boeren, S.; van Hooijdonk, T.; Vervoort, J.; Hettinga, K.A. The protein and lipid composition of the membrane of milk fat globules depends on their size. J. Dairy Sci. 2016, 99, 4726–4738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belizário, J.E.; Faintuch, J. Metabolic Interaction in Infection; Springer International Publishing: Cham, Switzerland, 2018; Volume 109, ISBN 978-3-319-74931-0. [Google Scholar]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Zhang Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [Green Version]
- Watrous, D.A.; Andrews, B.S. The metabolism and immunology of bone. Semin. Arthritis Rheum. 1989, 19, 45–65. [Google Scholar] [CrossRef]
- Krajmalnik-Brown, R.; Ilhan, Z.-E.; Kang, D.-W.; DiBaise, J.K. Effects of Gut Microbes on Nutrient Absorption and Energy Regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, T.C.; Marzorati, M.; Spence, L.; Weaver, C.M.; Williamson, P.S. New Frontiers in Fibers: Innovative and Emerging Research on the Gut Microbiome and Bone Health. J. Am. Coll. Nutr. 2017, 36, 218–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef] [Green Version]
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Rooks, M.G.; Veiga, P.; Wardwell-Scott, L.H.; Tickle, T.; Segata, N.; Michaud, M.; Gallini, C.A.; Beal, C.; van Hylckama-Vlieg, J.E.; Ballal, S.A.; et al. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 2014, 8, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, R.J.; Dowd, S.E.; Chamberlin, W.M.; Galandiuk, S.; Davis, B.; Glassing, A. Microbial population differentials between mucosal and submucosal intestinal tissues in advanced Crohn’s disease of the ileum. PLoS ONE 2015, 10, e0134382. [Google Scholar] [CrossRef]
- Sgambato, D.; Gimigliano, F.; De Musis, C.; Moretti, A.; Toro, G.; Ferrante, E.; Miranda, A.; De Mauro, D.; Romano, L.; Iolascon, G.; et al. Bone alterations in inflammatory bowel diseases. World J. Clin. Cases 2019, 7, 1908–1925. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Arora, S.; Li, J.; Rahmani, R.; Sun, L.; Steinlauf, A.F.; Mechanick, J.I.; Zaidi, M. Bone, Inflammation, and Inflammatory Bowel Disease. Curr. Osteoporos. Rep. 2011, 9, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Gao, W.; Wang, B.; Zhao, H.; Zeng, Y.; Ji, Y.; Hao, D. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ 2017, 5, e3450. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wu, T.; Li, N.; Wang, X.; Chen, G.; Lyu, X. Bilberry anthocyanin extract promotes intestinal barrier function and inhibits digestive enzyme activity by regulating the gut microbiota in aging rats. Food Funct. 2019, 10, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, F.; Lu, J.; Shi, J.; Guan, J.; Yan, F.; Li, B.; Huo, G. Probiotic Mixture of Lactobacillus plantarum Strains Improves Lipid Metabolism and Gut Microbiota Structure in High Fat Diet-Fed Mice. Front. Microbiol. 2020, 11, 512. [Google Scholar] [CrossRef] [Green Version]
- Jia, E.; Liu, Z.; Pan, M.; Lu, J.; Ge, Q. Regulation of bile acid metabolism-related signaling pathways by gut microbiota in diseases. J. Zhejiang Univ. B 2019, 20, 781–792. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-Mediated Bile Acid Sensing Controls Glucose Homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Schiellerup, S.P.; Skov-Jeppesen, K.; Windeløv, J.A.; Svane, M.S.; Holst, J.J.; Hartmann, B.; Rosenkilde, M.M. Gut Hormones and Their Effect on Bone Metabolism. Potential Drug Therapies in Future Osteoporosis Treatment. Front. Endocrinol. 2019, 10, 75. [Google Scholar] [CrossRef]
- Zhao, C.; Liang, J.; Yang, Y.; Yu, M.; Qu, X. The Impact of Glucagon-Like Peptide-1 on Bone Metabolism and Its Possible Mechanisms. Front. Endocrinol. 2017, 8, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavropoulou, M.; Yovos, J. Incretins and bone: Evolving concepts in nutrient-dependent regulation of bone turnover. Hormones 2013, 12, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Van Der Eerden, B.C.J.; Karperien, M.; Wit, J.M. Systemic and Local Regulation of the Growth Plate. Endocr. Rev. 2003, 24, 782–801. [Google Scholar] [CrossRef] [Green Version]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Luka, Đ.; Fu, A.; Muhvi, D.; Giacometti, J. The role of polyphenols on bone metabolism in osteoporosis. Food Res. Int. 2015, 77, 290–298. [Google Scholar] [CrossRef]
- Das, A.S.; Mukherjee, M.; Mitra, C. Evidence for a prospective anti-osteoporosis effect of black tea (Camellia Sinensis) extract in a bilaterally ovariectomized rat model. Asia Pac. J. Clin. Nutr. 2004, 13, 210–216. [Google Scholar]
- Choi, J.S.; Park, M.Y.; Kim, J.D.; Cho, H.R.; Choi, I.S.; Kim, J.W. Safety and efficacy of polycalcium for improving biomarkers of bone metabolism: A 4-week open-label clinical study. J. Med. Food 2013, 16, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, K.; Bashir, I.; Choi, J. Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future. Int. J. Mol. Sci. 2017, 18, 1906. [Google Scholar] [CrossRef]







| Control Diet | Unbalanced Diet | |
|---|---|---|
| Ingredient | g\kg Diet | g\kg Diet |
| Cornstarch | 397 | 438.7 |
| Casein (≥85% protein) | 200 | 105 |
| Dextrinized cornstarch (90–94% tetrasaccharides) | 132 | 145.7 |
| Sucrose | 100 | 110 |
| Soybean oil | 70 | 118.8 |
| Fiber | 50 | 54 |
| Mineral mix (AIN-93G-MX) | 35 | 18.9 |
| Vitamin mix (AIN-93-VX) | 10 | 5.4 |
| L-Cystine | 3 | 1.6 |
| Choline bitartrate (41.1% choline) | 2.5 | 1.34 |
| Tert-butylhydroquinone | 0.014 | 0.015 |
| Control Diet | Unbalanced Diet | |||
|---|---|---|---|---|
| Ingredient | Kcal\kg Diet | % Kcal | Kcal\kg Diet | % Kcal |
| Cornstarch | 1402 | 63.5 | 1559.4 | 65 |
| Dextrinized cornstarch (90–94% tetrasaccharides) | 465.5 | 518 | ||
| Sucrose | 352.7 | 392 | ||
| Casein (≥85% protein) | 705 | 20.5 | 374 | 10 |
| L-Cystine | 10.6 | 5.6 | ||
| Soybean oil | 555.5 | 16 | 949.9 | 25 |
| Total calories | 3491.3 | 3799 | ||
| Kcal/gr | 3.49 | 3.799 | ||
| Ingredient | g\kg Diet | Kcal\kg Diet |
|---|---|---|
| Spirulina algae | 5 | 19 |
| Pleurotus eryngii | 3.5 | 15.765 |
| Agaricus bisporus | 3.5 | 13.618 |
| CON | UBD | UBD + SP | UBD + PE | UBD + AB | UBD + MIX | |
|---|---|---|---|---|---|---|
| Femora trabecular bone microarchitecture | ||||||
| BV/TV (%) | 38.222 ± 2.81 a | 23.261 ± 2.79 c | 30.44 ± 3.49 b | 30.263 ± 2.99 b | 33.214 ± 1.29 b | 33.697 ± 3.19 b |
| Tb.Th (mm) | 0.119 ± 0.009 a | 0.105 ± 0.005 b | 0.118 ± 0.007 a | 0.117 ± 0.006 a | 0.117 ± 0.04 a | 0.121 ± 0.008 a |
| Tb.N (1/mm) | 3.203 ± 0.15 a | 2.205 ± 0.19 c | 2.583 ± 0.2 b | 2.592 ± 0.19 b | 2.841 ± 0.16 b | 2.771 ± 0.16 b |
| Tb.Sp (mm) | 0.264 ± 0.06 c | 0.572 ± 0.12 a | 0.466 ± 0.06 ab | 0.45 ± 0.05 b | 0.398 ± 0.07 b | 0.378 ± 0.03 b |
| 5th vertebra trabecular bone microarchitecture | ||||||
| BV/TV (%) | 36.797 ± 1.4 a | 30.568 ± 1.24 c | 32.841 ± 1.77 bc | 33.146 ± 2.41 bc | 35.011 ± 2.15 ab | 31.881 ± 2.57 c |
| Tb.Th (mm) | 0.128 ± 0.005 a | 0.111 ± 0.003 d | 0.116 ± 0.003 c | 0.115 ± 0.003 cd | 0.121 ± 0.003 b | 0.115 ± 0.003 cd |
| Tb.N (1/mm) | 2.871 ± 0.09 a | 2.762 ± 0.1 a | 2.84 ± 0.12 a | 2.869 ± 0.17 a | 2.901 ± 0.15 a | 2.775 ± 0.19 a |
| Tb.Sp (mm) | 0.297 ± 0.017 a | 0.328 ± 0.017 a | 0.312 ± 0.012 a | 0.307 ± 0.025 a | 0.313 ± 0.03 a | 0.322 ± 0.037 a |
| Femora cortical bone microarchitecture | ||||||
| Ct.Ar/Tt.Ar (%) | 48.925 ± 1.69 a | 40.378 ± 1.87 c | 43.534 ± 1.06 b | 42.945 ± 1.69 b | 44.6 ± 1.38 b | 44.565 ± 1.61 b |
| Cs.Th (mm) | 0.466 ± 0.02 a | 0.343 ± 0.02 c | 0.384 ± 0.01 b | 0.385 ± 0.02 b | 0.387 ± 0.01 b | 0.392 ± 0.01 b |
| Ma.Ar (mm2) | 5.324 ± 0.4 a | 5.108 ± 0.59 a | 5.081 ± 0.25 a | 5.299 ± 0.35 a | 4.828 ± 0.3 a | 4.981 ± 0.39 a |
| BMD (g/cm3) | 1.26 ± 0.03 a | 1.184 ± 0.01 c | 1.205 ± 0.02 bc | 1.199 ± 0.02 bc | 1.229 ± 0.03 ab | 1.23 ± 0.04 ab |
| Femora bone mechanical properties | ||||||
| Stiffness (N/mm) | 268.72 ± 45.5 a | 195.6 ± 43 b | 225.32 ± 22.9 ab | 197.34 ± 44 b | 228.49 ± 33.9 ab | 202.57 ± 39.1 b |
| Yield point (N) | 45.55 ± 3.9 a | 26.31 ± 4.2 c | 36.01 ± 3.7 b | 34.64 ± 2.7 b | 37.8 ± 2.6 b | 37.28 ± 3 b |
| Fracture load (N) | 74.13 ± 7.5 a | 42.69 ± 11.4 b | 52.06 ± 6.2 b | 41.81 ± 9.3 b | 51.25 ± 6.5 b | 50.21 ± 11.2 b |
| Max load (N) | 90.47 ± 7.7 a | 63.04 ± 9.3 c | 75.32 ± 6.7 b | 68.81 ± 5.7 bc | 70.01 ± 5.9 bc | 73.26 ± 6.2 bc |
| E to F (N × mm) | 83.01 ± 16.5 a | 54.93 ± 10.6 c | 71.8 ± 11.8 abc | 72.04 ± 11.6 abc | 63.2 ± 8.7 bc | 74.84 ± 17.5 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sides, R.; Griess-Fishheimer, S.; Zaretsky, J.; Shitrit, A.; Kalev-Altman, R.; Rozner, R.; Beresh, O.; Dumont, M.; Penn, S.; Shahar, R.; et al. The Use of Mushrooms and Spirulina Algae as Supplements to Prevent Growth Inhibition in a Pre-Clinical Model for an Unbalanced Diet. Nutrients 2021, 13, 4316. https://doi.org/10.3390/nu13124316
Sides R, Griess-Fishheimer S, Zaretsky J, Shitrit A, Kalev-Altman R, Rozner R, Beresh O, Dumont M, Penn S, Shahar R, et al. The Use of Mushrooms and Spirulina Algae as Supplements to Prevent Growth Inhibition in a Pre-Clinical Model for an Unbalanced Diet. Nutrients. 2021; 13(12):4316. https://doi.org/10.3390/nu13124316
Chicago/Turabian StyleSides, Roni, Shelley Griess-Fishheimer, Janna Zaretsky, Astar Shitrit, Rotem Kalev-Altman, Reut Rozner, Olga Beresh, Maïtena Dumont, Svetlana Penn, Ron Shahar, and et al. 2021. "The Use of Mushrooms and Spirulina Algae as Supplements to Prevent Growth Inhibition in a Pre-Clinical Model for an Unbalanced Diet" Nutrients 13, no. 12: 4316. https://doi.org/10.3390/nu13124316
APA StyleSides, R., Griess-Fishheimer, S., Zaretsky, J., Shitrit, A., Kalev-Altman, R., Rozner, R., Beresh, O., Dumont, M., Penn, S., Shahar, R., & Monsonego-Ornan, E. (2021). The Use of Mushrooms and Spirulina Algae as Supplements to Prevent Growth Inhibition in a Pre-Clinical Model for an Unbalanced Diet. Nutrients, 13(12), 4316. https://doi.org/10.3390/nu13124316






