The Association between Treatment Modality, Lipid Profile, Metabolic Control in Children with Type 1 Diabetes and Celiac Disease—Data from the International Sweet Registry
Abstract
:1. Introduction
2. Methods
2.1. Data Source and Participants
2.2. Statistical Analysis
3. Results
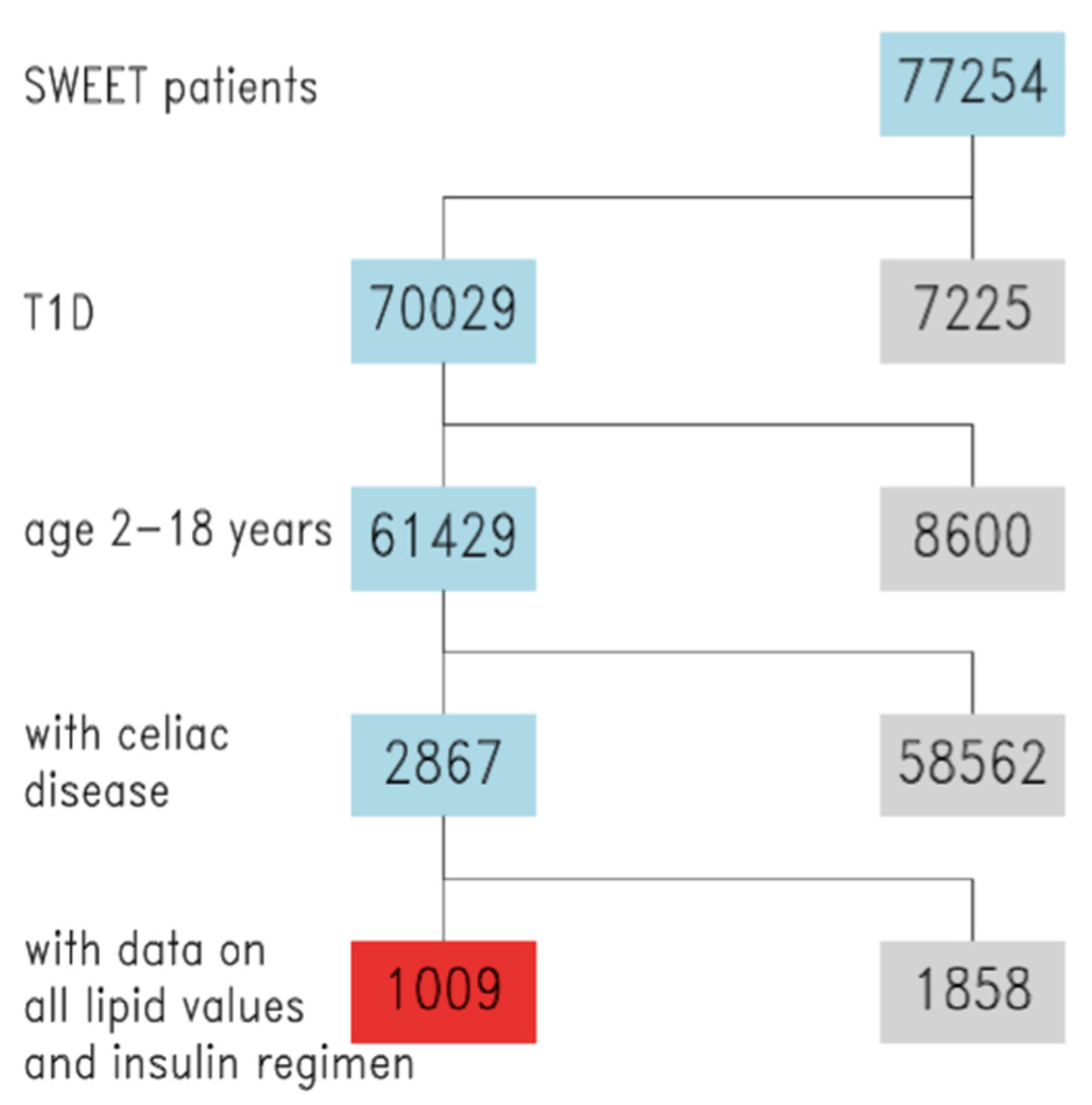
3.1. Descriptive Analysis
3.2. Results from Adjusted Regression Models
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHCL | advanced hybrid closed loop |
| BMI-SDS | body mass index—standard deviation score |
| CCT | cholesterol treatment trialists |
| CD | celiac disease |
| CSII | continuous subcutaneous insulin infusion |
| DCCT/EDIC | diabetes control and complications trial/epidemiology of diabetes interventions and complications |
| ESPGHAN | European Society for Paediatric Gastroenterology Hepatology and Nutrition |
| GFD | gluten-free diet |
| HbA1c | glycated hemoglobin |
| HDL | high-density lipoproteins |
| ISPAD | International Society of Pediatric and Adolescent Diabetes |
| IT | injections therapy years |
| LDL | low-density lipoproteins |
| MDI | multiple daily injections |
| SAP | sensor augmented therapy |
| SAS | statistical analysis system |
| T1D | type 1 diabetes |
| WHO | World Health Organisation |
References
- Lionetti, E.; Catassi, C. New Clues in Celiac Disease Epidemiology, Pathogenesis, Clinical Manifestations, and Treatment. Int. Rev. Immunol. 2011, 30, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Ludvigsson, J.F.; Brantner, T.L.; Murray, J.A.; Everhart, J.E. The Prevalence of Celiac Disease in the United States. Am. J. Gastroenterol. 2012, 107, 1538–1544. [Google Scholar] [CrossRef]
- Mahmud, F.H.; De Melo, E.N.; Noordin, K.; Assor, E.; Sahota, K.; Davies-Shaw, J.; Cutz, E.; Somers, G.; Lawson, M.; Mack, D.R.; et al. The Celiac Disease and Diabetes-Dietary Intervention and Evaluation Trial (CD-DIET) protocol: A randomised controlled study to evaluate treatment of asymptomatic coeliac disease in type 1 diabetes. BMJ Open 2015, 5, e008097. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, F.H.; Murray, J.A.; Kudva, Y.C.; Zinsmeister, A.R.; Dierkhising, R.A.; Lahr, B.D.; Dyck, P.J.; Kyle, R.A.; El-Youssef, M.; Burgart, L.J.; et al. Celiac Disease in Type 1 Diabetes Mellitus in a North American Community: Prevalence, Serologic Screening, and Clinical Features. Mayo Clin. Proc. 2005, 80, 1429–1434. [Google Scholar] [CrossRef] [Green Version]
- Lind, M.; Svensson, A.M.; Kosiborod, M.; Gudbjörnsdottir, S.; Pivodic, A.; Wedel, H.; Dahlqvist, S.; Clements, M.; Rosengren, A. Glycemic Control and Excess Mortality in Type 1 Diabetes. N. Engl. J. Med. 2015, 372, 1972–1982. [Google Scholar] [CrossRef]
- Margeirsdottir, H.D.; Stensaeth, K.H.; Larsen, J.R.; Brunborg, C.; Dahl-Jørgensen, K. Early signs of atherosclerosis in diabetic children on intensive insulin treatment: A population-based study. Diabetes Care. 2010, 33, 2043–2048. [Google Scholar] [CrossRef] [Green Version]
- Berenson, G.S.; Srinivasan, S.R.; Bao, W.; Newman, W.P.; Tracy, R.E.; Wattigney, W.A. Association between Multiple Cardiovascular Risk Factors and Atherosclerosis in Children and Young Adults. N. Engl. J. Med. 1998, 338, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Risk factors for Cardiovascular Disease in Type 1 Diabetes. N. Engl. J. Med. 2015, 372, 1722–1733. [Google Scholar]
- Mollazadegan, K.; Kugelberg, M.; Montgomery, S.M.; Sanders, D.S.; Ludvigsson, J.; Ludvigsson, J.F. A Population-Based Study of the Risk of Diabetic Retinopathy in Patients with Type 1 Diabetes and Celiac Disease. Diabetes Care 2013, 36, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Mollazadegan, K.; Fored, M.; Lundberg, S.; Ludvigsson, J.; Ekbom, A.; Montgomery, S.M.; Ludvigsson, J.F. Risk of renal disease in patients with both type 1 diabetes and coeliac disease. Diabetologia 2014, 57, 1339–1345. [Google Scholar] [CrossRef]
- Lionetti, E.; Antonucci, N.; Marinelli, M.; Bartolomei, B.; Franceschini, E.; Gatti, S.; Catassi, G.N.; Verma, A.K.; Monachesi, C.; Catassi, C. Nutritional Status, Dietary Intake, and Adherence to the Mediterranean Diet of Children with Celiac Disease on a Gluten-Free Diet: A Case-Control Prospective Study. Nutrients 2020, 12, 143. [Google Scholar] [CrossRef] [Green Version]
- Valvano, M.; Longo, S.; Stefanelli, G.; Frieri, G.; Viscido, A.; Latella, G. Celiac Disease, Gluten-Free Diet, and Metabolic and Liver Disorders. Nutrients. 2020, 12, 940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosteria, I.; Schwandt, A.; Davis, E.; Jali, S.; Prieto, M.; Rottembourg, D. Lipid profile is associated with treatment regimen in a large cohort of children and adolescents with Type 1 diabetes mellitus: A study from the international SWEET database. Diabetic Med. 2019, 36, 1294–1303. [Google Scholar] [CrossRef] [Green Version]
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.X.; Aschner, P.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatric Diabetes 2018, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the Diagnosis of Coeliac Disease. J. Pediatric Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- Maffeis, C.; Birkebaek, N.H.; Konstantinova, M.; Schwandt, A.; Vazeou, A.; Casteels, K.; Jali, S.; Limbert, C.; Pundziute-Lycka, A.; Toth-Heyn, P.; et al. Prevalence of underweight, overweight, and obesity in children and adolescents with type 1 diabetes: Data from the international SWEET registry. Pediatric Diabetes 2018, 19, 1211–1220. [Google Scholar] [CrossRef]
- Rosenbauer, J.; Dost, A.; Karges, B.; Hungele, A.; Stahl, A.; Bächle, C.; Gerstl, E.M.; Kastendieck, C.; Hofer, S.E.; Holl, R.W.; et al. Improved Metabolic Control in Children and Adolescents with Type 1 Diabetes: A trend analysis using prospective multicenter data from Germany and Austria. Diabetes Care 2012, 35, 80–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- US Department of Health and Human Services. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents; US Department of Health and Human Services: Washington, DC, USA, 2004; pp. 555–576.
- Rawshani, A.; Rawshani, A.; Franzén, S.; Eliasson, B.; Svensson, A.M.; Miftaraj, M.; McGuire, D.K.; Sattar, N.; Rosengren, A.; Gudbjörnsdottir, S. Range of Risk Factor Levels: Control, Mortality, and Cardiovascular Outcomes in Type 1 Diabetes Mellitus. Circulation 2017, 135, 1522–1531. [Google Scholar] [CrossRef]
- Tell, S.; Nadeau, K.J.; Eckel, R.H. Lipid management for cardiovascular risk reduction in type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 207–214. [Google Scholar] [CrossRef]
- Jarvisalo, M.J.; Raitakari, M.; Toikka, J.O.; Putto-Laurila, A.; Rontu, R.; Laine, S.; Lehtimaki, T.; Ronnemaa, T.; Viikari, J.; Raitakari, O.T. Endothelial Dysfunction and Increased Arterial Intima-Media Thickness in Children With Type 1 Diabetes. Circulation 2004, 109, 1750–1755. [Google Scholar] [CrossRef] [Green Version]
- Haller, M.J.; Stein, J.; Shuster, J.; Theriaque, D.; Silverstein, J.; Schatz, D.A.; Earing, M.G.; Lerman, A.; Mahmud, F.H. Peripheral artery tonometry demonstrates altered endothelial function in children with type 1 diabetes. Pediatric Diabetes 2007, 8, 193–198. [Google Scholar] [CrossRef]
- Shah, A.S.; Wadwa, R.P.; Dabelea, D.; Hamman, R.F.; D’Agostino, R., Jr.; Marcovina, S.; Daniels, S.R.; Dolan, L.M.; Fino, N.F.; Urbina, E.M. Arterial stiffness in adolescents and young adults with and without type 1 diabetes: The SEARCH CVD study. Pediatric Diabetes 2015, 16, 367–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawshani, A.; Rawshani, A.; Sattar, N.; Franzén, S.; McGuire, D.K.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; Rosengren, A.; et al. Relative Prognostic Importance and Optimal Levels of Risk Factors for Mortality and Cardiovascular Outcomes in Type 1 Diabetes Mellitus. Circulation 2019, 139, 1900–1912. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: A meta-analysis. Lancet 2008, 371, 117–125. [Google Scholar] [CrossRef]
- Foster, N.C.; Beck, R.W.; Miller, K.M.; Clements, M.A.; Rickels, M.R.; DiMeglio, L.A.; Maahs, D.M.; Tamborlane, W.V.; Bergenstal, R.; Smith, E.; et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol. Ther. 2019, 21, 66–72. [Google Scholar] [CrossRef]
- Karges, B.; Schwandt, A.; Heidtmann, B.; Kordonouri, O.; Binder, E.; Schierloh, U.; Boettcher, C.; Kapellen, T.; Rosenbauer, J.; Holl, R.W. Association of Insulin Pump Therapy vs. Insulin Injection Therapy with Severe Hypoglycemia, Ketoacidosis, and Glycemic Control Among Children, Adolescents, and Young Adults with Type 1 Diabetes. JAMA 2017, 318, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Catassi, C. Clinical practice. Celiac disease. N. Engl. J. Med. 2012, 367, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Penagini, F.; Dilillo, D.; Meneghin, F.; Mameli, C.; Fabiano, V.; Zuccotti, G. Gluten-Free Diet in Children: An Approach to a Nutritionally Adequate and Balanced Diet. Nutrients 2013, 5, 4553. [Google Scholar] [CrossRef] [Green Version]
- Fry, L.; Madden, A.M.; Fallaize, R. An investigation into the nutritional composition and cost of gluten-free versus regular food products in the UK. J. Hum. Nutr. Diet. 2018, 31, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Cornicelli, M.; Saba, M.; Machello, N.; Silano, M.; Neuhold, S. Nutritional composition of gluten-free food versus regular food sold in the Italian market. Dig. Liver Dis. 2018, 50, 1305–1308. [Google Scholar] [CrossRef]
- Larretxi, I.; Simon, E.; Benjumea, L.; Miranda, J.; Bustamante, M.A.; Lasa, A.; Eizaguirre, F.J.; Churruca, I. Gluten-free-rendered products contribute to imbalanced diets in children and adolescents with celiac disease. Eur. J. Nutr. 2019, 58, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Babio, N.; Alcázar, M.; Castillejo, G.; Recasens, M.; Martínez-Cerezo, F.; Gutiérrez-Pensado, V.; Masip, G.; Vaqué, C.; Vila-Martí, A.; Torres-Moreno, M.; et al. Patients with Celiac Disease Reported Higher Consumption of Added Sugar and Total Fat Than Healthy Individuals. J. Pediatric Gastroenterol. Nutr. 2017, 64, 63–69. [Google Scholar] [CrossRef]
- Kautto, E.; Ivarsson, A.; Norström, F.; Högberg, L.; Carlsson, A.; Hörnell, A. Nutrient intake in adolescent girls and boys diagnosed with coeliac disease at an early age is mostly comparable to their non-coeliac contemporaries. J. Hum. Nutr. Diet. 2014, 27, 41–53. [Google Scholar] [CrossRef]
- Zuccotti, G.; Fabiano, V.; Dilillo, D.; Picca, M.; Cravidi, C.; Brambilla, P. Intakes of nutrients in Italian children with celiac disease and the role of commercially available gluten-free products. J. Hum. Nutr. Diet. 2013, 26, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Gibson, P.R. Nutritional inadequacies of the gluten-free diet in both recently-diagnosed and long-term patients with coeliac disease. J. Hum. Nutr. Diet. 2013, 26, 349–358. [Google Scholar] [CrossRef]
- Öhlund, K.; Olsson, C.; Hernell, O.; Öhlund, I. Dietary shortcomings in children on a gluten-free diet. J. Hum. Nutr. Diet. 2010, 23, 294–300. [Google Scholar] [CrossRef]
- Hopman, E.G.D.; le Cessie, S.; von Blomberg, B.M.E.; Mearin, M.L. Nutritional Management of the Gluten-free Diet in Young People with Celiac Disease in The Netherlands. J. Pediatric Gastroenterol. Nutr. 2006, 43, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Bardella, M.T.; Fredella, C.; Prampolini, L.; Molteni, N.; Giunta, A.M.; Bianchi, P.A. Body composition and dietary intakes in adult celiac disease patients consuming a strict gluten-free diet. Am. J. Clin. Nutr. 2000, 72, 937–939. [Google Scholar] [CrossRef] [Green Version]
- Erciyas, F.; Taneli, F.; Arslan, B.; Uslu, Y. Glycemic control, oxidative stress, and lipid profile in children with type 1 diabetes mellitus. Arch. Med. Res. 2004, 35, 134–140. [Google Scholar] [CrossRef]
- Schreiver, C.; Jacoby, U.; Watzer, B.; Thomas, A.; Haffner, D.; Fischer, D.-C. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): A cross-sectional cohort study. Clin. Endocrinol. 2013, 79, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Gong, C.; Cao, B.; Peng, X.; Wu, D.; Gu, Y.; Wei, L.; Liang, X.; Liu, M.; Li, W.; et al. Glucose Fluctuations in Association with Oxidative Stress Among Children With T1DM: Comparison of Different Phases. J. Clin. Endocrinol. Metab. 2015, 100, 1828–1836. [Google Scholar] [CrossRef] [Green Version]
- Altıncık, A.; Tuğlu, B.; Demir, K.; Çatli, G.; Abacı, A.; Böber, E. Relationship between oxidative stress and blood glucose fluctuations evaluated with daily glucose monitoring in children with type 1 diabetes mellitus. J. Pediatric Endocrinol. Metab. 2016, 29, 435–439. [Google Scholar] [CrossRef]
- Mezzetti, A. Oxidative stress and cardiovascular complications in diabetes: Isoprostanes as new markers on an old paradigm. Cardiovasc. Res. 2000, 47, 475–488. [Google Scholar] [CrossRef] [Green Version]
- Davi, G.; Ciabattoni, G.; Consoli, A.; Mezzetti, A.; Falco, A.; Santarone, S.; Pennese, E.; Vitacolonna, E.; Bucciarelli, T.; Costantini, F.; et al. In Vivo Formation of 8-Iso-Prostaglandin F 2α and Platelet Activation in Diabetes Mellitus. Circulation 1999, 99, 224–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quagliaro, L.; Piconi, L.; Assaloni, R.; Martinelli, L.; Motz, E.; Ceriello, A. Intermittent High Glucose Enhances Apoptosis Related to Oxidative Stress in Human Umbilical Vein Endothelial Cells: The Role of Protein Kinase C and NAD(P)H-Oxidase Activation. Diabetes 2003, 52, 2795–2804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanik, M.H.; Xu, Y.; Skrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin Resistance and Hyperinsulinemia: Is hyperinsulinemia the cart or the horse? Diabetes Care 2008, 31 (Suppl. S2), S262–S268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braffett, B.H.; Dagogo-Jack, S.; Bebu, I.; Sivitz, W.I.; Larkin, M.; Kolterman, O.; Lachin, J.M. Association of Insulin Dose, Cardiometabolic Risk Factors, and Cardiovascular Disease in Type 1 Diabetes During 30 Years of Follow-up in the DCCT/EDIC Study. Diabetes Care 2019, 42, 657–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham-Short, A.; Donaghue, K.C.; Ambler, G.; Garnett, S.; Craig, M.E. Quality of Life in Type 1 Diabetes and Celiac Disease: Role of the Gluten-Free Diet. J. Pediatrics 2016, 179, 131–138. [Google Scholar] [CrossRef]
- Nansel, T.R.; Lipsky, L.M.; Liu, A. Greater diet quality is associated with more optimal glycemic control in a longitudinal study of youth with type 1 diabetes. Am. J. Clin. Nutr. 2016, 104, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Cherubini, V.; Bonfanti, R.; Casertano, A.; De Nitto, E.; Iannilli, A.; Lombardo, F.; Maltoni, G.; Marigliano, M.; Bassi, M.; Minuto, N.; et al. Time in Range in Children with Type 1 Diabetes Using Treatment Strategies Based on Nonautomated Insulin Delivery Systems in the Real World. Diabetes Technol. Ther. 2020, 22, 509–515. [Google Scholar] [CrossRef]
| All Subjects | |||
|---|---|---|---|
| N | Median/Percentage | Lower/Upper Quartile | |
| Demographics | |||
| % males | 1009 | 46 | N/A |
| age (years) | 1009 | 13.9 | 11.4/17.5 |
| duration of diabetes (years) | 1009 | 7.2 | 3.9/10.0 |
| height-SDS | 1004 | 0.29 | −0.34/0.99 |
| BMI-SDS | 1003 | 0.48 | −0.18/1.10 |
| % from Europe | 1009 | 75.2 | N/A |
| % from Asia/Africa a | 1009 | 3.8 | N/A |
| % from Australia b | 1009 | 4.9 | N/A |
| % from North America | 1009 | 12.8 | N/A |
| % from South America | 1009 | 3.4 | N/A |
| Complications and comorbidities | |||
| systolic blood pressure—SDS | 917 | 0.18 | −0.44/0.85 |
| systolic blood pressure—SDS | 916 | 0.22 | −0.25/0.69 |
| % nephropathy | 696 | 5.2 | N/A |
| % retinopathy | 531 | 4.3 | N/A |
| Diabetes Parameters | |||
| HbA1c (%) | 1006 | 7.86 | 6.9/8.5 |
| HbA1c (mmol/mol) | 1006 | 62.4 | 52.3/69.1 |
| total daily insulin dose (U/kg) | 920 | 0.83 | 0.67/0.99 |
| Lipid parameters | |||
| % Dyslipidemia | 1009 | 42 | N/A |
| TG (mg/dL) | 1009 | 89.4 | 54.9/105.4 |
| total Chol (mg/dL) | 1009 | 165.0 | 143.0/182.9 |
| HDL (mg/dL) | 1009 | 58.9 | 49.5/68.0 |
| LDL (mg/dL) | 1009 | 91.6 | 73.4/105.0 |
| Treatment Modality of T1D | |||
|---|---|---|---|
| CSII | IT | p-Value | |
| Sex (% males) | 44 | 49 | 0.502 |
| age (years) | 13.3 (10.9–16.9) | 14.6 (12.4–17.3) | <0.001 |
| DM duration (years) | 7.6 (4.5–10.2) | 6.7 (3.4–9.9) | 0.002 |
| Height-SDS (WHO) | 0.34 (−0.22, 1.07) | 0.18 (−0.47, 0.93) | 0.033 |
| BMI-SDS (WHO) | 0.41 (−0.21, 0.96) | 0.57 (−0.14, 1.30) | 0.058 |
| HbA1c (%) | 7.7 (6.8–8.3) | 8.1 (7.0–8.8) | <0.001 |
| daily insulin dose (U/kg) | 0.79 (0.65–0.93) | 0.88 (0.69–1.05) | <0.001 |
| triglyceride (mg/dL) | 88.2 (54–102) | 90.8 (55–106.3) | 1.000 |
| total cholesterol (mg/dL) | 164.3 (143–182) | 165.9 (143.1–184) | 1.000 |
| HDL (mg/dL) | 60.1 (50.3–69.6) | 57.6 (47.2–66.1) | 0.033 |
| LDL (mg/dL) | 89.9 (72–105) | 93.6 (74–105.9) | 0.499 |
| dyslipidemia (%) | 41 | 43 | 1.000 |
| Original Model Adjusted for Age, Gender, Diabetes Duration | Model 1 + Adjustment for BMI-SDS | Model 2 + Adjustment for HbA1c | Model 3 + Adjustment for BMI-SDS and HbA1c | |
|---|---|---|---|---|
| Triglycerides [mg/dL] | MDI: 91 [85; 96] | MDI: 90 [84; 96] | MDI: 90 [84; 95] | MDI: 89 [84; 95] |
| CSII: 88 [83; 93] | CSII: 89 [84; 94] | CSII: 89 [84; 94] | CSII: 90 [85; 95] | |
| p = 0.5086 | p = 0.7600 | p = 0.8880 | p = 0.8910 | |
| Cholesterol [mg/dL] | MDI: 167 [164; 170] | MDI: 166 [163; 169] | MDI: 166 [163; 169] | MDI: 166 [163; 169] |
| CSII: 164 [161; 93,166] | CSII: 164 [161; 167] | CSII: 164 [162; 167] | CSII: 165 [162; 167] | |
| p = 0.1305 | p = 0.2817 | p = 0.3429 | p = 0.5488 | |
| HDL [mg/dL] | MDI: 58 [56; 59] | MDI: 58 [57; 59] | MDI: 58 [56; 59] | MDI: 58 [57; 59] |
| CSII: 60 [59; 61] | CSII: 60 [59; 61] | CSII: 60 [59; 61] | CSII: 60 [59; 61] | |
| p = 0.0157 | p = 0.0526 | p = 0.0192 | p = 0.0548 | |
| LDL [mg/dL] | MDI: 94 [92; 97] | MDI: 94 [91; 96] | MDI: 94 [91; 96] | MDI: 93 [91; 96] |
| CSII: 89 [87; 92] | CSII: 90 [88; 92] | CSII: 90 [87; 92] | CSII: 90 [88; 92] | |
| p = 0.0062 | p = 0.0323 | p = 0.0224 | p = 0.0753 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, M.; Eckert, A.J.; Tell, S.; Krnic, N.; Deja, G.; Faber Rasmussen, V.; Coelho, R.; Todorovic, S.; Jefferies, C.A.; Sherif, E.; et al. The Association between Treatment Modality, Lipid Profile, Metabolic Control in Children with Type 1 Diabetes and Celiac Disease—Data from the International Sweet Registry. Nutrients 2021, 13, 4473. https://doi.org/10.3390/nu13124473
Marino M, Eckert AJ, Tell S, Krnic N, Deja G, Faber Rasmussen V, Coelho R, Todorovic S, Jefferies CA, Sherif E, et al. The Association between Treatment Modality, Lipid Profile, Metabolic Control in Children with Type 1 Diabetes and Celiac Disease—Data from the International Sweet Registry. Nutrients. 2021; 13(12):4473. https://doi.org/10.3390/nu13124473
Chicago/Turabian StyleMarino, Monica, Alexander J. Eckert, Shoshana Tell, Nevena Krnic, Grazyna Deja, Vinni Faber Rasmussen, Raquel Coelho, Sladjana Todorovic, Craig A. Jefferies, Eman Sherif, and et al. 2021. "The Association between Treatment Modality, Lipid Profile, Metabolic Control in Children with Type 1 Diabetes and Celiac Disease—Data from the International Sweet Registry" Nutrients 13, no. 12: 4473. https://doi.org/10.3390/nu13124473
APA StyleMarino, M., Eckert, A. J., Tell, S., Krnic, N., Deja, G., Faber Rasmussen, V., Coelho, R., Todorovic, S., Jefferies, C. A., Sherif, E., Martinez Mateu, C., & Elena Lionetti, M. (2021). The Association between Treatment Modality, Lipid Profile, Metabolic Control in Children with Type 1 Diabetes and Celiac Disease—Data from the International Sweet Registry. Nutrients, 13(12), 4473. https://doi.org/10.3390/nu13124473






