Nutrient Intake and Gut Microbial Genera Changes after a 4-Week Placebo Controlled Galacto-Oligosaccharides Intervention in Young Females
Abstract
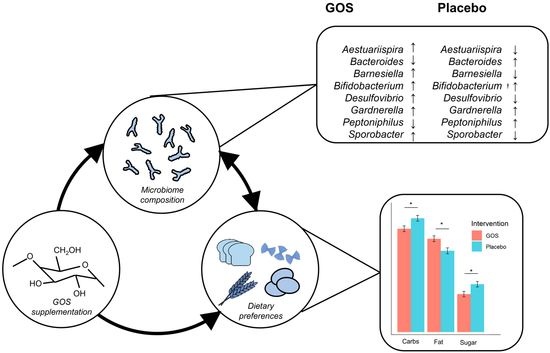
:1. Introduction
2. Methods and Materials
2.1. Participants
2.2. Protocol
2.3. Materials
2.4. Analysis
3. Results
3.1. Intervention Effects on Nutrient Outcomes
3.2. Exploring Intervention Effects on Gut Microbiota in Predicting Nutritional Intake
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Action Plan for the Prevention and Control of NCDs 2013-2020; WHO: Geneva, Switzerland, 2013; Available online: https://www.who.int/publications/i/item/9789241506236 (accessed on 2 December 2021).
- Zimmermann, M. Diet, Nutrition, and the Prevention of Chronic Diseases. Am. J. Clin. Nutr. 1994, 60, 644–645. [Google Scholar] [CrossRef] [Green Version]
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-Sweetened Beverages and Weight Gain in Children and Adults: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Department of Health and Social Care. Tackling Obesity: Empowering Adults and Children to Live Healthier Lives; Department of Health and Social Care: London, UK, 2020. Available online: https://www.gov.uk/government/publications/tackling-obesity-government-strategy/tackling-obesity-empowering-adults-and-children-to-live-healthier-lives (accessed on 2 December 2021).
- Department of Health and Social Care, Prime Minister’s Office, HM Treasury; Cabinet Office. Childhood Obesity: A Plan for Action. 2016. Available online: https://www.gov.uk/government/publications/childhood-obesity-a-plan-for-action (accessed on 2 December 2021).
- Zellner, D.A.; Loaiza, S.; Gonzalez, Z.; Pita, J.; Morales, J.; Pecora, D.; Wolf, A. Food Selection Changes under Stress. Physiol. Behav. 2006, 87, 789–793. [Google Scholar] [CrossRef]
- Yau, Y.H.C.; Potenza, M.N. Stress and Eating Behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic Effects: Metabolic and Health Benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [Green Version]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic Intake Reduces the Waking Cortisol Response and Alters Emotional Bias in Healthy Volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, N.; Milesi, C.; Burn, O.; van den Bogert, B.; Nauta, A.; Hart, K.; Sowden, P.; Burnet, P.W.J.; Cohen Kadosh, K. Anxiolytic Effects of a Galacto-Oligosaccharides Prebiotic in Healthy Females (18–25 Years) with Corresponding Changes in Gut Bacterial Composition. Sci. Rep. 2021, 11, 8302. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain–Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of Fermented Milk Product With Probiotic Modulates Brain Activity. Gastroenterology 2013, 144, 1394–1401.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna, R.A.; Foster, J.A. Gut Brain Axis: Diet Microbiota Interactions and Implications for Modulation of Anxiety and Depression. Curr. Opin. Biotechnol. 2015, 32, 35–41. [Google Scholar] [CrossRef]
- Parylak, S.L.; Koob, G.F.; Zorrilla, E.P. The Dark Side of Food Addiction. Physiol. Behav. 2011, 104, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Hume, M.P.; Nicolucci, A.C.; Reimer, R.A. Prebiotic Supplementation Improves Appetite Control in Children with Overweight and Obesity: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2017, 105, 790–799. [Google Scholar] [CrossRef] [Green Version]
- Michael, T.; Zetsche, U.; Margraf, J. Epidemiology of Anxiety Disorders. Psychiatry 2007, 6, 136–142. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The Gut Microbiota at the Intersection of Diet and Human Health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [Green Version]
- Sherwin, E.; Dinan, T.G.; Cryan, J.F. Recent Developments in Understanding the Role of the Gut Microbiota in Brain Health and Disease. Ann. N. Y. Acad. Sci. 2018, 1420, 5–25. [Google Scholar] [CrossRef]
- Plassmann, H.; Schelski, D.S.; Simon, M.C.; Koban, L. How We Decide What to Eat: Toward an Interdisciplinary Model of Gut–Brain Interactions. Wiley Interdiscip. Rev. Cogn. Sci. 2021, 11, e1562. [Google Scholar] [CrossRef] [PubMed]
- Amato, K.R.; Arrieta, M.-C.; Azad, M.B.; Bailey, M.T.; Broussard, J.L.; Bruggeling, C.E.; Claud, E.C.; Costello, E.K.; Davenport, E.R.; Dutilh, B.E.; et al. The Human Gut Microbiome and Health Inequities. Proc. Natl. Acad. Sci. USA 2021, 118, e2017947118. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment Dominates over Host Genetics in Shaping Human Gut Microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Bastiaanssen, T.F.S.; Gururajan, A.; van de Wouw, M.; Moloney, G.M.; Ritz, N.L.; Long-Smith, C.M.; Wiley, N.C.; Murphy, A.B.; Lyte, J.M.; Fouhy, F.; et al. Volatility as a Concept to Understand the Impact of Stress on the Microbiome. Psychoneuroendocrinology 2021, 124, 105047. [Google Scholar] [CrossRef] [PubMed]
- Cohen Kadosh, K.; Basso, M.; Knytl, P.; Johnstone, N.; Lau, J.Y.F.; Gibson, G.R. Psychobiotic Interventions for Anxiety in Young People: A Systematic Review and Meta-Analysis, with Youth Consultation. Transl. Psychiatry 2021, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and Probiotics for Depression and Anxiety: A Systematic Review and Meta-Analysis of Controlled Clinical Trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Romijn, A.R.; Rucklidge, J.J. Systematic Review of Evidence to Support the Theory of Psychobiotics. Nutr. Rev. 2015, 73, 675–693. [Google Scholar] [CrossRef] [Green Version]
- van de Guchte, M.; Blottière, H.M.; Doré, J. Humans as Holobionts: Implications for Prevention and Therapy. Microbiome 2018, 6, 81. [Google Scholar] [CrossRef]
- Johnstone, N.; Cohen Kadosh, K. Why a Developmental Cognitive Neuroscience Approach May Be Key for Future-Proofing Microbiota-Gut-Brain Research. Behav. Brain Sci. 2019, 42, e73. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. State-Trait Anxiety Inventory for Adults; Mind Garden Inc.: Palo Alto, CA, USA, 1983. [Google Scholar]
- Nutritics 2021. Research Edition (v5.64) [Computer Software]. Dublin. Available online: https://www.nutritics.com/p/home (accessed on 6 December 2012).
- Gloor, G.B.; Reid, G. Compositional Analysis: A Valid Approach to Analyze Microbiome High-Throughput Sequencing Data. Can. J. Microbiol. 2016, 62, 692–703. [Google Scholar] [CrossRef]
- Gloor, G.B.; Wu, J.R.; Pawlowsky-Glahn, V.; Egozcue, J.J. It’s All Relative: Analyzing Microbiome Data as Compositions. Ann. Epidemiol. 2016, 26, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Martín-Fernández, J.-A.; Hron, K.; Templ, M.; Filzmoser, P.; Palarea-Albaladejo, J. Bayesian-Multiplicative Treatment of Count Zeros in Compositional Data Sets. Stat. Model. Int. J. 2015, 15, 134–158. [Google Scholar] [CrossRef]
- Storey, J.D.; Bass, A.J.; Dabney, A.; Robinson, D. Qvalue: Q-Value Estimation for False Discovery Rate Control. Available online: http://github.com/StoreyLab/qvalue (accessed on 2 December 2021).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3re ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Riplley, B.; Venables, B.; Bates, D.M.; Firth, D.; Hornik, K.; Gebhardt, A. Package “MASS”. Support Functions and Datasets for Venables and Ripley’s MASS. 2018, p. 169. Available online: http://www.stats.ox.ac.uk/pub/MASS4/ (accessed on 2 December 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 2 December 2021).
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation 2021. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 2 December 2021).
- Robinson, D.; Hayes, A.; Couch, S. Broom: Convert Statistical Objects into Tidy Tibbles 2021. Available online: https://cran.r-project.org/web/packages/broom (accessed on 2 December 2021).
- Long, J.A. Jtools: Analysis and Presentation of Social Scientific Data 2020. Available online: https://cran.r-project.org/package=jtools (accessed on 2 December 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 2 December 2021).
- Chambers, E.S.; Byrne, C.S.; Frost, G. Carbohydrate and Human Health: Is It All about Quality? Lancet 2019, 393, 384–386. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; te Morenga, L. Carbohydrate Quality and Human Health: A Series of Systematic Reviews and Meta-Analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [Green Version]
- Murga-Garrido, S.M.; Hong, Q.; Cross, T.-W.L.; Hutchison, E.R.; Han, J.; Thomas, S.P.; Vivas, E.I.; Denu, J.; Ceschin, D.G.; Tang, Z.-Z.; et al. Gut Microbiome Variation Modulates the Effects of Dietary Fiber on Host Metabolism. Microbiome 2021, 9, 117. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary Fibre in Gastrointestinal Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Blaak, E.; Canfora, E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, E.S.; Morrison, D.J.; Frost, G. Control of Appetite and Energy Intake by SCFA: What Are the Potential Underlying Mechanisms? Proc. Nutr. Soc. 2015, 74, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef]
- Koh, A.; de Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Diether, N.E.; Willing, B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet–Microbe–Host Interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [Green Version]
- Schoeller, D.A. Limitations in the Assessment of Dietary Energy Intake by Self-Report. Metabolism 1995, 44, 18–22. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Zhou, L.-Z.; Fang, S.-T.; Long, H.-Y.; Chen, J.-Y.; Zhang, G.-X. Isolation of Desulfovibrio spp. from Human Gut Microbiota Using a Next-generation Sequencing Directed Culture Method. Lett. Appl. Microbiol. 2019, 68, 553–561. [Google Scholar] [CrossRef]
- Hong, Y.; Sheng, L.; Zhong, J.; Tao, X.; Zhu, W.; Ma, J.; Yan, J.; Zhao, A.; Zheng, X.; Wu, G.; et al. Desulfovibrio vulgaris, a Potent Acetic Acid-Producing Bacterium, Attenuates Nonalcoholic Fatty Liver Disease in Mice. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Arthur, J.C.; Jobin, C.; Keku, T.; Bruno-Barcena, J.M.; Azcarate-Peril, M.A. High Purity Galacto-Oligosaccharides Enhance Specific Bifidobacterium Species and Their Metabolic Activity in the Mouse Gut Microbiome. Benef. Microbes 2016, 7, 247–264. [Google Scholar] [CrossRef] [Green Version]
- Vulevic, J.; Juric, A.; Tzortzis, G.; Gibson, G.R. A Mixture of Trans-Galactooligosaccharides Reduces Markers of Metabolic Syndrome and Modulates the Fecal Microbiota and Immune Function of Overweight Adults. J. Nutr. 2013, 143, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Alhabeeb, H.; Chhina, N.; Morrison, D.J.; Preston, T.; Tedford, C.; Fitzpatrick, J.; Irani, C.; Busza, A.; et al. Increased Colonic Propionate Reduces Anticipatory Reward Responses in the Human Striatum to High-Energy Foods. Am. J. Clin. Nutr. 2016, 104, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.I.; Kambashi, B.; Rodriguez, J.; Cani, P.D.; et al. Effects of a Diet Based on Inulin-Rich Vegetables on Gut Health and Nutritional Behavior in Healthy Humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695. [Google Scholar] [CrossRef]
- Pedersen, C.; Lefevre, S.; Peters, V.; Patterson, M.; Ghatei, M.A.; Morgan, L.M.; Frost, G.S. Gut Hormone Release and Appetite Regulation in Healthy Non-Obese Participants Following Oligofructose Intake. A Dose-Escalation Study. Appetite 2013, 66, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Horner, K.; Hopkins, M.; Finlayson, G.; Gibbons, C.; Brennan, L. Biomarkers of Appetite: Is There a Potential Role for Metabolomics? Nutr. Res. Rev. 2020, 33, 271–286. [Google Scholar] [CrossRef] [PubMed]


| GOS (n = 23) | |||||||
|---|---|---|---|---|---|---|---|
| Measure | T1 M | (SD) | T2 M | (SD) | ∆M | (SD) | |
| Energy (Kcal) | 1631.68 | (338.09) | 1556.04 | (501.17) | −102.20 | (376.35) | ↓ |
| Protein (%E) | 16.18 | (4.54) | 16.62 | (4.09) | 0.29 | (2.77) | ↑ |
| Fat (%E) | 36.05 | (6.54) | 39.31 | (7.28) | 3.63 *B | (6.40) | ↑ |
| Monounsaturated fatty acid (%E) | 11.55 | 3.29 | 13.47 | 5.25 | 1.63 | 5.16 | ↑ |
| Saturated fatty acid (%E) | 12.58 | (3.40) | 13.40 | (3.41) | 1.11 | (2.84) | ↑ |
| Carbohydrate (%E) | 45.28 | (7.12) | 42.60 | (7.58) | −2.77 *A | (5.82) | ↓ |
| Free Sugars (%E) | 8.75 | (5.48) | 9.23 | (5.25) | 0.11 | (1.42) | ↑ |
| Sugars (%E) | 18.85 | (7.61) | 16.25 | (4.93) | −3.21 *A | (5.62) | ↓ |
| Fibre (%E) | 2.26 | (0.64) | 2.20 | (0.69) | −0.04 | (0.17) | ↓ |
| Placebo (n = 23) | |||||||
| T1 M | (SD) | T2 M | (SD) | ∆M | (SD) | ||
| Energy (Kcal) | 1921.62 | (418.33) | 1724.03 | (452.41) | −212.47 *A | (367.84) | ↓ |
| Protein (%E) | 15.35 | (4.33) | 16.10 | (4.45) | 0.74 | (2.87) | ↑ |
| Fat (%E) | 35.01 | (6.04) | 33.81 | (4.89) | −1.06 | (7.56) | ↓ |
| Monounsaturated fatty acid (%E) | 12.11 | 3.35 | 11.64 | 2.50 | −0.64 | 3.76 | ↓ |
| Saturated fatty acid (%E) | 11.65 | 3.14 | 11.91 | 3.27 | 0.38 | 3.13 | ↑ |
| Carbohydrate (%E) | 47.19 | (6.41) | 48.36 | (6.47) | 1.13 | (6.54) | ↑ |
| Free Sugars (%E) | 8.45 | (4.40) | 9.02 | (4.77) | 0.30 | (1.44) | ↑ |
| Sugars (%E) | 18.95 | (6.79) | 19.27 | (8.20) | 0.17 | (7.93) | ↑ |
| Fibre (%E) | 1.95 | (0.65) | 1.99 | (0.61) | 0.01 | (0.33) | ↑ |
| Measure | T1 M | (SD) | T2 M | (SD) | ∆M | (SD) | |
|---|---|---|---|---|---|---|---|
| GOS n = 21 | |||||||
| Aestuariispira | −3.30 | (1.54) | −2.77 | (2.35) | 0.54 | (1.82) | ↑ |
| Bacteroides | 5.69 | (1.13) | 5.49 | (1.53) | −0.20 | (0.71) | ↓ |
| Barnesiella | 1.23 | (2.39) | 1.82 | (2.25) | 0.59 | (1.51) | ↑ |
| Bifidobacterium | 3.82 | (1.96) | 4.62 | (1.37) | 0.80 **B | (1.28) | ↑ |
| Desulfovibrio | −1.45 | (2.30) | −1.30 | (2.57) | 0.15 | (0.87) | ↑ |
| Gardnerella | −3.83 | (0.65) | −3.64 | (0.75) | 0.18 | (0.59) | ↑ |
| Peptoniphilus | −3.32 | (1.10) | −3.36 | (1.21) | −0.04 | (1.41) | ↓ |
| Sporobacter | 0.11 | (1.75) | 0.48 | (1.72) | 0.37 | (1.15) | ↑ |
| Placebo n = 23 | |||||||
| Aestuariispira | −3.30 | (2.04) | −3.59 | (1.26) | −0.32 | (1.40) | ↓ |
| Bacteroides | 5.11 | (1.15) | 5.30 | (1.23) | 0.24 | (0.59) | ↑ |
| Barnesiella | 1.59 | (2.06) | 1.19 | (2.17) | −0.32 *B | (1.58) | ↓ |
| Bifidobacterium | 3.93 | (1.88) | 4.14 | (2.18) | 0.01 | (2.05) | ↑ |
| Desulfovibrio | −1.72 | (2.44) | −2.34 | (2.25) | −0.56 | (2.00) | ↓ |
| Gardnerella | −3.73 | (1.15) | −2.97 | (1.59) | 0.75 **B | (1.03) | ↑ |
| Peptoniphilus | −3.24 | (1.47) | −2.50 | (1.63) | 0.70 | (1.90) | ↑ |
| Sporobacter | 0.48 | (1.72) | 0.23 | (2.11) | −0.17 | (0.89) | ↓ |
| Carbohydrate | Fibre | Protein | Free Sugar | Saturated Fat | |
|---|---|---|---|---|---|
| (Intercept) | 12.20 | 0.08 | 0.04 | −0.79 | 1.10 * |
| [−2.04, 26.44] | [−0.09, 0.25] | [−0.80, 0.88] | [−2.22, 0.63] | [0.26, 1.94] | |
| BMI | −0.52 | ||||
| GOS | [−1.17, 0.13] | ||||
| Bifidobacterium | −2.70 ** | 0.07 | 0.86 * | −0.47 | |
| [−4.69, −0.71] | [−0.10, 0.23] | [0.06, 1.65] | [−1.78, 0.84] | ||
| Barnesiella | −1.60 | ||||
| [−3.27, 0.08] | |||||
| Desulfovibrio | 3.26 * | ||||
| [0.02, 6.49] | |||||
| Peptoniphilus | 0.82 | −0.66 | |||
| [−0.51, 2.14] | [−1.50, 0.19] | ||||
| Sporobacter | 1.34 | ||||
| [−0.23, 2.92] | |||||
| Placebo | |||||
| Bifidobacterium | −0.43 | −0.25 *** | −0.68 * | 0.92 * | |
| [−1.74, 0.89] | [−0.36, −0.14] | [−1.22, −0.15] | [0.05, 1.79] | ||
| Barnesiella | −0.20 | ||||
| [−1.97, 1.57] | |||||
| Desulfovibrio | 1.35 | ||||
| [−0.33, 3.04] | |||||
| Peptoniphilus | 1.04 * | −0.93 ** | |||
| [0.04, 2.04] | [−1.54, −0.32] | ||||
| Sporobacter | 1.60 | ||||
| [−0.53, 3.72] | |||||
| N | 44 | 44 | 44 | 44 | 44 |
| R2 | 0.34 | 0.34 | 0.22 | 0.31 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnstone, N.; Dart, S.; Knytl, P.; Nauta, A.; Hart, K.; Cohen Kadosh, K. Nutrient Intake and Gut Microbial Genera Changes after a 4-Week Placebo Controlled Galacto-Oligosaccharides Intervention in Young Females. Nutrients 2021, 13, 4384. https://doi.org/10.3390/nu13124384
Johnstone N, Dart S, Knytl P, Nauta A, Hart K, Cohen Kadosh K. Nutrient Intake and Gut Microbial Genera Changes after a 4-Week Placebo Controlled Galacto-Oligosaccharides Intervention in Young Females. Nutrients. 2021; 13(12):4384. https://doi.org/10.3390/nu13124384
Chicago/Turabian StyleJohnstone, Nicola, Susannah Dart, Paul Knytl, Arjen Nauta, Kathryn Hart, and Kathrin Cohen Kadosh. 2021. "Nutrient Intake and Gut Microbial Genera Changes after a 4-Week Placebo Controlled Galacto-Oligosaccharides Intervention in Young Females" Nutrients 13, no. 12: 4384. https://doi.org/10.3390/nu13124384
APA StyleJohnstone, N., Dart, S., Knytl, P., Nauta, A., Hart, K., & Cohen Kadosh, K. (2021). Nutrient Intake and Gut Microbial Genera Changes after a 4-Week Placebo Controlled Galacto-Oligosaccharides Intervention in Young Females. Nutrients, 13(12), 4384. https://doi.org/10.3390/nu13124384