A Low-Glucose Eating Pattern Improves Biomarkers of Postmenopausal Breast Cancer Risk: An Exploratory Secondary Analysis of a Randomized Feasibility Trial
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Cancer Research Fund; American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; The American Institute for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- American Cancer Society. Breast Cancer Facts & Figures 2019–2020; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- Harvie, M.; Howell, A.; Vierkant, R.A.; Kumar, N.; Cerhan, J.R.; Kelemen, L.E.; Folsom, A.R.; Sellers, T.A. Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women’s health study. Cancer Epidemiol. Biomark. Prev. 2005, 14, 656–661. [Google Scholar] [CrossRef] [Green Version]
- Lahmann, P.H.; Schulz, M.; Hoffmann, K.; Boeing, H.; Tjonneland, A.; Olsen, A.; Overvad, K.; Key, T.J.; Allen, N.E.; Khaw, K.T.; et al. Long-term weight change and breast cancer risk: The European prospective investigation into cancer and nutrition (EPIC). Br. J. Cancer 2005, 93, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Eliassen, A.H.; Colditz, G.A.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Adult weight change and risk of postmenopausal breast cancer. JAMA 2006, 296, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Schatzkin, A.; Lacey, J.V., Jr.; Albanes, D.; Ballard-Barbash, R.; Adams, K.F.; Kipnis, V.; Mouw, T.; Hollenbeck, A.R.; Leitzmann, M.F. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch. Intern. Med. 2007, 167, 2091–2102. [Google Scholar] [CrossRef] [Green Version]
- Manders, P.; Pijpe, A.; Hooning, M.J.; Kluijt, I.; Vasen, H.F.; Hoogerbrugge, N.; van Asperen, C.J.; Meijers-Heijboer, H.; Ausems, M.G.; van Os, T.A.; et al. Body weight and risk of breast cancer in BRCA1/2 mutation carriers. Breast Cancer Res. Treat. 2011, 126, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Hankinson, S.E.; Colditz, G.A.; Stampfer, M.J.; Hunter, D.J.; Manson, J.E.; Hennekens, C.H.; Rosner, B.; Speizer, F.E.; Willett, W.C. Dual effects of weight and weight gain on breast cancer risk. JAMA 1997, 278, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Ballard-Barbash, R.; Hunsberger, S.; Alciati, M.H.; Blair, S.N.; Goodwin, P.J.; McTiernan, A.; Wing, R.; Schatzkin, A. Physical activity, weight control, and breast cancer risk and survival: Clinical trial rationale and design considerations. J. Natl. Cancer Inst. 2009, 101, 630–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byers, T.; Sedjo, R.L. Does intentional weight loss reduce cancer risk? Diabetes Obes. Metab. 2011, 13, 1063–1072. [Google Scholar] [CrossRef]
- Reeves, M.M.; Terranova, C.O.; Eakin, E.G.; Demark-Wahnefried, W. Weight loss intervention trials in women with breast cancer: A systematic review. Obes. Rev. 2014, 15, 749–768. [Google Scholar] [CrossRef] [Green Version]
- Fabian, C.J.; Kimler, B.F.; Donnelly, J.E.; Sullivan, D.K.; Klemp, J.R.; Petroff, B.K.; Phillips, T.A.; Metheny, T.; Aversman, S.; Yeh, H.W.; et al. Favorable modulation of benign breast tissue and serum risk biomarkers is associated with >10% weight loss in postmenopausal women. Breast Cancer Res. Treat. 2013, 142, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Fabian, C.J.; Klemp, J.R.; Marchello, N.J.; Vidoni, E.D.; Sullivan, D.K.; Nydegger, J.L.; Phillips, T.A.; Kreutzjans, A.L.; Hendry, B.; Befort, C.A.; et al. Rapid Escalation of High-Volume Exercise during Caloric Restriction; Change in Visceral Adipose Tissue and Adipocytokines in Obese Sedentary Breast Cancer Survivors. Cancers 2021, 13, 4871. [Google Scholar] [CrossRef]
- Playdon, M.; Thomas, G.; Sanft, T.; Harrigan, M.; Ligibel, J.; Irwin, M. Weight Loss Intervention for Breast Cancer Survivors: A Systematic Review. Curr. Breast Cancer Rep. 2013, 5, 222–246. [Google Scholar] [CrossRef] [Green Version]
- Birks, S.; Peeters, A.; Backholer, K.; O’Brien, P.; Brown, W. A systematic review of the impact of weight loss on cancer incidence and mortality. Obes. Rev. 2012, 13, 868–891. [Google Scholar] [CrossRef]
- Burgess, E.; Hassmen, P.; Pumpa, K.L. Determinants of adherence to lifestyle intervention in adults with obesity: A systematic review. Clin. Obes. 2017, 7, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.W.Y.; Chan, R.S.M.; Sea, M.M.M.; Woo, J. An Overview of Factors Associated with Adherence to Lifestyle Modification Programs for Weight Management in Adults. Int. J. Environ. Res. Public Health 2017, 14, 922. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research, G.; Knowler, W.C.; Fowler, S.E.; Hamman, R.F.; Christophi, C.A.; Hoffman, H.J.; Brenneman, A.T.; Brown-Friday, J.O.; Goldberg, R.; Venditti, E.; et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef] [Green Version]
- Chaix, A.; Manoogian, E.N.C.; Melkani, G.C.; Panda, S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Ann. Rev. Nutr. 2019, 39, 291–315. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 2017, 39, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Yang, J.N.; Garcia, J.I.; Myers, S.; Bozzi, I.; Wang, W.; Buxton, O.M.; Shea, S.A.; Scheer, F.A. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E2225–E2234. [Google Scholar] [CrossRef] [Green Version]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buxton, O.M.; Cain, S.W.; O’Connor, S.P.; Porter, J.H.; Duffy, J.F.; Wang, W.; Czeisler, C.A.; Shea, S.A. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 2012, 4, 129ra43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gale, J.E.; Cox, H.I.; Qian, J.; Block, G.D.; Colwell, C.S.; Matveyenko, A.V. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J. Biol. Rhythm. 2011, 26, 423–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melkani, G.C.; Panda, S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J. Physiol. 2017, 595, 3691–3700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, S.; Kang, J.; Kim, S.H.; Chung, H.S.; Kim, Y.J.; Yu, J.M.; Cho, S.T.; Oh, C.M.; Kim, T. Beneficial Effects of Time-Restricted Eating on Metabolic Diseases: A Systemic Review and Meta-Analysis. Nutrients 2020, 12, 1267. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Cioffi, I.; Evangelista, A.; Ponzo, V.; Goitre, I.; Ciccone, G.; Ghigo, E.; Bo, S. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmi de Toledo, F.; Buchinger, A.; Burggrabe, H.; Holz, G.; Kuhn, C.; Lischka, E.; Lischka, N.; Lutzner, H.; May, W.; Ritzmann-Widderich, M.; et al. Fasting therapy—An expert panel update of the 2002 consensus guidelines. Forsch. Komplementmed. 2013, 20, 434–443. [Google Scholar] [CrossRef]
- Parr, E.B.; Devlin, B.L.; Radford, B.E.; Hawley, J.A. A Delayed Morning and Earlier Evening Time-Restricted Feeding Protocol for Improving Glycemic Control and Dietary Adherence in Men with Overweight/Obesity: A Randomized Controlled Trial. Nutrients 2020, 12, 505. [Google Scholar] [CrossRef] [Green Version]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef]
- French, S.A.; Epstein, L.H.; Jeffery, R.W.; Blundell, J.E.; Wardle, J. Eating behavior dimensions. Associations with energy intake and body weight. A review. Appetite 2012, 59, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Schembre, S.M.; Liao, Y.; Huh, J.; Keller, S. Using pre-prandial blood glucose to assess eating in the absence of hunger in free-living individuals. Eat. Behav. 2020, 38, 101411. [Google Scholar] [CrossRef]
- Jospe, M.R.; Brown, R.C.; Roy, M.; Taylor, R.W. Adherence to hunger training using blood glucose monitoring: A feasibility study. Nutr. Metab. 2015, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Ciampolini, M.; Lovell-Smith, D.; Sifone, M. Sustained self-regulation of energy intake. Loss of weight in overweight subjects. Maintenance of weight in normal-weight subjects. Nutr. Metab. 2010, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciampolini, M.; Bianchi, R. Training to estimate blood glucose and to form associations with initial hunger. Nutr. Metab. 2006, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- Jospe, M.R.; de Bruin, W.E.; Haszard, J.J.; Mann, J.I.; Brunton, M.; Taylor, R.W. Teaching people to eat according to appetite—Does the method of glucose measurement matter? Appetite 2020, 151, 104691. [Google Scholar] [CrossRef]
- Ciampolini, M.; Lovell-Smith, D.; Bianchi, R.; de Pont, B.; Sifone, M.; van Weeren, M.; de Hahn, W.; Borselli, L.; Pietrobelli, A. Sustained self-regulation of energy intake: Initial hunger improves insulin sensitivity. J. Nutr. Metab. 2010, 2010, 286952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jospe, M.R.; Taylor, R.W.; Athens, J.; Roy, M.; Brown, R.C. Adherence to Hunger Training over 6 Months and the Effect on Weight and Eating Behaviour: Secondary Analysis of a Randomised Controlled Trial. Nutrients 2017, 9, 1260. [Google Scholar] [CrossRef] [Green Version]
- De Bruin, W.E.; Ward, A.L.; Taylor, R.W.; Jospe, M.R. ‘Am I really hungry?’ A qualitative exploration of patients’ experience, adherence and behaviour change during hunger training: A pilot study. BMJ Open 2019, 9, e032248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ertunc, M.E.; Hotamisligil, G.S. Lipid signaling and lipotoxicity in metaflammation: Indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 2016, 57, 2099–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, D.P.; Gracheck, P.J.; Vona-Davis, L. The Interactions of Obesity, Inflammation and Insulin Resistance in Breast Cancer. Cancers 2015, 7, 2147–2168. [Google Scholar] [CrossRef] [PubMed]
- Schembre, S.M.; Jospe, M.R.; Bedrick, E.J.; Liang, L.; Brewster, A.M.; Levy, E.; Dirba, D.D.; Campbell, M.; Taylor, R.W.; Basen-Engquist, K.M. Hunger Training as a self-regulation strategy in a comprehensive weight loss program for breast cancer prevention: A randomized feasibility study. Cancer Prev. Res. 2021. under review. [Google Scholar] [CrossRef]
- Wing, R.R.; Hamman, R.F.; Bray, G.A.; Delahanty, L.; Edelstein, S.L.; Hill, J.O.; Horton, E.S.; Hoskin, M.A.; Kriska, A.; Lachin, J.; et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes. Res. 2004, 12, 1426–1434. [Google Scholar] [CrossRef]
- Stephens, S.K.; Cobiac, L.J.; Veerman, J.L. Improving diet and physical activity to reduce population prevalence of overweight and obesity: An overview of current evidence. Prev. Med. 2014, 62, 167–178. [Google Scholar] [CrossRef]
- Gail, M.H.; Brinton, L.A.; Byar, D.P.; Corle, D.K.; Green, S.B.; Schairer, C.; Mulvihill, J.J. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J. Natl. Cancer Inst. 1989, 81, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, N.R.; Oliver, N.S.; Choudhary, P.; Levy, J.C.; Hindmarsh, P.; Matthews, D.R. Normal Reference Range for Mean Tissue Glucose and Glycemic Variability Derived from Continuous Glucose Monitoring for Subjects Without Diabetes in Different Ethnic Groups. Diabetes Technol. Ther. 2011, 13, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Pendergast, F.J.; Ridgers, N.D.; Worsley, A.; McNaughton, S.A. Evaluation of a smartphone food diary application using objectively measured energy expenditure. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, V.; Voci, S.M.; Mendes-Netto, R.S.; da Silva, D.G. The relative validity of a food record using the smartphone application MyFitnessPal. Nutr. Diet. 2018, 75, 219–225. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-h time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- Hegedus, E.; Salvy, S.-J.; Wee, C.P.; Naguib, M.; Raymond, J.K.; Fox, D.S.; Vidmar, A.P. Use of continuous glucose monitoring in obesity research: A scoping review. Obes. Res. Clin. Pract. 2021, 15, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Connell, L.E.; Carey, R.N.; de Bruin, M.; Rothman, A.J.; Johnston, M.; Kelly, M.P.; Michie, S. Links Between Behavior Change Techniques and Mechanisms of Action: An Expert Consensus Study. Ann. Behav. Med. 2019, 53, 708–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Basen-Engquist, K.M.; Urbauer, D.L.; Bevers, T.B.; Hawk, E.; Schembre, S.M. Using Continuous Glucose Monitoring to Motivate Physical Activity in Overweight and Obese Adults: A Pilot Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 761–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
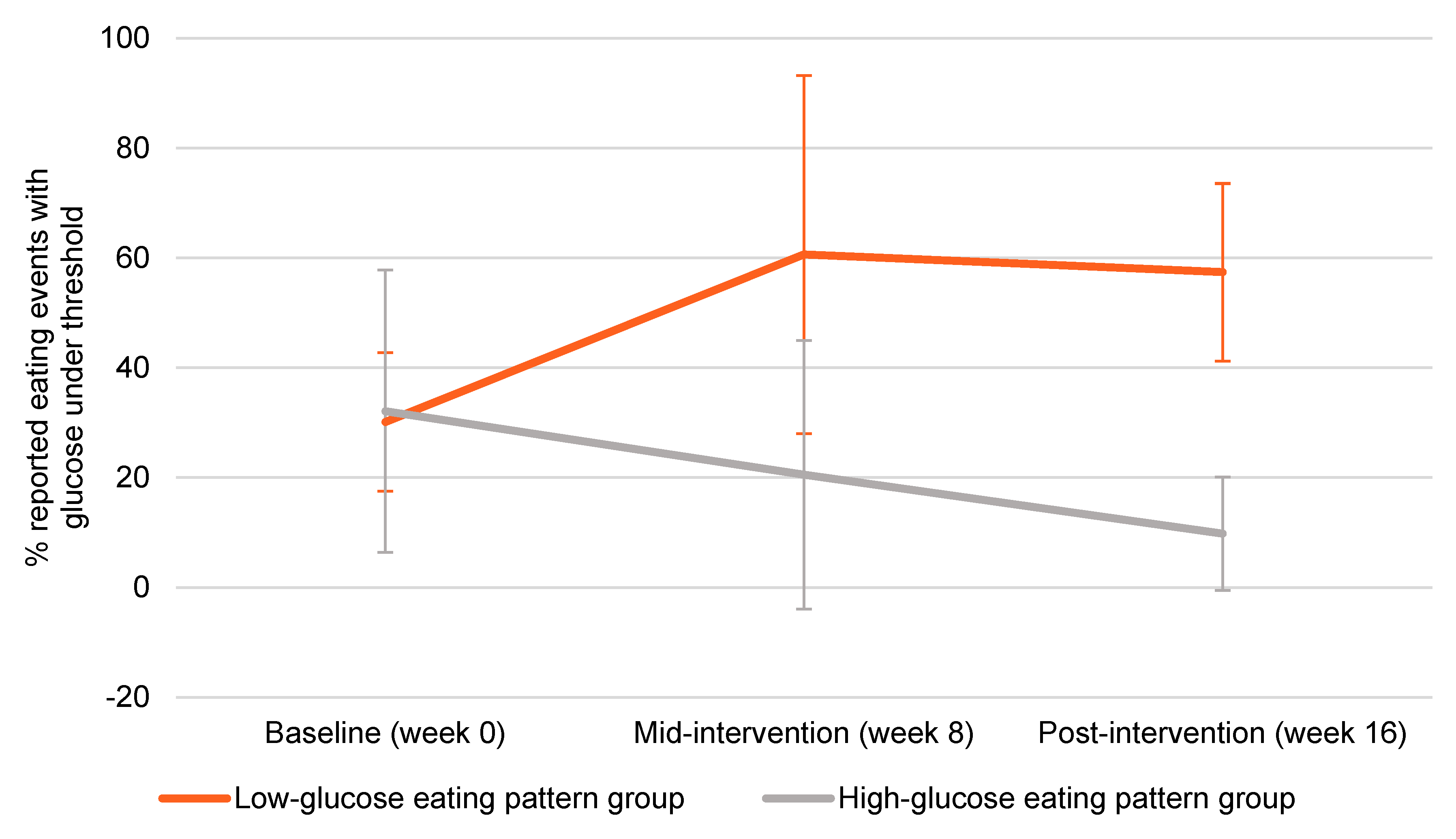


| Low-Glucose Eating Pattern | High-Glucose Eating Pattern | |
|---|---|---|
| N | N = 8 | N = 11 |
| DPP + GGE group, n (%) | 5 (63%) | 6 (55%) |
| White, non-Hispanic, n (%) | 8 (100%) | 11 (100%) |
| Married, n (%) | 7 (88%) | 11 (100%) |
| College educated, n (%) | 8 (100%) | 10 (90%) |
| Age (years) | 59.4 ± 7.0 | 61.9 ± 4.9 |
| Body mass index (kg/m2) | 32.6 ± 6.2 | 36.0 ± 7.0 |
| LGEP (4 Months) | TRF 4HR (2 Months) | TRF 6HR (2 Months) | TRF 8HR (3 Months) | ILI, >10% Weight Loss (6 Months) | |
|---|---|---|---|---|---|
| Current study | Cienfuegos, 2020 [50] | Cienfuegos, 2020 [50] | Gabel, 2018 [51] | Fabian, 2013 [12] | |
| N | N = 7 | N = 16 | N = 19 | N = 23 | N = 24 |
| Participants | Postmenopausal women at risk for BrCa without DM | 90% women | 90% women | 87% women | Postmenopausal women at risk for BrCa without DM |
| BMI inclusion (kg/m2) | >27 | >30 | >30 | >30 | >25 |
| Age (years), mean ± SD | 59 ± 7 | 49 ± 2 | 46 ± 3 | 50 ± 2 | 57 ± 5 |
| Body weight (kg) | −7.4 (−8%) | −3.0 (−3%) | −3.0 (−3%) | −3.0 (−3%) | −12.8, (−16%) |
| Fasting glucose (mg/dL) | −3.3, (−3%) | −5.0 (−6%) | −2.3 (−2%) | +3 (+4%) | −3.0, (−3.0%) |
| Fasting insulin (µIU/mL) | −6.6, (−32%) | −2.3 (−19%) | −1.9 (12%) | −2.6 (−31%) | −3.7, (−57%) |
| Insulin resistance (HOMA-IR) | −0.7, (−32%) | −0.8 (−29%) | −0.5 (−12%) | −0.6 (−38%) | −0.5, (−56%) |
| IGF-1 (nM) | +7.8, (+8%) | NR | NR | NR | +0.6, (+6%) |
| Adiponectin | +1.8 (+26%) | NR | NR | NR | +3.5, (+31%) |
| TNF-α (pg/mL) | NR | −2.4 (−29%) | −0.4 (−3%) | NR | −0.2, (−4%) |
| CRP (µg/mL) | −0.5 (−33%) | NR | NR | NR | −1.0, (−39%) |
| Energy intake (kcals) | −323 (−16%) | −528 (−30%) | −566 (−29%) | −341 (−20%) | −387, (−21%) |
| Macronutrient composition as percentage of energy intake (fat, carbohydrates, protein) | 36%, 47%, 18% | 36%, 46%, 18% | 40%, 40%, 20% | 37%, 46%, 17% | 20%, 60%, 21% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schembre, S.M.; Jospe, M.R.; Giles, E.D.; Sears, D.D.; Liao, Y.; Basen-Engquist, K.M.; Thomson, C.A. A Low-Glucose Eating Pattern Improves Biomarkers of Postmenopausal Breast Cancer Risk: An Exploratory Secondary Analysis of a Randomized Feasibility Trial. Nutrients 2021, 13, 4508. https://doi.org/10.3390/nu13124508
Schembre SM, Jospe MR, Giles ED, Sears DD, Liao Y, Basen-Engquist KM, Thomson CA. A Low-Glucose Eating Pattern Improves Biomarkers of Postmenopausal Breast Cancer Risk: An Exploratory Secondary Analysis of a Randomized Feasibility Trial. Nutrients. 2021; 13(12):4508. https://doi.org/10.3390/nu13124508
Chicago/Turabian StyleSchembre, Susan M., Michelle R. Jospe, Erin D. Giles, Dorothy D. Sears, Yue Liao, Karen M. Basen-Engquist, and Cynthia A. Thomson. 2021. "A Low-Glucose Eating Pattern Improves Biomarkers of Postmenopausal Breast Cancer Risk: An Exploratory Secondary Analysis of a Randomized Feasibility Trial" Nutrients 13, no. 12: 4508. https://doi.org/10.3390/nu13124508
APA StyleSchembre, S. M., Jospe, M. R., Giles, E. D., Sears, D. D., Liao, Y., Basen-Engquist, K. M., & Thomson, C. A. (2021). A Low-Glucose Eating Pattern Improves Biomarkers of Postmenopausal Breast Cancer Risk: An Exploratory Secondary Analysis of a Randomized Feasibility Trial. Nutrients, 13(12), 4508. https://doi.org/10.3390/nu13124508








