Effect of Ketogenic Diet on Quality of Life in Adults with Chronic Disease: A Systematic Review of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Review Design
2.2. Criteria for Study Inclusion
2.3. Search Strategy
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Data Synthesis
3. Results
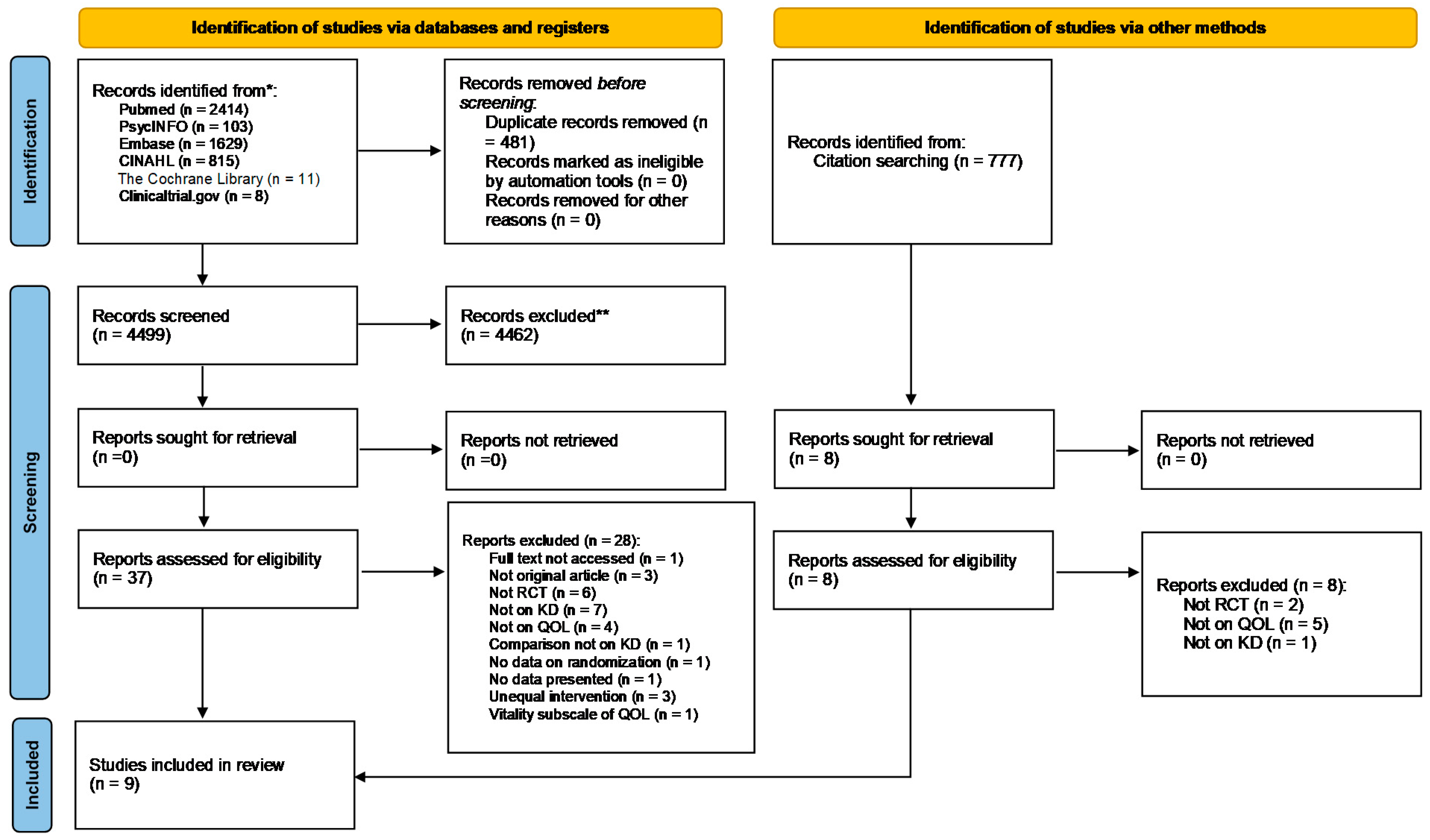
3.1. Search Results
3.2. Characteristics of Included Studies
| First Author, Year, Country | Study Population & Type of Chronic Disease | Age; %Male | Duration | Intervention: Features of KD | Control | Isocaloric Diets (arms) | Co-intervention | Assessment of Ketosis | Assessment of QOL |
|---|---|---|---|---|---|---|---|---|---|
| Cancer | |||||||||
| Augustus, 2021, Trinidad and Tobago (Trinidad) [36] | Stages 2 and 3 cancer patients, receiving chemotherapy or radiation, nonvegetarian, on a CHO-based diet (>40%) I: n = 20; 16 completers C: n = 20; 20 completers | Age: mean (SD): I: 49.80 ± 6.72 C: 51.80 ± 4.18 %Male: NR | 16 weeks | MKD: 7-day cyclic altered KD (≈10% CHO (50 g), 15% Protein (75 g), 75% Fat (167 g); 2000 Kcal); main source of Fat: MCT | Standard traditional diet | Not specified by study protocol | None | Urinary ketones: dip stick test and urine analyzer | EORTC QLQ-C30 |
| Cohen, 2018, Birmingham (USA) [42] | Women with ovarian or endometrial cancer, BMI ≥ 18.5 kg/m2 I: n = 37; 25 completers C: n = 36; 20 completers | Age: mean (SD): I: 61.5 ± 8.5 C: 58.6 ± 11.7 %Male: 0% | 12 weeks | KD: 5% CHO (≤20 g); 25% Protein (≤100 g); 70% Fat (≤125 g) | ACS diet | Neither group was instructed to alter total energy intake | None | Serum BHB: SIRRUS analyzer Urinary ketones: strips | SF-12 (PCS and MCS) |
| Khodabakhshi, 2020, Tehran (Iran) [40] | 80 women with locally advanced or metastatic breast cancer receiving chemotherapy for ≥12 weeks I: n = 40; 30 completers C: n = 40; 30 completers | Age Range: 18–70 I: 44.8 ± 8.4 C: 45.2 ± 15.0 %Male: 0% | 12 weeks | 6% CHO, 19% Protein, 20% MCT, 55% Fat | 55% CHO, 15% Protein, 30% Fat | Both diets calculated to be eucaloric | None | Blood BHB: home kit | EORTC QLQ-C30 and EORTC QLQ-BR23 |
| Martin-McGill, 2020, United Kingdom [39] | 12 patients with glioblastoma planning to go temozolomide chemotherapy and radiotherapy MKD: n = 6; 1 completed 12 weeks; 1 completed 12 months MCTKD: n = 6; 3 completed 12 weeks; 2 completed 12 months | Age Median: 57; Range: 44–66 %Male: 66.60% | 12 weeks 12 months | I1: MKD: 5% CHO, 80% Fat, 15% Protein I2: MCTKD: 10% CHO, 75% Fat (30% from MCT nutrition product), 15% Protein | None | Not specified by study protocol | None | Urinary ketones: dip stick test Blood ketones: home kit | EORTC QLQ C30 and BN20 |
| Neurological disorders | |||||||||
| Lee, 2020, Iowa (USA) [43] | 15 patients with relapsing remitting multiple sclerosis or progressive relapsing-remitting multiple sclerosis (expanded disability status ≥ 4.5) KD: n = 5; 4 analyzed (1: insufficient data) MPD: n = 6 Usual diet: n = 4 | Age Total: Range: 36–63 Mean (SD): 51.9 ± 9.5 KD: 51.8 ± 11.8 MPD: 50.3 ± 9.5 C: 54.5 ± 11.8 %Male: 50% | 12 weeks | MCT-based KD: ketogenic version of the modified Paleolithic diet with supplemental MCTs to achieve a daily goal of 70% of total Kcal from fat) | Modified Paleolithic diet C: Usual diet | Not specified by study protocol | Pre-study vitamins, supplements, and/or medications | Plasma BHB: NR | Multiple Sclerosis Quality of Life-54 |
| Philips, 2021, Hamilton (New Zealand) [41] | 26 patients with Alzheimer diseases BMI > 18.5 kg/m2 Phase 1 KD: n = 13; 11 completers Usual diet: n = 13; 13 completers Phase 2 KD: n = 13; 10 completers Usual diet: n = 13; 13 completers | Age Total: Range: 57–79 Mean (SD): 69.8 ± 6.0 KD > Usual diet: Range: 57–77 Mean(SD): 68.0 ± 5.4 Usual diet > KD: Range: 61–79 Mean(SD): 71.7 ± 6.2 %Male: Total: 62% KD > Usual diet: 77% Usual diet > KD: 46% | 12 weeks: I or C 10 weeks: washout | 58% Fat (26% SFA, 32% non-saturated), 29% Protein, 7% Fiber, 6% net CHO by weight | Usual diet 11% Fat (3% SFA, 8% non-saturated), 19% Protein, 8% Fiber, 62% net CHO by weight | Not specified by study protocol | Daily multivitamin | Serum BHB: ketone blood monitor | QOL-AD |
| Obesity and T2DM | |||||||||
| Brinkworth, 2016, Adelaide (Australia) [37] | Adults with T2DM (HbA1c ≥ 7.0% or taking a diabetes medication), overweight and obese (BMI: 26–45 kg/m2) I: n = 58; 41 completers C: n = 57; 37 completers | Age Range: 35–68 Mean (SD) I: 58 ± 72 C: 58 ± 7 %Male: I: 64% C: 51% | 12 months | Very-low CHO, high-fat diet: 14% CHO (<50 g); 28% Protein, 58% Fat (35% MUFA, 13% PUFA, <10% SFA) | High-CHO, low-fat diet: 53% CHO; 17% Protein; <30% Fat (15% MUFA, 9% PUFA, <10% SFA) | For I and C: ∼30% energy restriction (500–1000 Kcal/day) | 60-min, moderate-intensity, exercise: 3 days/week | Plasma BHB: D-3 Hydroxybutyrate kit | Diabetes-39 |
| Durrer, 2021, Southern British Columbia (Canada) [38] | Adults with T2DM, using glucose-lowering medication, obese (BMI ≥ 30 kg/m2) I: n = 98; 78 completers (98 ITT) C: n = 90; 60 completers (90 ITT) | Age: mean (SD): I: 58 ± 11 C: 59 ± 8 %Male: I: 44% C: 43% | 12 weeks | Low-CHO energy-restricted commercial weight loss plan supplemented with whole foods (<50 g CHO; 35–45 g Fat, 110–120 g Protein; 850–1100 Kcal) | Information conforming with 2013 Diabetes Canada Clinical Practice Guidelines | Not specified by study protocol | None | Capillary blood ketones: NR | SF-20 |
| Knee Osteoarthritis | |||||||||
| Strath, 2020, Birmingham (USA) [44] | 21 adults with knee osteoarthritis LCD: n = 8 LFD: n = 6 C: n = 7 | Age Range: 65–75 Mean (SD) LCD: 71.00 ± 3.12 LFD: 72.33 ± 1.97 C: 68.71 ± 7.11 %Male: LCD: 60%; LFD: 75%; C: 80% (Completers: LCD: 60%; LFD: 100%; C: 75%) | 12 weeks | Kcal: unlimited; Fat: unlimited; CHO: 20 g; Proteins: 100 g | LFD: Kcal: 800–1200; Fat: 50–67 g; CHO: unlimited; Proteins: 100 g C: Kcal, Fat, CHO, Proteins: unlimited | No | None | Not measured | Knee Injury and Osteoarthritis Outcome Score quality of life |
3.3. Assessment of Risk of Bias
3.4. Results of Included Studies
| (a) | ||||
| First Author, Year | Effect on QOL | Conclusion | ||
| Cancer | ||||
| Augustus, 2021 [36] | Mean change: I: +28 (Sig.); C: +0.6 (NS) Sig. between-group difference over time; effect size: 0.268 (medium) Inverse association between urinary ketones and QOL (b = −3.175, 95% CI = −5.723, −0.626) | Keto-adapted patients on a MKD had an improvement in self-reported QOL over time KD may improve QOL of cancer patients (not inclusive of advanced stage cancer) compared with patients on a standard traditional diet | ||
| Cohen, 2018 [42] | Sig. within-group increase in PCS in I (+11%); NS change in C Sig. between-group difference in adjusted PCS, NS between-group difference in MCS NS association between PCS or MCS and serum BHB | In women with ovarian or endometrial cancer, a KD does not negatively affect quality of life and may improve physical function | ||
| Khodabakhshi, 2020 [40] | Mean difference (95% CI): Physical functioning: 9.9 (−0.7, 20) (NS) Role functioning: 8.9 (−6, 23) (NS) Cognitive functioning: 5.5 (−8, 14) Emotional functioning: 2 (−10, 14) Social functioning 3.5 (−4.6, 5.9) (NS) Global quality of life: 8.1 (−5.7, 3.3) (NS) | After adjusting baseline values and chemotherapy status, NS differences in all domains of QOL between I and C KD diet combined to chemotherapy in patients with breast cancer does not bring additional benefit | ||
| Martin-McGill, 2020 [39] | Week 6 onward, GHS improved for the patient following MKD and reduced for patients following MCTKD | For retained patients at 12 months, GHS reduced within the MCTKD group and improved in the MKD group | ||
| Neurological disorders | ||||
| Lee, 2020 [43] | NS between-group differences in mental health and physical health | NS differences in mental health and physical health QOL scores among groups Suggested clinically sig. improvements in mental health and physical health QOL with Modified Paleolithic diet (change > 5) Suggested clinically sig. decline in mental health and physical health QOL with usual diet | ||
| Philips, 2021 [41] | Treatment effect (mean ± SD) Phase 1: KD > Usual diet: +2.86 ± 4.64; Usual diet > KD: −1.15 ± 5.41 Phase 2: KD > Usual diet: +0.31 ± 3.68; Usual diet > KD: +3.03 ± 7.52 All patients: KD > Usual diet: +2.95 ± 6.12; Usual diet > KD: −0.42 ± 4.60 Overall treatment effect: +3.37 ± 6.86 (Sig. change) | Patients on KD had improved QOL compared to those on usual diet High rates of retention and adherence are achievable in applying a 12-week MKD to patients with Alzheimer’s disease and adverse effects are mild | ||
| Obesity and T2DM | ||||
| Brinkworth, 2016 [37] | NS between-group differences in anxiety and worry, social burden, sexual functioning, and energy and mobility | In overweight and obese adults with T2DM, both high and low CHO diets achieved comparable improvements in QOL | ||
| Durrer, 2021 [38] | Treatment effect (95% CI): Physical Functioning: 0.7 (−7.7, 9.9) * Role Functioning: 13.6 (2.4, 26.3) * Social Functioning: 6.1 (−2, 14.3) * Mental Health: 6.9 (1.9, 12.7) * Health Perceptions: 19.2 (13.2, 25.4) (NS) Pain: −7.5 (−17.2, −0.1) * (* a precise p-value could not be obtained) | In obese patients with T2DM, there was sig. improvement in role functioning, mental health, health perceptions, and pain with low-CHO energy-restricted diet compared with the usual diet | ||
| Knee Osteoarthritis | ||||
| Strath, 2020 [44] | LCD: sig. withing-group change (≈−0.6) LFD: sig. withing-group change (≈−0.2) C: sig. withing-group change (≈−0.4) NS time * diet interaction and NS differences in LCD and LFD group after post hoc analysis | NS differences in LCD and LFD group were noted after post hoc analysis | ||
| (b) | ||||
| First Author, Year | Compliance | Ketosis | Adverse events/Side effects | Attrition |
| Cancer | ||||
| Augustus, 2021 [36] | Three-day food diaries (2 weekdays and 1 weekend) obtained at the weeks 6 and 12 | Sig. rise in urinary ketones in I vs. C | I: side-effects related to keto-adaptation (first 2–6 weeks; sig. reduced 6 weeks post treatment): fatigue, dizziness, reduced energy C: headaches/migraines Unable to determine whether reduced energy or fatigue are attributed to I or by natural progression of the disease | I: 2% [n = 4: nausea and vomiting related to I affecting subjects’ palatability (n = 2); inability to complete testing at all follow-up times (n = 1); mortality not related to medical treatment nor I (n = 1)] C: 0% |
| Cohen, 2018 [42] | Weekly phone calls/emails from the study dietitian to review food records and discuss strategies to enhance participants’ adherence | BHB (mmol/L) I: Sig. increase C: NS change | NR | I: n = 6 did not enroll due to scheduling conflicts; n = 6 withdrew: 1 scheduling conflicts; 1 no longer wishing to comply with dietary requirements; 3 cancer recurrence; 1 death C: n = 10 did not enroll due to scheduling conflicts; n = 6 withdrew: 3 scheduling conflicts; 2 no longer wishing to comply with dietary requirements, 1 death |
| Khodabakhshi, 2020 [40] | BHB every 3 weeks and dietary intake | Serum ketones > 0.5 mmol/L: 66.7% Sig. increase in serum ketones in I | None reported in both groups | I: n = 10 withdrew after beginning assigned diet (2 nausea and hypoglycemia; 3 weakness and hunger; 1 refusal to participate; 2 unable to stick to diet; 2 lack of energy and oiliness of the diet) C: n = 3 patients withdrew before beginning assigned diet; n = 7 withdrew after beginning assigned diet (5 frequent blood sampling; 1 surgery; 1 diabetes) |
| Martin-McGill, 2020 [39] | Assessment of diet adherence: food diaries Assessment of ketosis: urinary ketones and blood ketones (at home) | Blood ketones: ≥4 mmol/L During the first 6 weeks: MCTKD: 79.7%; MKD: 79.3% | Hypokalemia (n = 2), hypernatremia (n = 1), hypocalcemia (n = 1), partial seizure (n = 1), post-operative wound infection (n = 1) seizure (n = 1), back pain (n = 1) [none related to the dietary intervention] Gastrointestinal side effects: First 6 weeks: MCT KD group: diarrhea (n = 1), nausea (n = 1), vomiting (n = 1), dyspepsia (n = 1); MKD group: vomiting (n = 1) and a dry mouth (n = 1) At month 6: MCTKD: diarrhea, dyspepsia, constipation (n = 1); MKD: constipation (n = 1) | MCTKD: 6 randomized: 1 withdrew prior to commencing (changed mind); 5 commenced; 2 withdrew (1 dietary burden; 1 recruited to another trial); 3 completed 12 weeks; 1 withdrew (GI intolerance); 2 completed 12 months MKD: 6 randomized: 1 withdrew prior to commencing (non-related SAE); 5 commenced; 4 withdrew (2 dietary burden; 1 tumor progression; 1 nausea); 1 completed 12 weeks; 1 completed 12 months |
| Neurological disorders | ||||
| Lee, 2020 [43] | Plasma BHB | Plasma BHB: ≥0.50 mmol/L Sig. higher BHB in KD than MPD and C | None reported | n = 1 in KD not analyzed because of large amount of missing data |
| Philips, 2021 [41] | Assessment of diet adherence: 3-day (2 weekdays, 1 weekend day) food record Assessment of ketosis: Bedtime ketone monitoring | Serum BHB ≥ 0.6 mmol/L 85.7% of patients who completed KD achieved sustained physiological ketosis | I: Increased irritability: 19%; Increased fatigue: 23%; Sugar craving: 8%; Insomnia: 4%; Muscle cramp: 12%; Constipation: 4%; Feeling light headed: 15%; Increased back pain: 4%; Excessive hunger: 8%; Excessive thirst: 4%; Diarrhea: 4%; Palpitations: 4% C: Increased irritability: 35%; Increased fatigue: 27%; Sugar craving: 23%; Insomnia: 19%; Muscle cramp: 4%; Constipation: 15%; Feeling light headed: 12%; Increased back pain: 12%; Nausea: 8%; Headache: 12%; Heart burn: 8%; Palpitations: 4%; Urinary calculus: 4%; Psychotic episode: 4% | Phase 1 I: n = 13; 2 withdrew (1 declined to remove daily sugar; 1 excess coconut oil and diarrhea); 11 completers C: n = 13; 13 completers Phase 2 I: n = 13; 10 completers; 3 withdrew (1 declined to remove daily sugar; 1 declined to remove daily beer; 1 declined most of the food) C: n = 13; 13 completers |
| Obesity and T2DM | ||||
| Brinkworth, 2016 [37] | Good compliance in both groups to prescribed diets throughout the study assessed by dietary intake | Plasma BHB increased more with I after 4 weeks and remained higher over 52 weeks than C (Sig.) | Musculoskeletal ailments: I: n = 8; C: n = 13 [Associated with exercise training: I: n = 6; C: n = 8]; Gastrointestinal disorders (constipation and diverticulitis): I: n = 2; C: n = 1, Esophageal ulcers with Helicobacter pylori infection: C: n = 1; Non-hospitalized hypoglycemia incident: I: n = 1; Hospitalization for arrhythmia with suspected heart failure: C: n = 1; Prostate cancer and melanoma: I: n = 1; C: n = 1; Non-study related workplace injuries: I: n = 3; C: n = 1; Hospitalization for pneumonia: I: n = 1; Malignant hyperthermia: I: n = 1; Anaphylactic reaction to the influenza vaccine: C: n = 1; Motor vehicle accident: C: n = 1 | I: n = 17 (6 lost to follow-up; 4 time constraints; 3 work commitments; 2 unable to comply with diet; 2 personal reasons; 1 health issue external to study) C: n = 21 (4 lost to follow-up; 1 time constraints; 3 work commitments; 5 unable to comply with diet; 5 personal reasons; 3 health issue external to study) |
| Durrer, 2021 [38] | I: non-adherence: 2.12% Assessment of food intake: fasting blood sample and a 3-day diet | NR | I: n = 4: mild hypoglycemic events (n = 2 when participants were reluctant to reduce insulin dosages by the recommended amount; resolved with recommended medication); Hypoglycemic symptoms (n = 1 might be due to waiting too long between meals; resolved after solving this issue); Cardiac event (n = 1 occurred 3 weeks into the study; deemed not related to I by data and safety monitoring board) C: n = 0 | Drop-out prior to commencing the trial: I: n = 4 (1 ineligible; 3 lost contact) C: n = 15 (2 ineligible; 1 moved away; 12 lost contact) Attrition I: n = 16 (2 family issues; 2 could not adhere; 2 unrelated health issues; 1 travel; 9 lost contact) C: n = 15 (15 lost contact) |
| Knee Osteoarthritis | ||||
| Strath, 2020 [44] | Adherence verbally confirmed; food journals assessed by a dietician and the study administrator at each visit | Not measured | NR | LFD: 1 lost to nonadherence C: 2 failed to complete the study |
3.5. Dietary Compliance with the Ketogenic Diet
3.6. Adverse Events
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Reynolds, R.; Dennis, S.; Hasan, I.; Slewa, J.; Chen, W.; Tian, D.; Bobba, S.; Zwar, N. A systematic review of chronic disease management interventions in primary care. BMC Fam. Pract. 2018, 19, 11. [Google Scholar] [CrossRef]
- Schmidt, H. Chronic Disease Prevention and Health Promotion. In Public Health Ethics: Cases Spanning the Globe; Dawson, A., Saenz, C., Reis, A., Bolan, G., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Cugusi, L.; Prosperini, L.; Mura, G. Exergaming for Quality of Life in Persons Living with Chronic Diseases: A Systematic Review and Meta-analysis. PM&R 2021, 13, 756–780. [Google Scholar]
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Felce, D.; Perry, J. Quality of life: Its definition and measurement. Res. Dev. Disabil. 1995, 16, 51–74. [Google Scholar] [CrossRef]
- World Health Organization. Programme on Mental Health: WHOQOL User Manual; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Fallowfield, L. What is Quality of Life. Available online: http://www.bandolier.org.uk/painres/download/What%20is%202009/What_is_QOL.pdf (accessed on 6 October 2021).
- Cella, D.F. Quality of life: Concepts and definition. J. Pain Symptom Manag. 1994, 9, 186–192. [Google Scholar] [CrossRef]
- Walker, A.E. Multiple chronic diseases and quality of life: Patterns emerging from a large national sample, Australia. Chronic Illn. 2007, 3, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Lopez, A.D.; World Health Organization. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020: Summary; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Yach, D.; Hawkes, C.; Gould, C.L.; Hofman, K.J. The global burden of chronic diseases: Overcoming impediments to prevention and control. JAMA 2004, 291, 2616–2622. [Google Scholar] [CrossRef]
- Glozman, J.M. Quality of life of caregivers. Neuropsychol. Rev. 2004, 14, 183–196. [Google Scholar] [CrossRef]
- Berry, R.A.; Murphy, J.F. Well-being of caregivers of spouses with Parkinson’s disease. Clin. Nurs. Res. 1995, 4, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.H.; Stewart, B.J.; Archbold, P.G.; Inoue, I.; Jaglin, J.; Lannon, M.; Rost-Ruffner, E.; Tennis, M.; McDermott, M.P.; Amyot, D.; et al. Living with a person who has Parkinson’s disease: The spouse’s perspective by stage of disease. Parkinson’s Study Group. Mov. Disord. Off. J. Mov. Disord. Soc. 1998, 13, 20–28. [Google Scholar] [CrossRef]
- Bruce, S.; Devlin, A.; Air, L.; Cook, L. Changes in quality of life as a result of ketogenic diet therapy: A new approach to assessment with the potential for positive therapeutic effects. Epilepsy Behav. 2017, 66, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Brundage, M.; Leis, A.; Bezjak, A.; Feldman-Stewart, D.; Degner, L.; Velji, K.; Zetes-Zanatta, L.; Tu, D.; Ritvo, P.; Pater, J. Cancer patients’ preferences for communicating clinical trial quality of life information: A qualitative study. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2003, 12, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Bouteldja, N.; Andersen, L.T.; Møller, N.; Gormsen, L.C. Using positron emission tomography to study human ketone body metabolism: A review. Metab. Clin. Exp. 2014, 63, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Dhamija, R.; Eckert, S.; Wirrell, E. Ketogenic diet. Can. J. Neurol. Sci. 2013, 40, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. The ketogenic diet for the treatment of childhood epilepsy: A randomised controlled trial. Lancet Neurol. 2008, 7, 500–506. [Google Scholar] [CrossRef]
- Coppola, G.; D’Aniello, A.; Messana, T.; Di Pasquale, F.; della Corte, R.; Pascotto, A.; Verrotti, A. Low glycemic index diet in children and young adults with refractory epilepsy: First Italian experience. Seizure 2011, 20, 526–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, S.K.; Neal, E.G.; Camfield, C.S.; Kossoff, E.H. Dietary therapies for epilepsy: Future research. Epilepsy Behav. 2011, 22, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.L.P.; Mattingly, S.; Schirrmacher, R.; Sawyer, M.B.; Fine, E.J.; Prado, C.M. A Nutritional Perspective of Ketogenic Diet in Cancer: A Narrative Review. J. Acad. Nutr. Diet. 2018, 118, 668–688. [Google Scholar] [CrossRef]
- Sremanakova, J.; Sowerbutts, A.M.; Burden, S. A systematic review of the use of ketogenic diets in adult patients with cancer. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2018, 31, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Field, R.; Pourkazemi, F.; Rooney, K. Effects of a low-carbohydrate ketogenic diet on reported pain, blood biomarkers and quality of life in patients with chronic pain: A pilot randomised clinical trial rationale, study design and protocol. Eur. J. Integr. Med. 2021, 45, 101346. [Google Scholar] [CrossRef]
- Rho, J.M. How does the ketogenic diet induce anti-seizure effects? Neurosci. Lett. 2017, 637, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.J.; Villamena, F.A.; Volek, J.S. Nutritional Ketosis and Mitohormesis: Potential Implications for Mitochondrial Function and Human Health. J. Nutr. Metab. 2018, 2018, 5157645. [Google Scholar] [CrossRef] [Green Version]
- Masino, S.A.; Ruskin, D.N. Ketogenic diets and pain. J. Child Neurol. 2013, 28, 993–1001. [Google Scholar] [CrossRef] [Green Version]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Bernell, S.; Howard, S.W. Use Your Words Carefully: What Is a Chronic Disease? Front. Public Health 2016, 4, 159. [Google Scholar] [CrossRef] [Green Version]
- GACD. Non-Communicable Diseases: Key Facts and Figures. Available online: https://www.gacd.org/about/what-we-do/what-are-ncds/key-facts-and-figures (accessed on 6 October 2021).
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Augustus, E.; Granderson, I.; Rocke, K.D. The Impact of a Ketogenic Dietary Intervention on the Quality of Life of Stage II and III Cancer Patients: A Randomized Controlled Trial in the Caribbean. Nutr. Cancer 2021, 73, 1590–1600. [Google Scholar] [CrossRef] [PubMed]
- Brinkworth, G.D.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.; Wilson, C.J. Long-term effects of very low-carbohydrate and high-carbohydrate weight-loss diets on psychological health in obese adults with type 2 diabetes: Randomized controlled trial. J. Intern. Med. 2016, 280, 388–397. [Google Scholar] [CrossRef]
- Durrer, C.; McKelvey, S.; Singer, J.; Batterham, A.M.; Johnson, J.D.; Gudmundson, K.; Wortman, J.; Little, J.P. A randomized controlled trial of pharmacist-led therapeutic carbohydrate and energy restriction in type 2 diabetes. Nat. Commun. 2021, 12, 5367. [Google Scholar] [CrossRef] [PubMed]
- Martin-McGill, K.J.; Marson, A.G.; Tudur Smith, C.; Young, B.; Mills, S.J.; Cherry, M.G.; Jenkinson, M.D. Ketogenic diets as an adjuvant therapy for glioblastoma (KEATING): A randomized, mixed methods, feasibility study. J. Neuro-Oncol. 2020, 147, 213–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodabakhshi, A.; Seyfried, T.N.; Kalamian, M.; Beheshti, M.; Davoodi, S.H. Does a ketogenic diet have beneficial effects on quality of life, physical activity or biomarkers in patients with breast cancer: A randomized controlled clinical trial. Nutr. J. 2020, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C.L.; Deprez, L.M.; Mortimer, G.M.N.; Murtagh, D.K.J.; McCoy, S.; Mylchreest, R.; Gilbertson, L.J.; Clark, K.M.; Simpson, P.V.; McManus, E.J.; et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimer’s Res. Ther. 2021, 13, 51. [Google Scholar] [CrossRef]
- Cohen, C.W.; Fontaine, K.R.; Arend, R.C.; Soleymani, T.; Gower, B.A. Favorable Effects of a Ketogenic Diet on Physical Function, Perceived Energy, and Food Cravings in Women with Ovarian or Endometrial Cancer: A Randomized, Controlled Trial. Nutrients 2018, 10, 1187. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Titcomb, T.J.; Bisht, B.; Rubenstein, L.M.; Louison, R.; Wahls, T.L. A Modified MCT-Based Ketogenic Diet Increases Plasma β-Hydroxybutyrate but Has Less Effect on Fatigue and Quality of Life in People with Multiple Sclerosis Compared to a Modified Paleolithic Diet: A Waitlist-Controlled, Randomized Pilot Study. J. Am. Coll. Nutr. 2021, 40, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Strath, L.J.; Jones, C.D.; Philip George, A.; Lukens, S.L.; Morrison, S.A.; Soleymani, T.; Locher, J.L.; Gower, B.A.; Sorge, R.E. The Effect of Low-Carbohydrate and Low-Fat Diets on Pain in Individuals with Knee Osteoarthritis. Pain Med. 2020, 21, 150–160. [Google Scholar] [CrossRef]
- McDonald, T.J.W.; Cervenka, M.C. Lessons learned from recent clinical trials of ketogenic diet therapies in adults. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 418–424. [Google Scholar] [CrossRef]
- Brown, A.J. Low-carb diets, fasting and euphoria: Is there a link between ketosis and gamma-hydroxybutyrate (GHB)? Med. Hypotheses 2007, 68, 268–271. [Google Scholar] [CrossRef]
- Cash, C.D. Gamma-hydroxybutyrate: An overview of the pros and cons for it being a neurotransmitter and/or a useful therapeutic agent. Neurosci. Biobehav. Rev. 1994, 18, 291–304. [Google Scholar] [CrossRef]
- Kolotkin, R.L.; Andersen, J.R. A systematic review of reviews: Exploring the relationship between obesity, weight loss and health-related quality of life. Clin. Obes. 2017, 7, 273–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida Roediger, M.; de Fátima Nunes Marucci, M.; Duim, E.L.; Santos, J.L.F.; de Oliveira Duarte, Y.A.; de Oliveira, C. Inflammation and quality of life in later life: Findings from the health, well-being and aging study (SABE). Health Qual. Life Outcomes 2019, 17, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowakowski, A.C. Chronic inflammation and quality of life in older adults: A cross-sectional study using biomarkers to predict emotional and relational outcomes. Health Qual. Life Outcomes 2014, 12, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shingler, E.; Perry, R.; Mitchell, A.; England, C.; Perks, C.; Herbert, G.; Ness, A.; Atkinson, C. Dietary restriction during the treatment of cancer: Results of a systematic scoping review. BMC Cancer 2019, 19, 811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, D.D.; Aminzadeh-Gohari, S.; Tulipan, J.; Catalano, L.; Feichtinger, R.G.; Kofler, B. Ketogenic diet in the treatment of cancer-Where do we stand? Mol. Metab. 2020, 33, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Pavón, S.; Lázaro, E.; Martínez, O.; Amayra, I.; López-Paz, J.F.; Caballero, P.; Al-Rashaida, M.; Luna, P.M.; García, M.; Pérez, M.; et al. Ketogenic diet and cognition in neurological diseases: A systematic review. Nutr. Rev. 2021, 79, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef]
- Martin, C.; Preedy, V.; Rajendram, R. (Eds.) Factors Affecting Neurological Aging; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Dhatariya, K. Blood Ketones: Measurement, Interpretation, Limitations, and Utility in the Management of Diabetic Ketoacidosis. Rev. Diabet. Stud. RDS 2016, 13, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Nieman, D. Nutritional Assessment, 7th ed.; McGraw-Hill: New York, NY, USA, 2019. [Google Scholar]
- Grammatikopoulou, M.G.; Goulis, D.G.; Gkiouras, K.; Theodoridis, X.; Gkouskou, K.K.; Evangeliou, A.; Dardiotis, E.; Bogdanos, D.P. To Keto or Not to Keto? A Systematic Review of Randomized Controlled Trials Assessing the Effects of Ketogenic Therapy on Alzheimer Disease. Adv. Nutr. 2020, 11, 1583–1602. [Google Scholar] [CrossRef]
- Bostock, E.C.S.; Kirkby, K.C.; Taylor, B.V.; Hawrelak, J.A. Consumer Reports of “Keto Flu” Associated With the Ketogenic Diet. Front. Nutr. 2020, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batch, J.T.; Lamsal, S.P.; Adkins, M.; Sultan, S.; Ramirez, M.N. Advantages and Disadvantages of the Ketogenic Diet: A Review Article. Cureus 2020, 12, e9639. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abboud, M.; AlAnouti, F.; Georgaki, E.; Papandreou, D. Effect of Ketogenic Diet on Quality of Life in Adults with Chronic Disease: A Systematic Review of Randomized Controlled Trials. Nutrients 2021, 13, 4463. https://doi.org/10.3390/nu13124463
Abboud M, AlAnouti F, Georgaki E, Papandreou D. Effect of Ketogenic Diet on Quality of Life in Adults with Chronic Disease: A Systematic Review of Randomized Controlled Trials. Nutrients. 2021; 13(12):4463. https://doi.org/10.3390/nu13124463
Chicago/Turabian StyleAbboud, Myriam, Fatme AlAnouti, Evridiki Georgaki, and Dimitrios Papandreou. 2021. "Effect of Ketogenic Diet on Quality of Life in Adults with Chronic Disease: A Systematic Review of Randomized Controlled Trials" Nutrients 13, no. 12: 4463. https://doi.org/10.3390/nu13124463
APA StyleAbboud, M., AlAnouti, F., Georgaki, E., & Papandreou, D. (2021). Effect of Ketogenic Diet on Quality of Life in Adults with Chronic Disease: A Systematic Review of Randomized Controlled Trials. Nutrients, 13(12), 4463. https://doi.org/10.3390/nu13124463










