Timing of Meals and Exercise Affects Hormonal Control of Glucoregulation, Insulin Resistance, Substrate Metabolism, and Gastrointestinal Hormones, but Has Little Effect on Appetite in Postmenopausal Women
Abstract
1. Introduction
- (1)
- Increase hunger when exercise was performed before meals;
- (2)
- Decrease satiation, insulin and gut hormones when exercise was performed after meals;
- (3)
- Produce different effects on hunger and satiation in the afternoon than in the morning.
2. Materials and Methods
2.1. Subjects
2.2. General Experimental Protocol
2.3. Study Design
2.4. Exercise Intensity and Substrate Metabolism
2.5. Meals
2.6. Appetite Assessment
2.7. Blood Collection
2.8. Hormone and Metabolite Measurements
2.9. Statistical Analyses
3. Results
3.1. Exercise Parameters
3.2. Exercise- and Meal-Timing Effects on Circulating Metabolic Fuels
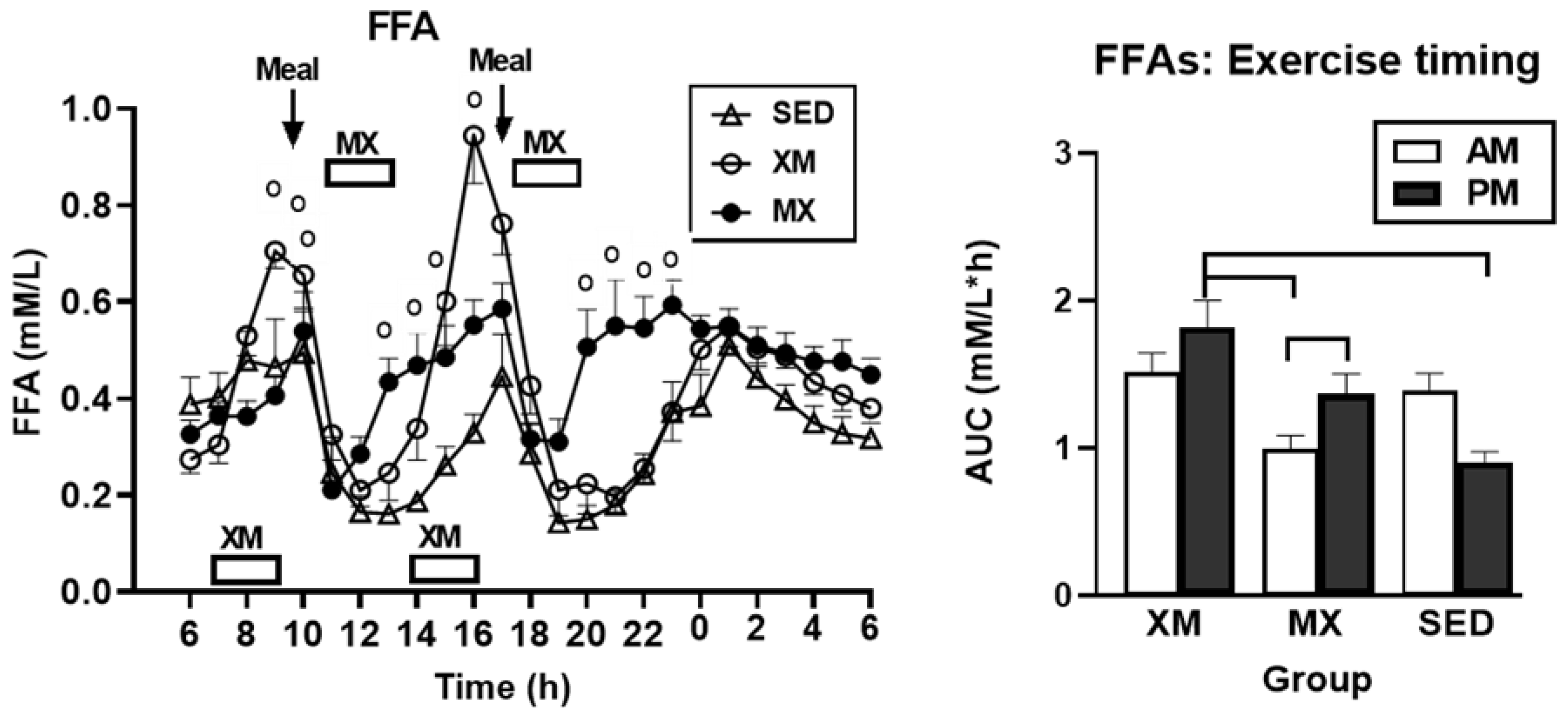
3.2.1. Exercise- and Meal-Timing Effects on Free Fatty Acids (FFAs)
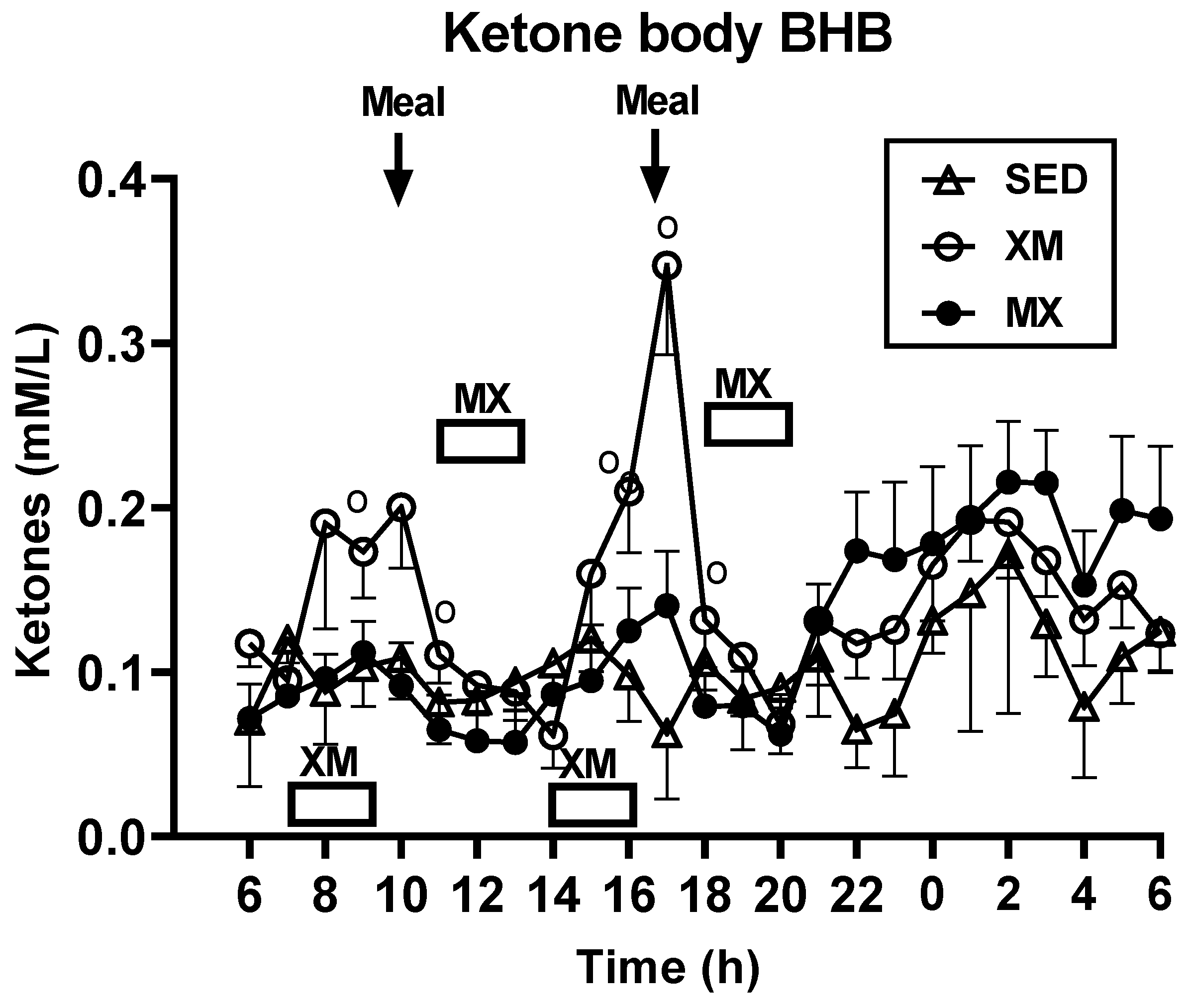
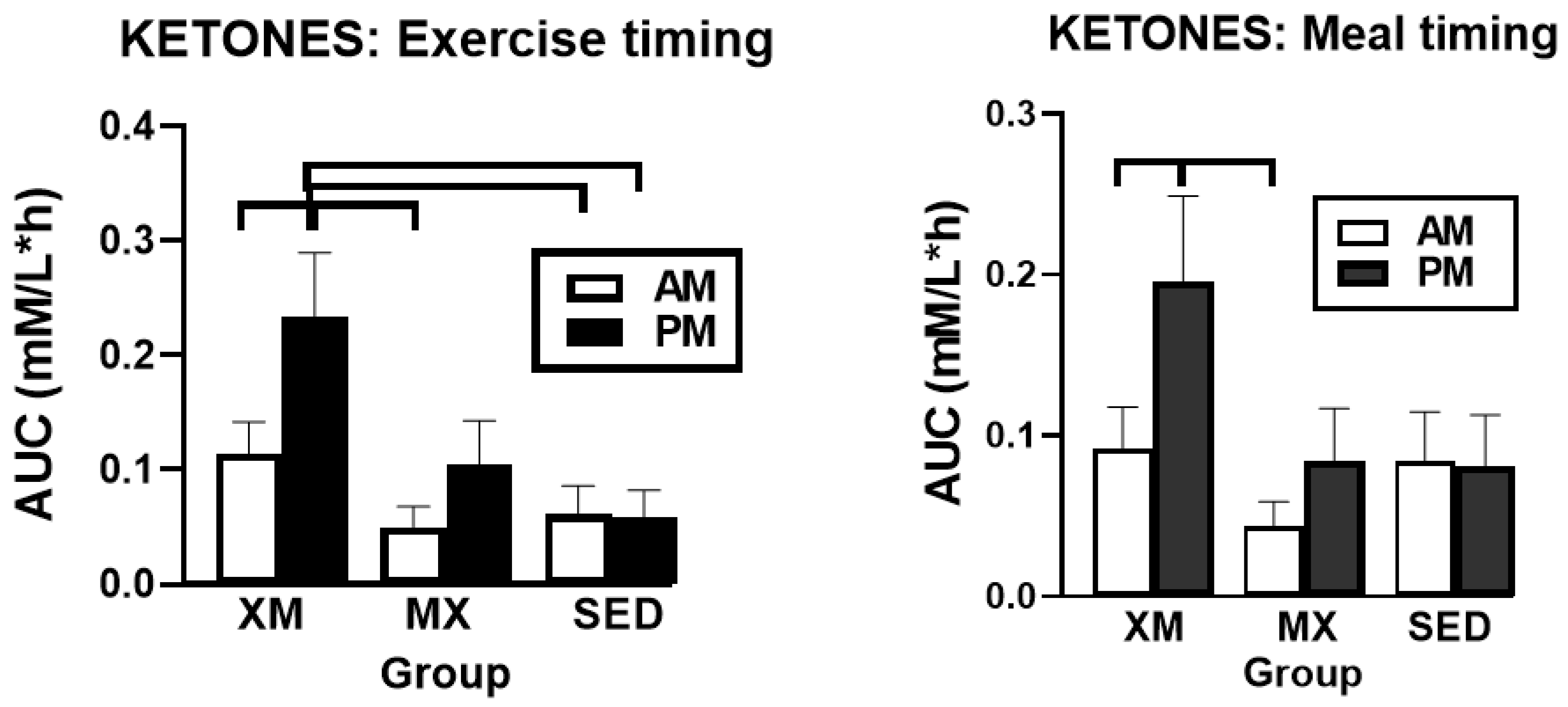
3.2.2. Exercise- and Meal-Timing Effects on D-ß-Hydroxybutyrate (BHB) Concentrations
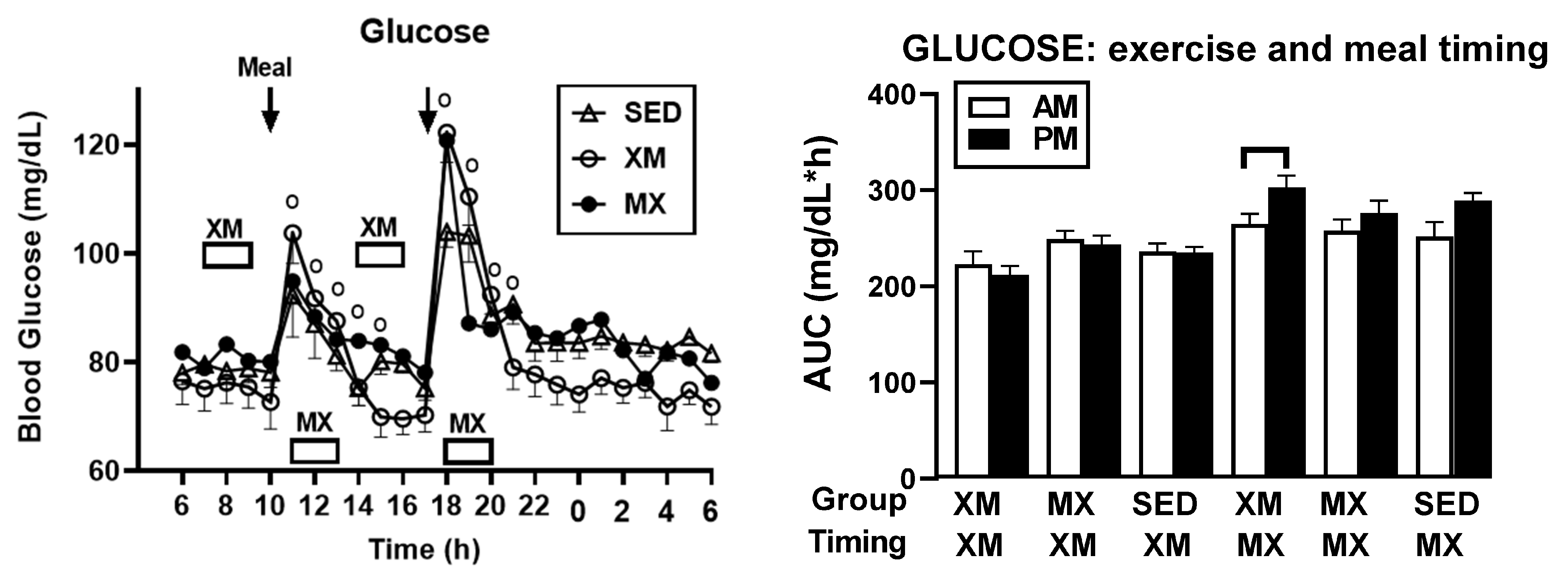
3.2.3. Exercise- and Meal-Timing Effects on Glucose Concentrations and AUCs
3.3. Exercise- and Meal-Timing Effects on Hormonal Control of Glucoregulation
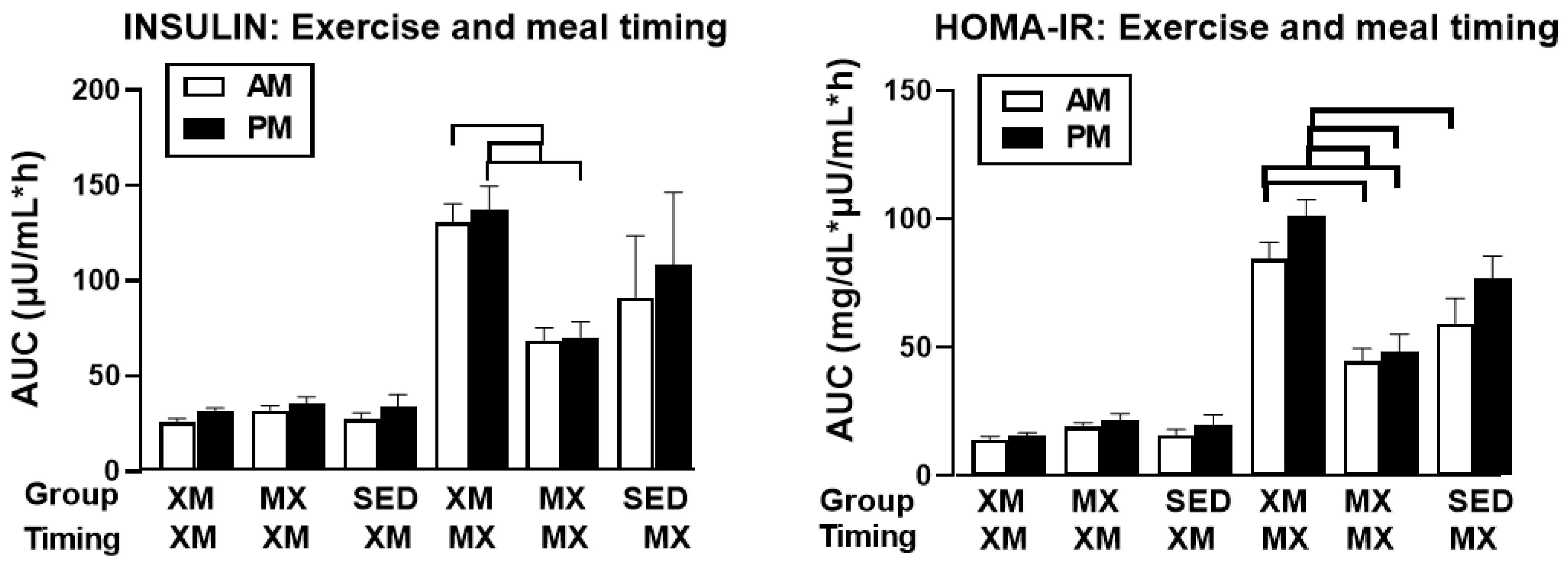
3.3.1. Exercise- and Meal-Timing Effects on Insulin
3.3.2. Exercise- and Meal-Timing Effects on HOMA-IR Measure of Insulin Resistance
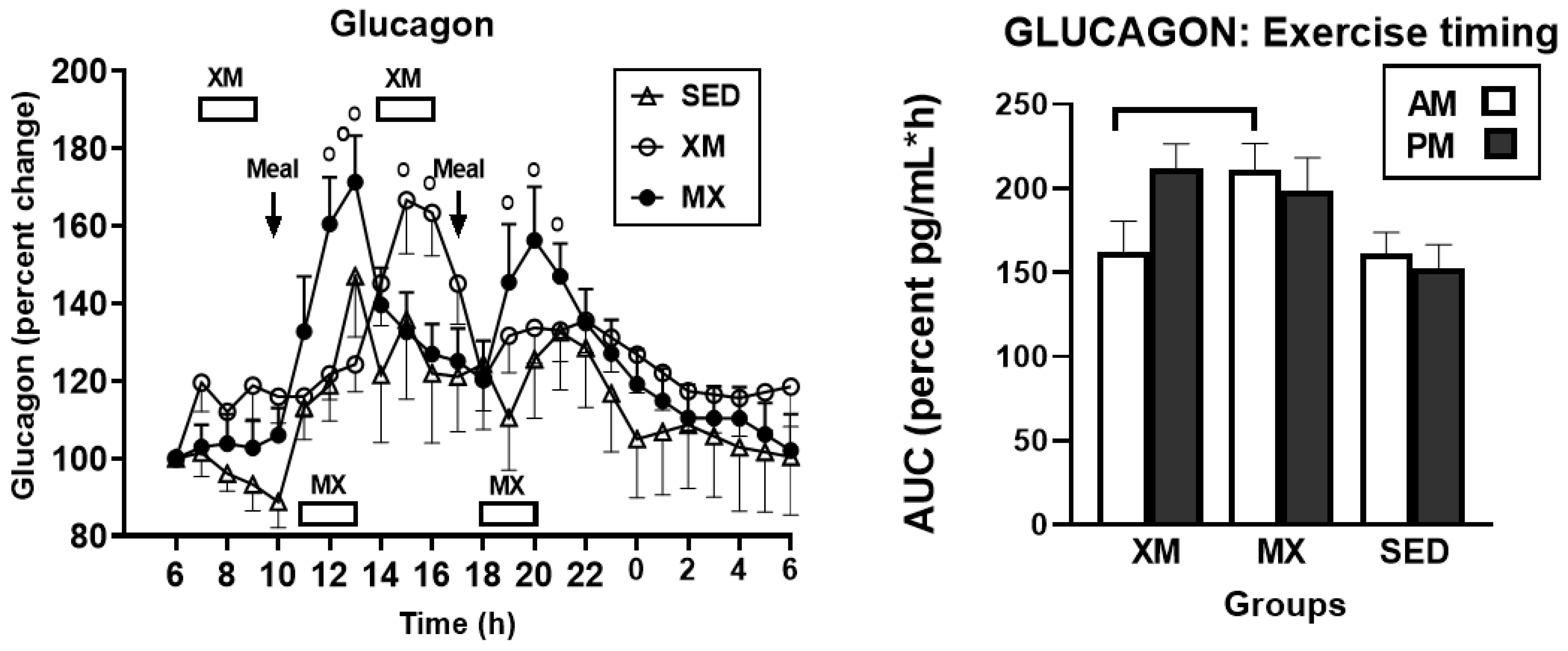
3.3.3. Exercise- and Meal-Timing Effects on Glucagon
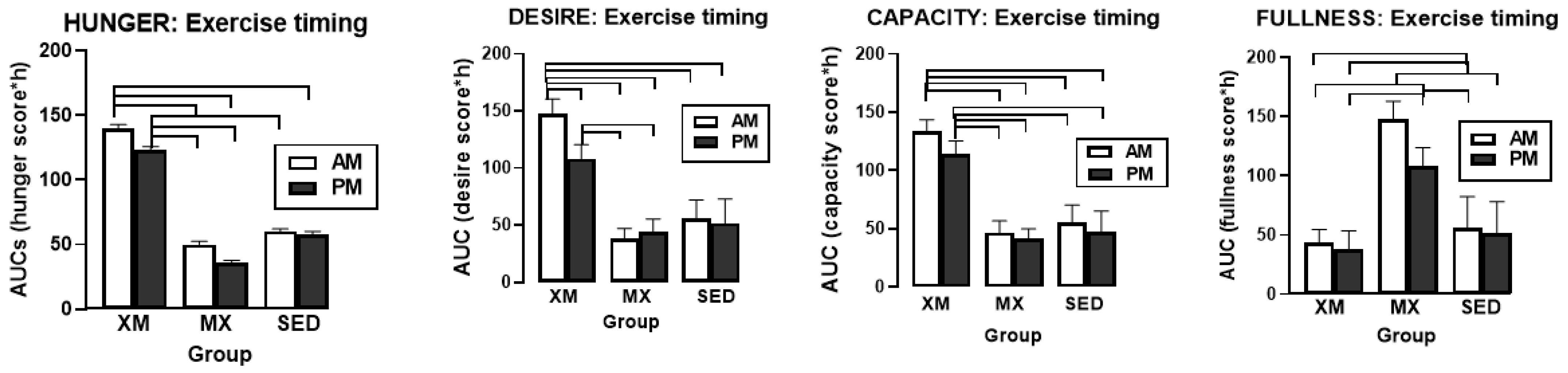
3.4. Exercise- and Meal-Timing Effects on VAS Appetite Ratings
3.5. Exercise- and Meal-Timing Effects on Gastro-Intestinal Hormones GIP, GLP-1, PYY, Ghrelin, Leptin, and CCK
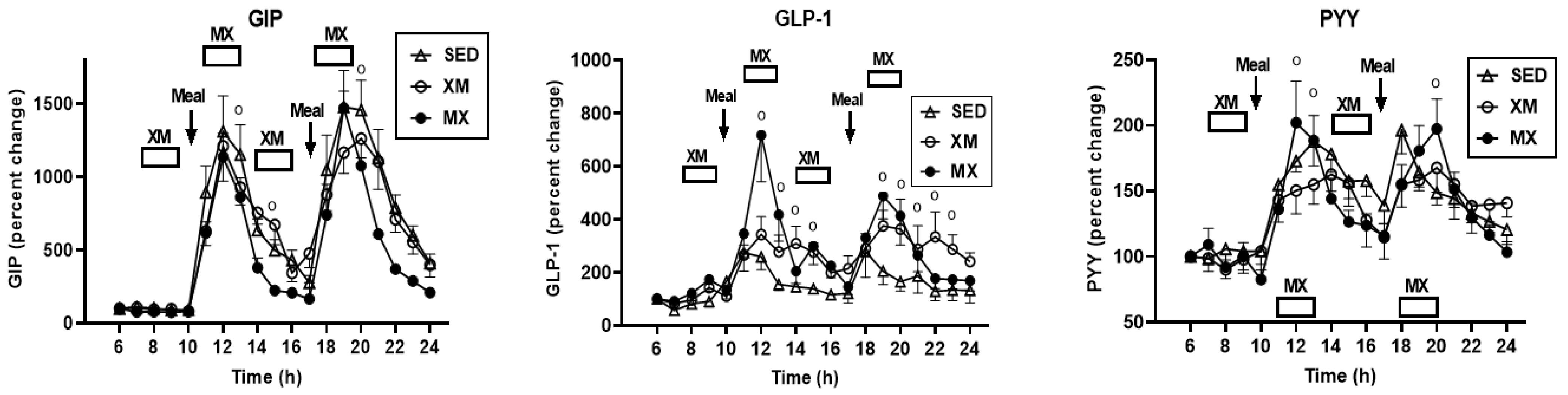
3.5.1. Exercise- and Meal-Timing Effects on GIP, GLP-1, and PYY
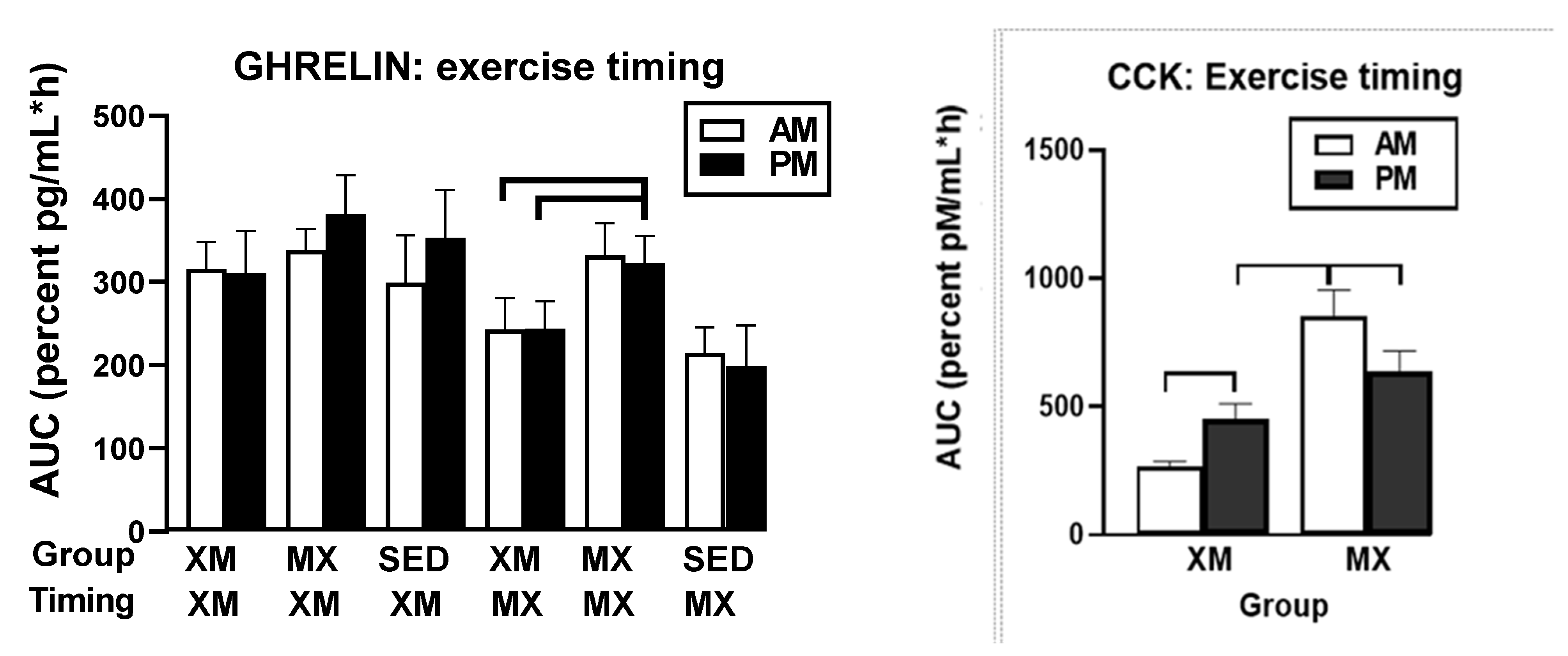
3.5.2. Exercise- and Meal-Timing Effects on Total Ghrelin, Leptin, and CCK
4. Discussion
4.1. Testing of Testing of Hypothesis 1
4.2. Testing of Hypothesis 2
4.3. Testing of Hypothesis 3
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dwyer-Lindgren, L.; Freedman, G.; Engell, R.E.; Fleming, T.D.; Lim, S.S.; Murray, C.J.L.; Mokdad, A.H. Prevalence of physical activity and obesity in US counties, 2001–2011: A road map for action. Popul. Health Metr. 2013, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Guo, J.; Dore, M.; Chow, C.C. The progressive increase of food waste in America and its environmental impact. PLoS ONE 2009, 4, e7940. [Google Scholar] [CrossRef]
- Adult Obesity Facts. Available online: www.cdc.gov/obesity/data/adult.html (accessed on 27 June 2021).
- Fryar, C.; Carroll, M.D.; Afful, J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. NCHS Health E-Stats 2020. Available online: https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm (accessed on 27 June 2021).
- Cannon, W.B.; Washburn, A.L. An explanation of hunger. Am. J. Physiol. 1912, 29, 441–454. [Google Scholar] [CrossRef]
- Cummings, D.E.; Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Investig. 2007, 117, 13–23. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, C.; Blom, W.A.M.; Smeets, P.A.M.; Stafleu, A.; Hendricks, H.F.J. Biomarkets of satiation and satiety. Am. J. Clin. Nutr. 2004, 79, 946–961. [Google Scholar] [CrossRef]
- Bellisle, F.; Drewnowski, A.; Anderson, G.H.; Westerterp-Platenga, M. Sweetness, satiation, and satiety. J. Nutr. 2012, 142, 11495–11542. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A. Brain glucose metabolism: Integration of energetics and function. Physiol. Rev. 2019, 99, 9491045. [Google Scholar] [CrossRef]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Fuchs, C.J.; Betts, J.A.; van Loon, L.J.C. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E543–E553. [Google Scholar] [CrossRef]
- Kovacs, E.M.R.; Westerterp-Plantenga, M.S.; Saris, W.H.M.; Melanson, K.J.; Goossens, I.; Geurten, P.; Brouns, F. Associations between spontaneous meal initiations and blood glucose dynamics in overweight men in negative energy balance. Br. J. Nutr. 2002, 87, 39–45. [Google Scholar] [CrossRef][Green Version]
- King, N.A.; Burley, V.J.; Blundell, J.E. Exercise-induced suppression of appetite: Effects on food intake and implications for energy balance. Eur. J. Clin. Nutr. 1994, 48, 715–724. [Google Scholar] [PubMed]
- Cummings, E.; Purnell, J.Q.; Frayo, S.R.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef]
- Wren, A.M.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 5992–5995. [Google Scholar] [CrossRef]
- Zanchi, D.; Depoortr, A.; Egloff, L.; Haller, S.; Mahlmann, L.; Lang, U.E.; Drewew, J.; Beglinger, C.; Schmdt, A.; Borgwardt, S. The impact of gut hormones on the neural circuit of appetite and satiety: A systematic review. Neurosci. Biobehav. Rev. 2017, 80, 457–475. [Google Scholar] [CrossRef]
- Berthoud, H.R. Vagal and hormonal gut-brain communication: From satiation to satisfaction. Neurogastroenterol. Motil. 2008, 20 (Suppl. 1), 64–72. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P.; Gibbs, J. Satiating effect of cholecystokinin. Ann. N. Y. Acad. Sci. 1985, 448, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Astrup, A.; Holst, J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Investig. 1998, 101, 515–520. [Google Scholar] [CrossRef]
- Batterham, R.F.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY (3-36) physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef]
- Woods, S.C. Gastrointestinal satiety signals, an overview of gastrointestinal signals that influence food intake. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G7–G13. [Google Scholar] [CrossRef]
- Helou, N.; Obeid, O.; Azar, S.T.; Hwalla, N. Variation of postprandial PYY 3-36 response following ingestion of differing macronutrient meals in obese females. Ann. Nutr. Metab. 2008, 52, 188–195. [Google Scholar] [CrossRef]
- Spiller, R.C.; Trotman, I.F.; Adrian, T.E.; Bloom, S.R.; Misiewicz, J.J.; Silk, D.B. Further characterisation of the ‘ileal brake’ reflex in man--effect of ileal infusion of partial digests of fat, protein, and starch on jejunal motility and release of neurotensin, enteroglucagon, and peptide yy. Gut 1988, 29, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Koeslag, J.H. Post-exercise ketosis and the hormone response to exercise: A review. Med. Sci. Sports Exerc. 1982, 14, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Koeslag, J.H.; Noakes, T.D.; Sloan, A.W. Post-exercise ketosis. J. Physiol. 1980, 301, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, I.S.; Jebb, S.A.; Langmack, G.; Lawrence, E.; Cheetham, C.H.; Prentice, A.M.; Highes, I.A.; McCamish, M.A.; O’Rahilly, S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 1999, 341, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Greenberg, A.S.; Fujioka, K.; Dixon, R.M.; Kushner, R.; Hunt, T.; Lubina, J.A.; Patane, J.; Self, B.; Hunt, P.; et al. Recombinant leptin for weight loss in obese and lean adults: A randomized, controlled, dose-escalation trial. JAMA 1999, 282, 1568–1575. [Google Scholar] [CrossRef]
- Wuorinen, E.C.; Borer, K.T. Circadian and ultradian components of hunger in human non-homeostatic meal-to-meal eating. Physiol. Behav. 2013, 122, 8–16. [Google Scholar] [CrossRef]
- Langendonk, J.G.; Pijl, H.; Toornvliet, A.C.; Burggraaf, J.; Frölich, M.; Schoemaker, R.C.; Doornbos, J.; Cohen, A.F.; Meinders, A.E. Circadian rhythm of plasma leptin levels in upper and lower body obese women: Influence of body fat distribution and weight loss. J. Clin. Endocrinol. Metab. 1998, 83, 1706–1712. [Google Scholar] [CrossRef]
- Geliebter, A.; Hashim, S.A. Gastric capacity in normal, obese, and bulimic women. Physiol. Behav. 2001, 74, 743–746. [Google Scholar] [CrossRef]
- Smoliga, J.M. Modelling themaximal active consumption rate and its plasticity in humans-perspectives from hot dog eating competitions. Biol. Lett. 2020, 16, 20200096. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Shott, M.E.; Stoddard, J.; Swindle, S.; Pryor, T. Association of Brain Reward Response With Body Mass Index and Ventral Striatal-Hypothalamic Circuitry Among Young Women With Eating Disorders. JAMA Psychiatry 2021, 51, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.K.; Fleming, L.W. Features of a successful therapeutic fast of 382 days duration. Postgrad. Med. 1973, 49, 203–209. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 1989, 21, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-J.; Borer, K.T. Third exposure to a reduced carbohydrate meal lowers evening postprandial insulin and GIP responses and HOMA-IR estimate of insulin resistance. PLoS ONE 2016, 11, e0165378. [Google Scholar] [CrossRef]
- Hill, A.J.; Blundell, J.E. Nutrients and behavior: Research strategies for the investigation of taste characteristics, food preferences, hunger sensations and eating patterns in man. Psychiatr. Res. 1982, 17, 203–212. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int. J. Sports Med. 2005, 26 (Suppl. 1), S28–S37. [Google Scholar] [CrossRef]
- US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 7th ed.; U.S. Government Printing Office: Washington, DC, USA, 2010.
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Caumo, A.; Bergman, R.N.; Cobelli, C. Insulin sensitivity from meal tolerance tests in normal subjects: A minimal model index. J. Clin. Endocrinol. Metab. 2000, 85, 4396–4402. [Google Scholar] [CrossRef] [PubMed]
- Aloulou, I.; Brun, J.-F.; Mercier, J. Evaluation of insulin sensitivity and glucose effecytiveness during standardized breakfast test: Comparison with the minimal model analysis of intravenous glucose tolerance test. Metab. Clin. Exp. 2006, 55, 676–690. [Google Scholar] [CrossRef]
- Bado, A.; Levasseur, S.; Attoub, S.; Kermorgant, S.; Laigneau, J.P.; Bortoluzzi, M.N.; Moizo, L.; Lehy, T.; Guerre-Millo, M.; Le Marchand-Brustel, Y.; et al. The stomach is the source of leptin. Nature 1998, 394, 790–793. [Google Scholar] [CrossRef]
- Cammisotto, P.G.; Bendayan, M. Leptin secretion by white adipose tissue and gastric mucosa. Histol. Histopathol. 2007, 22, 199–210. [Google Scholar]
- Romijn, J.A.; Coyle, E.F.; Sidosis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. Endocrinol. Metab. 1993, 28, E380–E391. [Google Scholar] [CrossRef] [PubMed]
- Borer, K.T.; Wuorinen, E.; Ku, K.; Burant, C. Appetite responds to changes in meal content, whereas ghrelin, leptin, and insulin track changes in energy availability. J. Clin. Endocrinol. Metab. 2009, 94, 2290–2298. [Google Scholar] [CrossRef]
- Aarnio, P.; Lauritsen, T.; Dela, F. Insulin secretion and glucose kinetics during exercise with and without pharmacological alpha(1)- and alpha(2)-receptor blockade. Diabetes 2001, 50, 1834–1843. [Google Scholar] [CrossRef]
- Ueda, S.; Yoshikawa, T.; Katsura, Y.; Usui, T.; Fujimoto, S. Comparable effects of moderate intensity exercise on changes in anorectic gut hormone levels and energy intake to high intensity exercise. J. Endocrinol. 2009, 203, 357–364. [Google Scholar] [CrossRef]
- Hulver, M.W.; Houmard, J.A. Plasma leptin and exercise: Recent findings. Sports Med. 2003, 33, 473–482. [Google Scholar] [CrossRef]
- King, J.A.; Wasse, L.K.; Broom, D.R.; Stensel, D.J. Influence of brisk walking on appetite, energy intake and plasma acylated ghrelin. Med. Sci. Sports Exerc. 2010, 42, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.-E.-S.; Shah, M.; Ali, A.; Malik, M.O.; Rehman, F.; Badshah, H.; Ehtesham, E.; Vitale, S.G. Treatment with metformin and combination of metformin plus pioglitazone on serum levels of IL-6 and IL-8 in polycystic ovarian syndrome: A randomized clinical trial. Horm. Metab. Res. 2019, 51, 714–722. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism 2016, 65, 20–29. [Google Scholar] [CrossRef]
- Borror, A.; Zieff, G.; Battaglini, C.; Stoner, L. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: A systematic review. Sports Med. 2018, 48, 1479–1491. [Google Scholar] [CrossRef]
- Chacko, E. Timing and intensity of exercise for glucose control. Diabetologia 2014, 57, 2425–2426. [Google Scholar] [CrossRef]
- DiPietro, L.; Gribok, A.; Stevens, M.S.; Hamm, L.F.; Rumpler, W. Three 15-min bouts of moderate postmeal walking significantly improves 24-h glycemic control in older people at risk for impaired glucose tolerance. Diabetes Care 2013, 36, 3262–3268. [Google Scholar] [CrossRef]
- Carroll, K.F.; Nestel, P.J. Diurnal variation in glucose tolerance and in insulin secretion in man. Diabetes 1973, 22, 333–348. [Google Scholar] [CrossRef]
- Jarrett, R.J.; Baker, I.A.; Keen, H.; Oakley, N.W. Diurnal variation in oral glucose tolerance: Blood sugar and plasma insulin levels morning, afternoon, and evening. Br. Med. J. 1972, 1, 199–201. [Google Scholar] [CrossRef]
- Zimmet, P.Z.; Wall, J.R.; Rome, R.; Stimmler, L.; Jarrett, R.J. Diurnal variation in glucose tolerance: Associated changes in plasma insulin, growth hormone, and non-esterified fatty acids. Br. Med. J. 1974, 1, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Dalla Man, C.; Nandy, D.K.; Levine, J.A.; Bharucha, A.E.; Rizza, R.A.; Basu, R.; Carter, R.E.; Cobelli, C.; Kudva, Y.C.; et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012, 61, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Whichelow, M.J.; Sturge, R.A.; Keen, H.; Jarrett, R.J.; Stimmler, L.; Grainger, S. Diurnal variation in response to intravenous glucose. Br. Med. J. 1974, 1, 488–491. [Google Scholar] [CrossRef]
- Tato, F.; Tato, S.; Beyer, J.; Schrezenmeir, J. Circadian variation of basal and postprandial insulin sensitivity in healthy individuals and patients with type-1 diabetes. Diabetes Res. 1991, 17, 13–24. [Google Scholar] [PubMed]
- Morris, C.J.; Yang, J.N.; Garcia, J.I.; Myers, S.; Bozzi, I.; Wang, W.; Buxton, O.M.; Shea, S.A.; Scheer, F.A.J.L. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E2225–E2234. [Google Scholar] [CrossRef]
- Daniela Jakubowicz, D.; Wainstein, J.; Ahrén, B.; Bar-Dayan, Y.; Landau, Z.; Rabinovitz, H.R.; Froy, O. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: A randomised clinical trial. Diabetologia 2015, 58, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Hoeks, J.; van Herpen, N.A.; Mensink, M.; Moonen-Kornips, E.; van Beurden, D.; Hesselink, M.K.C.; Patrick, S. Prolonged fasting identifies skeletal muscle mitochondrial dysfunction as consequence rather than cause of human insulin resistance. Diabetes 2010, 59, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Zhang, T.; Wu, X.; Qiu, J.Y. Ketone production by ketogenic diet and by intermittent fasting has different effects on the gut microbiota and disease progression in an Alzheimer’s disease rat model. J. Clin. Biochem. Nutr. 2020, 67, 188–198. [Google Scholar] [CrossRef] [PubMed]





| Variable | Sedentary | Exercise before Meals (XM) | Exercise after Meals (MX) | F(df=2,51), p T(df=51), p |
|---|---|---|---|---|
| Subjects | N = 8 | N = 23 | N = 23 | |
| Age (years) | 55.0 ± 1.1 | 59.0 ± 0.8 | 57.6 ± 1.0 | F = 2.89, p = 0.0619 |
| Weight (kg) | 66.1 ± 2.2 | 70.2 ± 2.3 | 68.3 ± 2.4 | F = 0.4969, p = 0.61 |
| BMI (kg/m2) | 23.6 ± 0.9 | 25.4 ± 0.8 | 22.2 ± 0.9 | F = 4.098, p = 0.023 |
| Body fat (%) | 35.1 ± 2.2 | 38.0 ± 1.6 | 36.3 ± 1.6 | F = 0.548, p = 0.582 |
| Fasting glucose (mg/dL) | 78.0 ± 2.4 | 77.9 ± 3.4 | 78.7 ± 1.1 | F = 0.048, p = 0.95 |
| Fasting insulin (μU/mL) | 10.6 ± 1.5 | 10.9 ± 1.2 | 10.5 ± 1.0 | F = 0.148, p = 0.86 |
| Fasting HOMA-IR | 2.0 ± 0.2 | 2.0 ± 0.1 | 2.0 ± 0.2 | F = 1.0, p = 0.379 |
| Fitness level (VO2/min * kg) | 25.6 ± 3.7 | 23.8 ± 1.8 | 27.7 ± 4.08 | F = 1.475, p = 0.864 |
| Energy intake (EI) in 2 meals (kcal) | 1614.6 ± 84.9 | 1582.2 ± 79.7 | T = 0.279, p = 0.785 | |
| Energy expended (EE) in 4 h of exercise (kcal) | 822.3 ± 66.26 | 977.7 ± 116.83 | T = 1.157, p = 0.267 | |
| Energy balance (EI-EE, kcal) | 815.8 ± 88.1 | 604.4 ± 119.46 | T = 1.424, p = 0.177 |
| Food Items | Wt (g) | CHO (g) | PRO (g) | FAT (g) | KCAL |
|---|---|---|---|---|---|
| Egg salad plate, multi grain bun | 1 plate; 156 g | 26 | 13 | 15 | 291 |
| Wheat roll | 0.5 each; 18.4 g | 8.6 | 1.7 | 0.8 | 48.4 |
| Margarine Country Crock | 0.5 tub; 2.4 g | 1.2 | 10.8 | ||
| New coleslaw | 85 g | 11 | 1 | 3 | 75 |
| Carrot sticks | 1 serving; 86 g | 4 | 1 | 0 | 20 |
| Skim milk | 1 cup; 243 g | 11 | 8 | 0 | 76 |
| Orange juice | 0.5 carton, 63 g | 7.5 | 0 | 0 | 30 |
| 1 banana | 70 g | 16 | 0.7 | 0 | 66.8 |
| 1 serving fresh fruit | Variable kind | 15 | 0.8 | 0.2 | 65 |
| Graham crackers | 4 squares; 28 g | 22 | 2 | 3 | 123 |
| Total | 121.1 | 28.2 | 23.2 | 806 | |
| Percent macronutrients | 60.1 | 14.0 | 25.9 | ||
| Glycemic index = 58 |
| Food Items | Wt (g) | CHO (g) | PRO (g) | FAT (g) | KCAL |
|---|---|---|---|---|---|
| Bacon, cheese, & ham sandwich | |||||
| Wheat toast | 2 slices; 73.4 g | 28 | 6 | 2 | 154 |
| Slivered ham | 50 g | 0 | 8 | 1.4 | 44.6 |
| Bacon | 1 slice; 4.7 g | 0 | 1.5 | 2.7 | 30.3 |
| Cheddar cheese | 1 slice; 22.7 g | 0 | 6 | 8 | 96 |
| Tomatoes | 2.5 slices; 55 g | 4 | 0.8 | 0 | 19.2 |
| Lettuce, green leaf, raw | 14 g | 0 | 0 | 0 | 0 |
| Diet mayonnaise | 1 pkg.; 12 g | 0 | 0 | 0 | 0 |
| Ketchup or mustard optional | 1 pkg.; 10 g | 0 | 0 | 0 | 0 |
| Broccoli, cauliflower, carrots (cooked, salt can be added) | 0.5 cup; 86 g | 7 | 1 | 0 | 32 |
| 1.6 oz Tossed Greens using romaine blend | 1.6 oz; 45.5 g | 5 | 1 | 0 | 24 |
| Diet french dressing | 1 package; 12 g | 2 | 0 | 0.5 | 12.5 |
| 1 serving fresh fruit | Variable kind | 22 | 1.2 | 0.3 | 95.5 |
| Cranberry Juice cocktail | 0.9 carton,110 g | 17 | 0 | 0 | 68 |
| Rold Gold pretzels | 1 oz; 28.3 g | 23 | 2 | 1 | 109 |
| Vanilla ice cream | 57 g | 13 | 1.8 | 6 | 113.2 |
| Total | 121 | 29.3 | 21.9 | 798.3 | |
| Percent macronutrients Glycemic index = 60 | 60.6 | 14.7 | 24.7 |
| Variable | XM Exercise before Meals | MX Exercise after Meals | t(df=19); p |
|---|---|---|---|
| Subjects | N = 10 | N = 10 | |
| Walking speed (km/h) | 4.5 ± 0.1 | ||
| Walking speed (m/s) | 2.0 ± 0.1 | ||
| Distance walked (km) | 8.7 ± 0.2 | ||
| RMR (kcal/h) | 53.1 ± 2.3 | 54.4 ± 4.1 | t = 0.151, p = 0.8848 |
| AM exercise EE (kcal/2 h) | 413.7 ± 24.55 | 486.5 ± 57.97 | t = 1.156, p = 0.267 |
| Carbohydrate utilization (%) during AM exercise | 43 ± 5.4 | 63 ± 5.8 | t = 2.524, p = 0.024 |
| Fat utilization (%) during AM exercise | 57 ± 5.4 | 37 ± 5.8 | t = 2.524, p = 0.024 |
| PM exercise EE (kcal/2 h) | 408.6 ± 24.5 | 491.2 ± 58.9 | t = 1.295, p = 0.216 |
| Carbohydrate utilization (%) during PM exercise | 60 ± 3.3 | 67 ±3.7 | t = 1.412, p = 0.180 |
| Fat utilization (%) during PM exercise | 40 ± 3.3 | 33 ± 3.7 | t = 1.412, p = 0.180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borer, K.T.; Lin, P.-J.; Wuorinen, E. Timing of Meals and Exercise Affects Hormonal Control of Glucoregulation, Insulin Resistance, Substrate Metabolism, and Gastrointestinal Hormones, but Has Little Effect on Appetite in Postmenopausal Women. Nutrients 2021, 13, 4342. https://doi.org/10.3390/nu13124342
Borer KT, Lin P-J, Wuorinen E. Timing of Meals and Exercise Affects Hormonal Control of Glucoregulation, Insulin Resistance, Substrate Metabolism, and Gastrointestinal Hormones, but Has Little Effect on Appetite in Postmenopausal Women. Nutrients. 2021; 13(12):4342. https://doi.org/10.3390/nu13124342
Chicago/Turabian StyleBorer, Katarina T., Po-Ju Lin, and Elizabeth Wuorinen. 2021. "Timing of Meals and Exercise Affects Hormonal Control of Glucoregulation, Insulin Resistance, Substrate Metabolism, and Gastrointestinal Hormones, but Has Little Effect on Appetite in Postmenopausal Women" Nutrients 13, no. 12: 4342. https://doi.org/10.3390/nu13124342
APA StyleBorer, K. T., Lin, P.-J., & Wuorinen, E. (2021). Timing of Meals and Exercise Affects Hormonal Control of Glucoregulation, Insulin Resistance, Substrate Metabolism, and Gastrointestinal Hormones, but Has Little Effect on Appetite in Postmenopausal Women. Nutrients, 13(12), 4342. https://doi.org/10.3390/nu13124342







