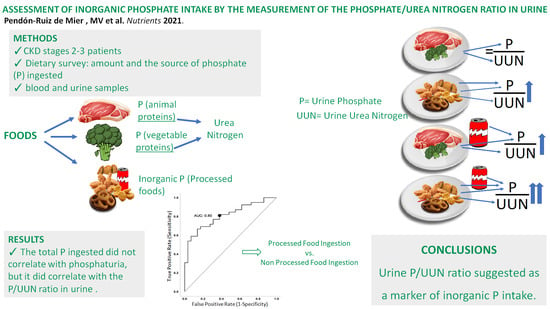
Assessment of Inorganic Phosphate Intake by the Measurement of the Phosphate/Urea Nitrogen Ratio in Urine
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Patients
2.5. Blood and Urine Chemistries
2.6. Animal Studies
2.6.1. Animals
2.6.2. Dietary Inorganic Phosphorus Modulation
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isakova, T.; Wolf, M.S. FGF23 or PTH: Which comes first in CKD? Kidney Int. 2010, 78, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Wheeler, D.C.; Persky, M.S.; Kestenbaum, B.; Ketteler, M.; Spiegel, D.M.; Allison, M.A.; Asplin, J.; Smits, G.; Hoofnagle, A.N.; et al. Effects of Phosphate Binders in Moderate CKD. JASN 2012, 23, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Gutekunst, L.; Mehrotra, R.; Kovesdy, C.P.; Bross, R.; Shinaberger, C.S.; Noori, N.; Hirschberg, R.; Benner, D.; Nissenson, A.R.; et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Vervloet, M.G.; Sezer, S.; Massy, Z.A.; Johansson, L.; Cozzolino, M.; Fouque, D. On behalf of the ERA–EDTA Working Group on Chronic Kidney Disease—Mineral and Bone Disorders and the European Renal Nutrition Working Group; The role of phosphate in kidney disease. Nat. Rev. Nephrol. 2017, 13, 27–38. [Google Scholar] [CrossRef]
- St-Jules, D.E.; Goldfarb, D.S.; Pompeii, M.L.; Sevick, M.A. Phosphate Additive Avoidance in Chronic Kidney Disease. Diabetes Spectr. 2017, 30, 101–106. [Google Scholar] [CrossRef]
- Vervloet, M.G.; van Ballegooijen, A.J. Prevention and treatment of hyperphosphatemia in chronic kidney disease. Kidney Int. 2018, 93, 1060–1072. [Google Scholar] [CrossRef]
- Moe, S.M.; Zidehsarai, M.P.; Chambers, M.A.; Jackman, L.A.; Radcliffe, J.S.; Trevino, L.L.; Donahue, S.E.; Asplin, J.R. Vegetarian Compared with Meat Dietary Protein Source and Phosphorus Homeostasis in Chronic Kidney Disease. CJASN 2011, 6, 257–264. [Google Scholar] [CrossRef]
- Karp, H.; Ekholm, P.; Kemi, V.; Itkonen, S.; Hirvonen, T.; Närkki, S.; Lamberg-Allardt, C. Differences Among Total and In Vitro Digestible Phosphorus Content of Plant Foods and Beverages. J. Renal Nutr. 2012, 22, 416–422. [Google Scholar] [CrossRef]
- Calvo, M.S.; Uribarri, J. Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am. J. Clin. Nutr. 2013, 98, 6–15. [Google Scholar] [CrossRef]
- Moorthi, R.N.; Armstrong, C.L.H.; Janda, K.; Ponsler-Sipes, K.; Asplin, J.R.; Moe, S.M. The Effect of a Diet Containing 70% Protein from Plants on Mineral Metabolism and Musculoskeletal Health in Chronic Kidney Disease. Am. J. Nephrol. 2014, 40, 582–591. [Google Scholar] [CrossRef]
- Chang, A.R.; Anderson, C. Dietary Phosphorus Intake and the Kidney. Annu. Rev. Nutr. 2017, 37, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Selamet, U.; Tighiouart, H.; Sarnak, M.J.; Beck, G.; Levey, A.S.; Block, G.; Ix, J.H. Relationship of dietary phosphate intake with risk of end-stage renal disease and mortality in chronic kidney disease stages 3–5: The Modification of Diet in Renal Disease Study. Kidney Int.Int. 2016, 89, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Sacks, F.; Pfeffer, M.; Gao, Z.; Curhan, G. Cholesterol And Recurrent Events Trial Investigators Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005, 112, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R. Relations of Serum Phosphorus and Calcium Levels to the Incidence of Cardiovascular Disease in the Community. Arch. Intern. Med. 2007, 167, 879. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.; Kestenbaum, B.; Chonchol, M. Phosphate and Cardiovascular Disease. Adv. Chronic Kidney Dis. 2011, 18, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Vart, P.; Nigatu, Y.T.; Jaglan, A.; van Zon, S.K.R.; Shafique, K. Joint Effect of Hypertension and Elevated Serum Phosphorus on the Risk of Mortality in National Health and Nutrition Examination Survey-III. JAHA 2015, 4. [Google Scholar] [CrossRef]
- Santamaría, R.; Díaz-Tocados, J.M.; Pendón-Ruiz de Mier, M.V.; Robles, A.; Salmerón-Rodríguez, M.D.; Ruiz, E.; Vergara, N.; Aguilera-Tejero, E.; Raya, A.; Ortega, R.; et al. Increased Phosphaturia Accelerates The Decline in Renal Function: A Search for Mechanisms. Sci. Rep. 2018, 8, 13701. [Google Scholar] [CrossRef] [PubMed]
- Ritz, E.; Hahn, K.; Ketteler, M.; Kuhlmann, M.K.; Mann, J. Phosphate Additives in Food. Deutsches Aerzteblatt Online 2012. [Google Scholar] [CrossRef]
- Uribarri, J.; Calvo, M.S. Dietary Phosphorus Excess: A Risk Factor in Chronic Bone, Kidney, and Cardiovascular Disease? Adv. Nutr. 2013, 4, 542–544. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, X.; Song, M.; Zhou, Q.; Yu, J.; Zhou, B.; Wu, Y.; He, Y.; Huang, L. Fibroblast growth factor 23 as a predictor of cardiovascular and all-cause mortality in prospective studies. Atherosclerosis 2017, 261, 1–11. [Google Scholar] [CrossRef]
- Perlmutter, M. Urine-serum urea nitrogen ratio: Simple test of renal function in acute azotemia and oligu-ria. JAMA 1959, 170, 1533. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Mataix Verdú, J. Food Composition Table, 5th ed.; University of Granada: Granada, Spain, 2009; ISBN 978-84-338-4980-9. [Google Scholar]
- Spanish Food Composition Database published by the BEDCA Network of the Ministry of Science and Innovation. Available online: https://www.bedca.net/bdpub/ (accessed on 12 July 2020).
- European Food Information Resource. Available online: http://www.eurofir.org (accessed on 12 July 2020).
- EFSA NDA Panel General principles for the collection of national food consumption data in the view of a pan-European dietary survey. EFSA J. 2009, 7, 1435. [CrossRef]
- Yoshida, K.; Bartel, A.; Chipman, J.J.; Bohn, J.; DAgostino McGowan, L.; Barrett, M.; Christensen, R.H.B. Tableone: Create “Table 1” to Describe Baseline Characteristics with or without Propensity Score Weights. 2020. Available online: https://CRAN.R-project.org/package=tableone (accessed on 12 July 2020).
- Taiyun, W.; Viliam, S. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84). 2017. Available online: https://github.com/taiyun/corrplot (accessed on 12 July 2020).
- Christian, T. Cutpointr: Determine and Evaluate Optimal Cutpoints in BinaryClassification Tasks. R package Version 1.0.32. 2020. Available online: https://CRAN.R-project.org/package=cutpointr (accessed on 12 July 2020).
- Bingham, S.A. Urine Nitrogen as a Biomarker for the Validation of Dietary Protein Intake. J. Nutr. 2003, 133, 921S–924S. [Google Scholar] [CrossRef]
- Caravaca, F.; Caravaca-Fontán, F.; Azevedo, L.; Luna, E. Cómo estimar la eficacia de un captor del fósforo. Nefrología 2017, 37, 311–319. [Google Scholar] [CrossRef]
- Palomino, H.L.; Rifkin, D.E.; Anderson, C.; Criqui, M.H.; Whooley, M.A.; Ix, J.H. 24-Hour Urine Phosphorus Excretion and Mortality and Cardiovascular Events. CJASN 2013, 8, 1202–1210. [Google Scholar] [CrossRef]
- Ix, J.H.; Anderson, C.A.; Smits, G.; Persky, M.S.; Block, G.A. Effect of dietary phosphate intake on the circadian rhythm of serum phosphate concentrations in chronic kidney disease: A crossover study. Am. J. Clin. Nutr. 2014, 100, 1392–1397. [Google Scholar] [CrossRef]
- Stremke, E.R.; McCabe, L.D.; McCabe, G.P.; Martin, B.R.; Moe, S.M.; Weaver, C.M.; Peacock, M.; Hill Gallant, K.M. Twenty-Four-Hour Urine Phosphorus as a Biomarker of Dietary Phosphorus Intake and Absorption in CKD: A Secondary Analysis from a Controlled Diet Balance Study. CJASN 2018, 13, 1002–1012. [Google Scholar] [CrossRef]
- EFSA NDA Panel Scientific Opinion on Dietary Reference Values for phosphorus. EFSA J. 2015, 13, 54.
- Gutiérrez, O.M. Sodium- and Phosphorus-Based Food Additives: Persistent but Surmountable Hurdles in the Management of Nutrition in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2013, 20, 150–156. [Google Scholar] [CrossRef]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D.; et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s changed and why it matters. Kidney Int. 2017, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Cai, X.; Lee, J.; Xie, D.; Wang, X.; Mehta, R.; Allen, N.B.; Scialla, J.J.; Pencina, M.J.; Anderson, A.H.; et al. Longitudinal FGF23 Trajectories and Mortality in Patients with CKD. JASN 2018, 29, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castañeda, J.R.; Herencia, C.; Pendón-Ruiz de Mier, M.V.; Rodriguez-Ortiz, M.E.; Diaz-Tocados, J.M.; Vergara, N.; Martínez-Moreno, J.M.; Salmerón, M.D.; Richards, W.G.; Felsenfeld, A.; et al. Differential regulation of renal Klotho and FGFR1 in normal and uremic rats. FASEB J. 2017, 31, 3858–3867. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Wolf, M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat. Rev. Nephrol. 2014, 10, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-C.; Wu, H.-Y.; Peng, Y.-S.; Hsu, S.-P.; Chiu, Y.-L.; Chen, H.-Y.; Yang, J.-Y.; Ko, M.-J.; Pai, M.-F.; Tu, Y.-K.; et al. Effects of lower versus higher phosphate diets on fibroblast growth factor-23 levels in patients with chronic kidney disease: A systematic review and meta-analysis. Nephrol.Dial. Transplant. 2018, 33, 1977–1983. [Google Scholar] [CrossRef]
- Minakuchi, H.; Yoshida, T.; Kaburagi, N.; Fujino, T.; Endo, S.; Takemitsu, T.Y.; Yamashita, N.; Itoh, H.; Oya, M. Proton pump inhibitors may hinder hypophosphatemic effect of lanthanum carbonate, but not of ferric citrate hydrate or sucroferric oxyhydroxide, in hemodialysis patients. Ren. Fail. 2020, 42, 799–806. [Google Scholar] [CrossRef]
- Afshan, S.; Farah Musa, A.R.; Echols, V.; Lerant, A.A.; Fülöp, T. Persisting Hypocalcemia After Surgical Parathyroidectomy: The Differential Effectiveness of Calcium Citrate Versus Calcium Carbonate With Acid Suppression. Am. J. Med. Sci. 2017, 353, 82–86. [Google Scholar] [CrossRef]




| Characteristic | Mean ± SD |
|---|---|
| Age (years) | 61 ± 9 |
| Gender (male, n/%) | 51/72 |
| Type 2 diabetes (n/%) | 35/49 |
| Dyslipidemia (n/%) | 55.0/77 |
| Hyperuricemia (n/%) | 43.0/61 |
| Smokers (n/%) | 8.0/11 |
| Ischemic cardiopathy (n/%) | 8.0/11 |
| Peripheral arteriopathy (n/%) | 9.0/13 |
| Cardiovascular events (n/%) | 12.0/17 |
| Hypertension (n/%) | 71.0/100 |
| ACEI (n/%) | 7.0/10 |
| ARB (n/%) | 61.0/86 |
| Diuretics (n/%) | 41.0/58 |
| CCB (D) (n/%) | 45.0/63 |
| Beta-blocker (n/%) | 28.0/39 |
| Central Alfa-Blocker (n/%) | 31.0 /44 |
| Other antihypertensive drugs (n/%) | 4.0/6 |
| Body weight (kg) | 90.6 ± 13.0 |
| Body mass index (kg/m2) | 32.0 ± 4.0 |
| Waist circumference (cm) in men | 110.3 ± 10.1 |
| Waist circumference (cm) in women | 108.0 ± 10.1 |
| Systolic blood pressure (mmHg) | 136.3 ± 25.5 |
| Diastolic blood pressure (mmHg) | 84.1 ± 16.6 |
| Mean blood pressure (mmHg) | 108.4 ± 19.0 |
| Heart rate (bpm) | 64.0 ± 13.3 |
| Serum | Mean ± SD |
|---|---|
| Creatinine (mmol/L) | 0.105 ± 0.036 |
| Urea (mmol/L) | 17.1 ± 5.7 |
| Glucose (mmol/L) | 7.14 ± 2.71 |
| Sodium (mEq/L) | 139.4 ± 2.4 |
| Potassium (mEq/L) | 4.4 ± 0.5 |
| Chloride (mEq/L) | 106.1 ± 3.3 |
| Calcium (mmol/L) | 2.37 ± 0.07 |
| Phosphate (mmol/L) | 1 ± 0.16 |
| Magnesium (mmol/L) | 0.8 ± 0.1 |
| Triglycerides (mmol/L) | 1.70 ± 0.74 |
| Cholesterol (mmol/L) | 4.60 ± 0.96 |
| HDL-Cholesterol (mmol/L) | 1.01 ± 0.23 |
| LDL-Cholesterol (mmol/L) | 2.80 ± 0.83 |
| Albumin (µmol/L) | 652 ± 30 |
| CRP (µg/L) | 26,000 (12,000–55,000) |
| Iron (µmol/L) | 14.9 ± 6 |
| Ferritin (µg/L) | 74 (39–130) |
| PTH (pmol/L) | 6.1 (4.2–8.5) |
| c-FGF23 (RU/mL) | 77.0 (61.8–112.6) |
| URINE (24-h Collection) | Mean ± SD |
| Creatinine (mg/day) | 1402.3 ± 618.4 |
| UUN (g/day) | 12.2 ± 4.5 |
| Sodium (mEq/day) | 183 ± 76 |
| Potassium (mEq/day) | 67 ± 28 |
| Calcium (mg/day) | 106 ± 79 |
| Urine P Parameters (24-h Collection) | Mean ± SD |
| Urine Phosphate excretion (mg/day) | 797.2 ± 324.7 |
| Fractional excretion of phosphate (FeP) (%) | 23.3 ± 10.0 |
| Urine Phosphate excretion/eGFR (mg/day/eGFR) | 13.1 ± 7.1 |
| Phosphate/Creatinine ratio (mg/mg) | 0.60 ± 0.17 |
| Phosphate/UUN ratio (mg/g) | 66.5 ± 16.3 |
| Variable | Spearman Coefficient | Significance (p) |
|---|---|---|
| (A) Total P intake (mg/day) | ||
| Urine P Excretion (mg/day) | 0.189 | 0.128 |
| FeP (%) | −0.088 | 0.484 |
| Urine P Excretion/eGFR (mg/day/GFR) | −0.032 | 0.797 |
| P/Creatinine ratio (mg/mg) | 0.110 | 0.373 |
| UUN (g/day) | −0.11 | 0.927 |
| UUN/Creatinine ratio(mg/mg) | −0.088 | 0.471 |
| P/UUN ratio (mg/g) | 0.284 | 0.018 |
| (B) P/UUN ratio (mg/g) | ||
| P from Animal protein (mg) | 0.160 | 0.189 |
| P from Vegetable protein (mg) | 0.223 | 0.066 |
| Inorganic P (mg) | 0.332 | 0.005 |
| Variable | T1 (<58.9 mg/g) n = 24 | T2 (58.9–71.1 mg/g) n = 23 | T3 (>71.1 mg/g) n = 24 | p |
|---|---|---|---|---|
| Age (years) | 62 ± 10 | 62 ± 8 | 61 ± 9 | 0.863 |
| GFR (CKD-EPI) (mL/min/1.73 m2) | 65 ± 23 | 66 ± 20 | 73 ± 19 | 0.441 |
| Total intake of P (mg/day) | 979.3 ± 348.5 | 1042.1 ± 355.2 | 1227.2 ± 347.3 | 0.047 |
| P intake from animal source (mg/day) | 628.5 ± 98.9 | 673.0 ± 726.2 | 726.2 ± 164.8 | 0.243 |
| P intake from vegetable source (mg/day) | 194.0 ± 98.9 | 257.3 ± 143.9 | 255.4 ± 137.2 | 0.185 |
| Inorganic P intake (mg/day) | 160.1 ± 211.1 | 113.4 ± 103.6 | 247.1 ± 253.1 | 0.038 |
| FeP (%) | 22.9 ± 12.3 | 24.1 ± 10.6 | 22.9 ± 6.8 | 0.653 |
| Urine P Excretion (mg/day) | 696.7 ± 237.2 | 770.1 ± 313.7 | 923.6 ± 356.1 | 0.049 |
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Characteristic | OR | 95 % CI | p-Value | OR | 95% CI | p-Value |
| Gender (F vs. M) | 0.939 | 0.232, 3.696 | 0.93 | |||
| Age (Years) | 0.885 | 0.811, 0.95 | 0.002 | 0.883 | 0.807, 0.95 | 0.003 |
| P/UUN ratio (mg/g) | 1.058 | 1.015, 1.111 | 0.013 | 1.061 | 1.019, 1.112 | 0.007 |
| P from animal sources (mg/day) | 1.000 | 0.997, 1.003 | 0.82 | |||
| P from vegetal sources (mg/day) | 1.002 | 0.997, 1007 | 0.52 | |||
| GFR (CKD-EPI) (mL/min/1.73 m2) | 1.000 | 0.97, 1.029 | 0.99 |
| Variable | Spearman Coefficient | Significance (p) |
|---|---|---|
| Urine P excretion (mg/day) | ||
| c-FGF23 (RU/mL) | −0.149 | 0.225 |
| PTH (pg/mL) | −0.096 | 0.435 |
| Urine P/Cr ratio (mg/mg) | ||
| c-FGF23 (RU/mL) | −0.009 | 0.938 |
| PTH (pg/mL) | 0.148 | 0.221 |
| Urine P/UUN ratio (mg/g) | ||
| c-FGF23 (RU/mL) | −0.022 | 0.859 |
| PTH (pg/mL) | 0.147 | 0.228 |
| eGFR (CKD-EPI) | ||
| c-FGF23 (RU/mL) | −0.534 | <0.001 |
| PTH (pg/mL) | −0.265 | 0.050 |
| FeP (%) | ||
| c-FGF23 (RU/mL) | 0.367 | 0.002 |
| PTH (pg/mL) | 0.381 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pendón-Ruiz de Mier, M.V.; Vergara, N.; Rodelo-Haad, C.; López-Zamorano, M.D.; Membrives-González, C.; López-Baltanás, R.; Muñoz-Castañeda, J.R.; Caravaca, F.; Martín-Malo, A.; Felsenfeld, A.J.; et al. Assessment of Inorganic Phosphate Intake by the Measurement of the Phosphate/Urea Nitrogen Ratio in Urine. Nutrients 2021, 13, 292. https://doi.org/10.3390/nu13020292
Pendón-Ruiz de Mier MV, Vergara N, Rodelo-Haad C, López-Zamorano MD, Membrives-González C, López-Baltanás R, Muñoz-Castañeda JR, Caravaca F, Martín-Malo A, Felsenfeld AJ, et al. Assessment of Inorganic Phosphate Intake by the Measurement of the Phosphate/Urea Nitrogen Ratio in Urine. Nutrients. 2021; 13(2):292. https://doi.org/10.3390/nu13020292
Chicago/Turabian StylePendón-Ruiz de Mier, María Victoria, Noemí Vergara, Cristian Rodelo-Haad, María Dolores López-Zamorano, Cristina Membrives-González, Rodrigo López-Baltanás, Juan Rafael Muñoz-Castañeda, Francisco Caravaca, Alejandro Martín-Malo, Arnold J. Felsenfeld, and et al. 2021. "Assessment of Inorganic Phosphate Intake by the Measurement of the Phosphate/Urea Nitrogen Ratio in Urine" Nutrients 13, no. 2: 292. https://doi.org/10.3390/nu13020292
APA StylePendón-Ruiz de Mier, M. V., Vergara, N., Rodelo-Haad, C., López-Zamorano, M. D., Membrives-González, C., López-Baltanás, R., Muñoz-Castañeda, J. R., Caravaca, F., Martín-Malo, A., Felsenfeld, A. J., De la Torre, E. J., Soriano, S., Santamaría, R., & Rodríguez, M. (2021). Assessment of Inorganic Phosphate Intake by the Measurement of the Phosphate/Urea Nitrogen Ratio in Urine. Nutrients, 13(2), 292. https://doi.org/10.3390/nu13020292