Rind from Purple Mangosteen (Garcinia mangostana) Attenuates Diet-Induced Physiological and Metabolic Changes in Obese Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Garcinia mangostana Rind Powder Preparation and Analyses
2.2. Rats and Diets
2.3. Rat Measurements
2.4. Statistical Analysis
3. Results
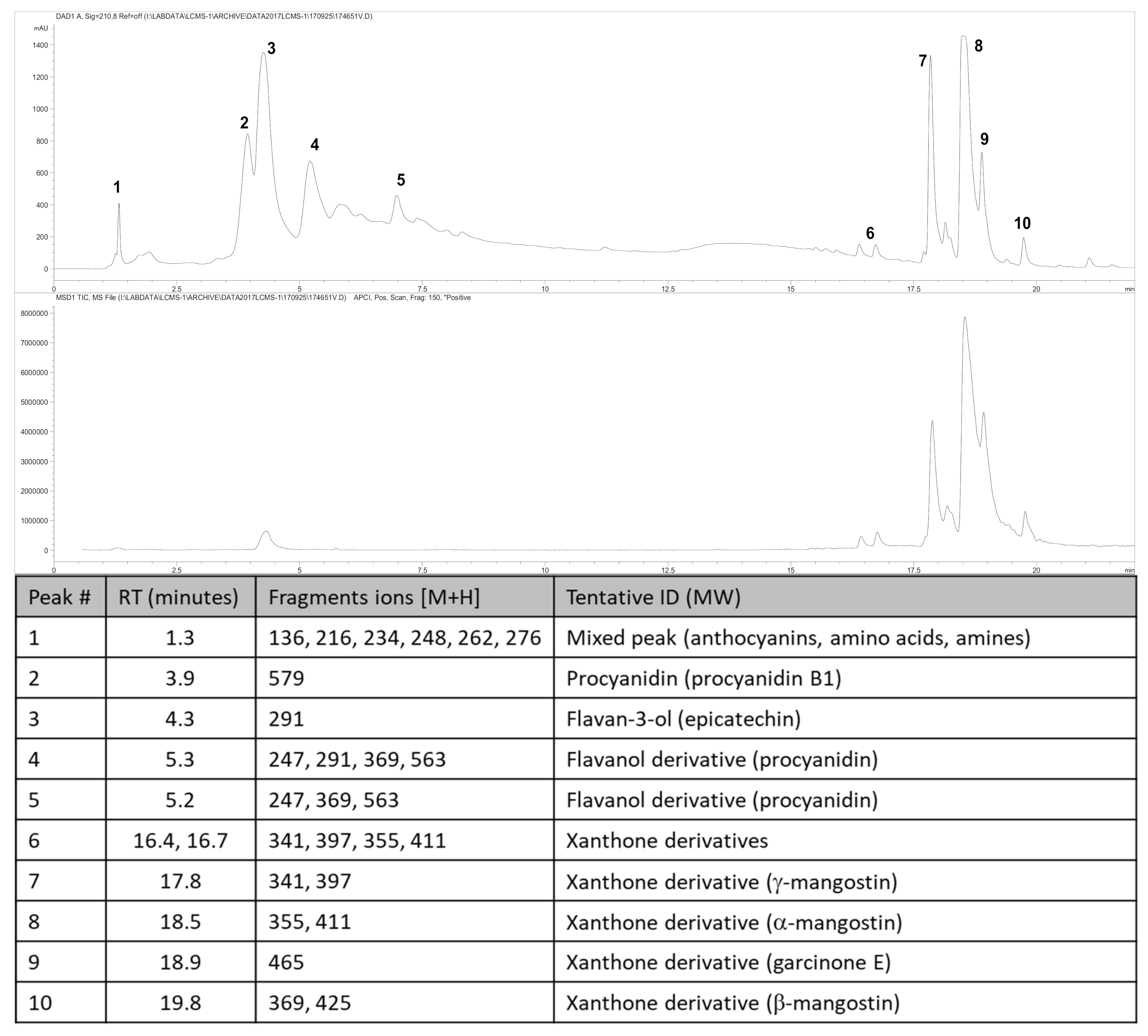
3.1. Weight and Phytochemical Analysis of Garcinia mangostana Rind
3.2. Metabolic Parameters
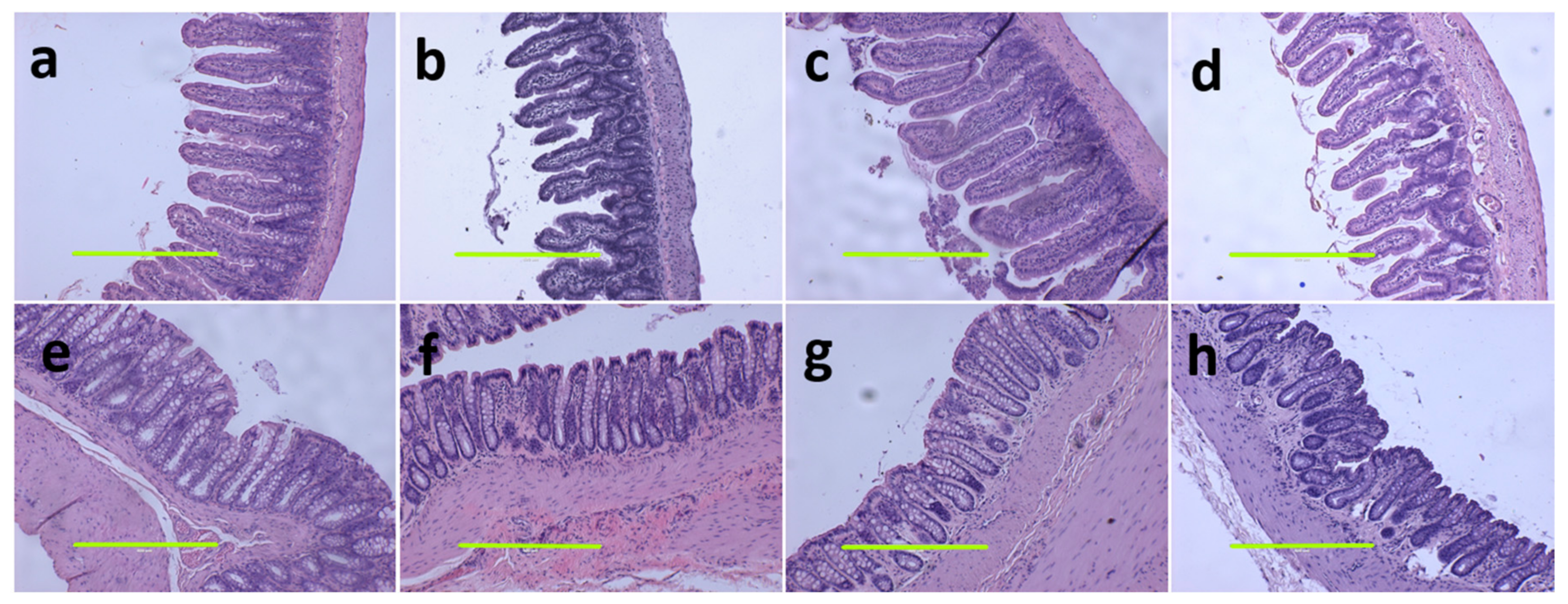
3.3. Liver and Gastrointestinal Structure and Function
3.4. Cardiovascular Structure and Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hemshekhar, M.; Sunitha, K.; Santhosh, M.S.; Devaraja, S.; Kemparaju, K.; Vishwanath, B.; Niranjana, S.; Girish, K. An overview on genus Garcinia: Phytochemical and therapeutical aspects. Phytochem. Rev. 2011, 10, 325–351. [Google Scholar] [CrossRef]
- John, O.D.; Brown, L.; Panchal, S.K. Chapter 3. Garcinia fruits: Their potential to combat metabolic syndrome. In Nutraceuticals and Natural Product Derivatives: Disease Prevention & Drug Discovery; Ullah, M.F., Ahmad, A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 39–80. [Google Scholar] [CrossRef]
- Asyifah, M.R.; Lu, K.; Ting, H.L.; Zhang, D. Hidden potential of tropical fruit waste components as a useful source of remedy for obesity. J. Agric. Food Chem. 2014, 62, 3505–3516. [Google Scholar] [CrossRef] [PubMed]
- Obolskiy, D.; Pischel, I.; Siriwatanametanon, N.; Heinrich, M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother. Res. 2009, 23, 1047–1065. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Orozco, F.; Failla, M.L. Biological activities and bioavailability of mangosteen xanthones: A critical review of the current evidence. Nutrients 2013, 5, 3163–3183. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Li, P.; Li, N.; Zhang, Q.; Bai, X.; Wang, L.; Xiao, Y.; Sun, L.; Yang, Q.; Yan, J. Xanthones from the pericarp of Garcinia mangostana. Molecules 2017, 22, 683. [Google Scholar] [CrossRef] [Green Version]
- Ovalle-Magallanes, B.; Eugenio-Pérez, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017, 109, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Tousian Shandiz, H.; Razavi, B.M.; Hosseinzadeh, H. Review of Garcinia mangostana and its xanthones in metabolic syndrome and related complications. Phytother. Res. 2017, 31, 1173–1182. [Google Scholar] [CrossRef]
- Taher, M.; Tg Zakaria, T.M.F.S.; Susanti, D.; Zakaria, Z.A. Hypoglycaemic activity of ethanolic extract of Garcinia mangostana Linn. in normoglycaemic and streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2016, 16, 135. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Watanabe, M.; Gangitano, E.; Francomano, D.; Addessi, E.; Toscano, R.; Costantini, D.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G. Mangosteen extract shows a potent insulin sensitizing effect in obese female patients: A prospective randomized controlled pilot study. Nutrients 2018, 10, 586. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Sintara, M.; Chang, T.; Ou, B. Daily consumption of a mangosteen-based drink improves in vivo antioxidant and anti-inflammatory biomarkers in healthy adults: A randomized, double-blind, placebo-controlled clinical trial. Food Sci. Nutr. 2015, 3, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Wang, Y.T.; Lin, L.G. New insights into the anti-obesity activity of xanthones from Garcinia mangostana. Food Funct. 2015, 6, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Risi, R.; Masi, D.; Caputi, A.; Balena, A.; Rossini, G.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Manfrini, S.; et al. Current evidence to propose different food supplements for weight loss: A comprehensive review. Nutrients 2020, 12, 2873. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet–induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.B. HPLC analysis of selected xanthones in mangosteen fruit. J. Sep. Sci. 2007, 30, 1229–1234. [Google Scholar] [CrossRef]
- John, O.; Wanyonyi, S.; Mouatt, P.; Panchal, S.; Brown, L. Achacha (Garcinia humilis) rind improves cardiovascular function in rats with diet-induced metabolic syndrome. Nutrients 2018, 10, 1425. [Google Scholar] [CrossRef] [Green Version]
- John, O.D.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Physiological and metabolic effects of yellow mangosteen (Garcinia dulcis) rind in rats with diet-induced metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 272. [Google Scholar] [CrossRef] [Green Version]
- Sekar, S.; Shafie, S.R.; Prasadam, I.; Crawford, R.; Panchal, S.K.; Brown, L.; Xiao, Y. Saturated fatty acids induce development of both metabolic syndrome and osteoarthritis in rats. Sci. Rep. 2017, 7, 46457. [Google Scholar] [CrossRef] [Green Version]
- Parlee, S.D.; Lentz, S.I.; Mori, H.; MacDougald, O.A. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 2014, 537, 93–122. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Good, C.A.; Kramer, H.; Somogyi, M. The determination of glycogen. J. Biol. Chem. 1933, 100, 485–491. [Google Scholar] [CrossRef]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Palapol, Y.; Ketsa, S.; Stevenson, D.; Cooney, J.M.; Allan, A.C.; Ferguson, I.B. Colour development and quality of mangosteen (Garcinia mangostana L.) fruit during ripening and after harvest. Postharvest Biol. Technol. 2009, 51, 349–353. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaovanalikit, A.; Mingmuang, A.; Kitbunluewit, T.; Choldumrongkool, N.; Sondee, J.; Chupratum, S. Anthocyanin and total phenolics content of mangosteen and effect of processing on the quality of mangosteen products. Int. Food Res. J. 2012, 19, 1047–1053. [Google Scholar]
- Lundbom, J. Adipose tissue and liver. J. Appl. Physiol. (1985) 2018, 124, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [Green Version]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial dysfunction in obesity-induced inflammation: Molecular mechanisms and clinical implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.M.; Kim, Y.M.; Huh, J.H.; Lee, E.S.; Kwon, M.H.; Lee, B.R.; Ko, H.-J.; Chung, C.H. α-Mangostin ameliorates hepatic steatosis and insulin resistance by inhibition C-C chemokine receptor 2. PLoS ONE 2017, 12, e0179204. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Liu, Q.; Lu, X.; Li, Z.; Wang, C.; Leung, C.-H.; Wang, Y.; Peng, C.; Lin, L. α-Mangostin remodels visceral adipose tissue inflammation to ameliorate age-related metabolic disorders in mice. Aging 2019, 11, 11084–11110. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Bae, J.K.; Chae, H.-S.; Kim, Y.-M.; Sreymom, Y.; Han, L.; Jang, H.Y.; Chin, Y.-W. α-Mangostin regulates hepatic steatosis and obesity through SirT1-AMPK and PPARγ pathways in high-fat diet-induced obese mice. J. Agric. Food Chem. 2015, 63, 8399–8406. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Wang, Y.; Ma, X.; Liang, Y.; Tian, W.; Ma, Q.; Jiang, H.; Zhao, Y. α-Mangostin induces apoptosis and suppresses differentiation of 3T3-L1 cells via inhibiting fatty acid synthase. PLoS ONE 2012, 7, e33376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taher, M.; Mohamed Amiroudine, M.Z.A.; Tengku Zakaria, T.M.F.S.; Susanti, D.; Ichwan, S.J.; Kaderi, M.A.; Ahmed, Q.U.; Zakaria, Z.A. α-Mangostin improves glucose uptake and inhibits adipocytes differentiation in 3T3-L1 cells via PPARγ, GLUT4, and leptin expressions. Evid. Based Complement. Alternat. Med. 2015, 2015, 740238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Bhatt, P.C.; Kaithwas, G.; Rashid, M.; Al-abbasi, F.; Khan, J.A.; Anwar, F.; Verma, A. α-Mangostin mediated pharmacological modulation of hepatic carbohydrate metabolism in diabetes induced Wistar rat. Beni. Suef. Univ. J. Basic Appl. Sci. 2016, 5, 255–276. [Google Scholar] [CrossRef] [Green Version]
- Chae, H.-S.; Kim, Y.-M.; Bae, J.-K.; Sorchhann, S.; Yim, S.; Han, L.; Paik, J.H.; Choi, Y.H.; Chin, Y.-W. Mangosteen extract attenuates the metabolic disorders of high-fat-fed mice by activating AMPK. J. Med. Food 2016, 19, 148–154. [Google Scholar] [CrossRef]
- Gu, L.; Cai, N.; Lyu, Y.; Yao, L.; Wang, F.; Xu, H.; Hu, Z.; Li, H.; Xu, X. γ-Mangostin ameliorates free fatty acid-induced lipid accumulation via the SIRT1/LKB1/AMPK pathway in HepG2 and L02 cells. J. Agric. Food Chem. 2019, 67, 13929–13938. [Google Scholar] [CrossRef]
- Catorce, M.N.; Acero, G.; Pedraza-Chaverri, J.; Fragoso, G.; Govezensky, T.; Gevorkian, G. Alpha-mangostin attenuates brain inflammation induced by peripheral lipopolysaccharide administration in C57BL/6J mice. J. Neuroimmunol. 2016, 297, 20–27. [Google Scholar] [CrossRef]
- Izumida, Y.; Yahagi, N.; Takeuchi, Y.; Nishi, M.; Shikama, A.; Takarada, A.; Masuda, Y.; Kubota, M.; Matsuzaka, T.; Nakagawa, Y. Glycogen shortage during fasting triggers liver–brain–adipose neurocircuitry to facilitate fat utilization. Nat. Commun. 2013, 4, 2316. [Google Scholar] [CrossRef]
- Gonzalez-Abuin, N.; Pinent, M.; Casanova-Marti, A.; Arola, L.; Blay, M.; Ardevol, A. Procyanidins and their healthy protective effects against type 2 diabetes. Curr. Med. Chem. 2015, 22, 39–50. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, Z.; Dai, X.; Jiang, Y.; Bao, L.; Li, Y.; Li, Y. Grape seed proanthocyanidins ameliorate pancreatic b-cell dysfunction and death in low-dose streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats partially by regulating endoplasmic reticulum stress. Nutr. Metab. (Lond.) 2013, 10, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonprom, P.; Boonla, O.; Chayaburakul, K.; Welbat, J.U.; Pannangpetch, P.; Kukongviriyapan, U.; Kukongviriyapan, V.; Pakdeechote, P.; Prachaney, P. Garcinia mangostana pericarp extract protects against oxidative stress and cardiovascular remodeling via suppression of p47phox and iNOS in nitric oxide deficient rats. Ann. Anat. 2017, 212, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Devi Sampath, P.; Vijayaraghavan, K. Cardioprotective effect of α-mangostin, a xanthone derivative from mangosteen on tissue defense system against isoproterenol-induced myocardial infarction in rats. J. Biochem. Mol. Toxicol. 2007, 21, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Buelna-Chontal, M.; Correa, F.; Hernández-Reséndiz, S.; Zazueta, C.; Pedraza-Chaverri, J. Protective effect of α-mangostin on cardiac reperfusion damage by attenuation of oxidative stress. J. Med. Food. 2011, 14, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Segovia, S.A.; Vickers, M.H.; Gray, C.; Reynolds, C.M. Maternal obesity, inflammation, and developmental programming. Biomed. Res. Int. 2014, 2014, 418975. [Google Scholar] [CrossRef]
- Abdallah, H.M.; El-Bassossy, H.M.; Mohamed, G.A.; El-Halawany, A.M.; Alshali, K.Z.; Banjar, Z.M. Phenolics from Garcinia mangostana alleviate exaggerated vasoconstriction in metabolic syndrome through direct vasodilatation and nitric oxide generation. BMC Complement. Altern. Med. 2016, 16, 359. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against oxidative stress: From molecular mechanisms to clinical applications. Biomed Res. Int. 2018, 2018, 8584136. [Google Scholar] [CrossRef] [Green Version]
- Handayani, O.; Sargowo, D.; Rohman, M.S.; Satrijo, B.; Tjahjono, C.T.; Hendrawan, D. The effect of add-on Garcinia mangostana L. extract on endothelial dysfunction in type 2 diabetes mellitus subjects with high risk Framingham Score: A cohort study. Heart Sci. J. 2020, 1, 21–25. [Google Scholar] [CrossRef]
- Chae, H.S.; Kim, E.Y.; Han, L.; Kim, N.R.; Lam, B.; Paik, J.H.; Yoon, K.D.; Choi, Y.H.; Chin, Y.W. Xanthones with pancreatic lipase inhibitory activity from the pericarps of Garcinia mangostana L. (Guttiferae). Eur. J. Lipid Sci. Technol. 2016, 118, 1416–1421. [Google Scholar] [CrossRef]
- Vishnu Priya, V.; Jainu, M.; Mohan, S.K.; Karthik, B.; Saraswathi, P.; Chandra Sada, G. Toxicity study of Garcinia mangostana Linn. pericarp extract in rats. Asian J. Exp. Biol. Sci. 2010, 1, 633–637. [Google Scholar]
- Chivapat, S.; Chavalittumrong, P.; Wongsinkongman, P.; Phisalpong, C.; Rungsipipat, A. Chronic toxicity study of Garcinia mangostana Linn. pericarp extract. Thai J. Vet. Med. 2011, 41, 45–54. [Google Scholar]
- Lluís, L.; Muñoz, M.; Nogués, M.R.; Sánchez-Martos, V.; Romeu, M.; Giralt, M.; Valls, J.; Solà, R. Toxicology evaluation of a procyanidin-rich extract from grape skins and seeds. Food Chem. Toxicol. 2011, 49, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Bhaswant, M.; Fanning, K.; Netzel, M.; Mathai, M.L.; Panchal, S.K.; Brown, L. Cyanidin 3-glucoside improves diet-induced metabolic syndrome in rats. Pharmacol. Res. 2015, 102, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Pourrat, H.; Bastide, P.; Dorier, P.; Tronche, P. Préparation et activité thérapeutique de quelques glycosides d’anthocyanes. Chim Thérap 1967, 2, 33–38. [Google Scholar]
- Chang, C.-W.; Huang, T.-Z.; Chang, W.-H.; Tseng, Y.-C.; Wu, Y.-T.; Hsu, M.-C. Acute Garcinia mangostana (mangosteen) supplementation does not alleviate physical fatigue during exercise: A randomized, double-blind, placebo-controlled, crossover trial. J. Int. Soc. Sports Nutr. 2016, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Chitchumroonchokchai, C.; Riedl, K.M.; Suksumrarn, S.; Clinton, S.K.; Kinghorn, A.D.; Failla, M.L. Xanthones in mangosteen juice are absorbed and partially conjugated by healthy adults. J. Nutr. 2012, 142, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef] [Green Version]
- Márquez, F.; Babio, N.; Bulló, M.; Salas-Salvadó, J. Evaluation of the safety and efficacy of hydroxycitric acid or Garcinia cambogia extracts in humans. Crit. Rev. Food Sci. Nutr. 2012, 52, 585–594. [Google Scholar] [CrossRef]
- Panda, V.; Ashar, H.; Srinath, S. Antioxidant and hepatoprotective effect of Garcinia indica fruit rind in ethanol-induced hepatic damage in rodents. Interdiscip. Toxicol. 2012, 5, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Guillén-Enríquez, C.; López-Teros, V.; Martín-Orozco, U.; López-Díaz, J.A.; Del Hierro-Ochoa, J.; Ramos-Jiménez, A.; Astiazarán-García, H.; Martínez-Ruiz, N.D.R.; Wall-Medrano, A. Selected physiological effects of a Garcinia gummi-gutta extract in rats fed with different hypercaloric diets. Nutrients 2018, 10, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-J.; Choi, M.-S.; Park, Y.B.; Kim, S.R.; Lee, M.-K.; Jung, U.J. Garcinia cambogia attenuates diet-induced adiposity but exacerbates hepatic collagen accumulation and inflammation. World J. Gastroenterol. 2013, 19, 4689–4701. [Google Scholar] [CrossRef] [PubMed]
- Ateş, A.; Esen Gürsel, F.; Bilal, T.; Altıner, A. Effect of dietary Garcinia cambogia extract on serum lipid profile and serum enzymes in rats fed high-lipid diet. Iran. J. Vet. Res. 2012, 13, 1–7. [Google Scholar] [CrossRef]




| Variables | C | CM | H | HM | p Value | ||
|---|---|---|---|---|---|---|---|
| Diet | Treatment | Diet × Treatment | |||||
| Physiological parameters | |||||||
| Initial body weight, g | 338 ± 1 a | 339 ± 1 a | 337 ± 1 a | 337 ± 1 a | 0.21 | 0.41 | 0.39 |
| Body weight at 8 weeks, g | 358 ± 7 b | 371 ± 5 b | 425 ± 7 a | 416 ± 6 a | <0.0001 | 0.76 | 0.08 |
| Body weight at 16 weeks, g | 407 ± 8 b | 346 ± 7 c | 515 ± 13 a | 438 ± 12 b | <0.0001 | <0.0001 | 0.44 |
| Food intake, g/day | 41.0 ± 2.1 a | 36.3 ± 2.3 a | 27.5 ± 2.9 b | 24.3 ± 1.9 b | <0.0001 | 0.10 | 0.75 |
| Water intake, g/day | 24.6 ± 2.6 a | 25.6 ± 2.5 a | 28.4 ± 3.4 a | 32.3 ± 3.8 a | 0.09 | 0.44 | 0.64 |
| α-mangostin intake, mg/kg/day | - | 280 ± 4 | - | 168 ± 10 | - | - | - |
| Procyanidin intake, mg/kg/day | - | 653 ± 8 | - | 355 ± 6 | - | - | - |
| Anthocyanin intake, mg/kg/day | - | 7.3 ± 0.09 | - | 3.9 ± 0.06 | - | - | - |
| Hydroxycitric acid intake, mg/kg/day | - | 21.8 ± 0.28 | - | 11.8 ± 0.18 | - | - | - |
| Energy intake, kJ/day | 460 ± 26 bc | 401 ± 28 c | 605 ± 48 a | 543 ± 39 ab | 0.0003 | 0.10 | 0.96 |
| Feed efficiency, g/kJ | 0.11 ± 0.01 b | −0.06 ± 0.01 d | 0.15 ± 0.02 a | 0.02 ± 0.01 c | <0.0001 | <0.0001 | 0.25 |
| Body weight gain (8–16 weeks), % | 13.7 ± 0.9 b | −6.7 ± 0.8 d | 21.4 ± 2.8 a | 3.0 ± 1.9 c | <0.0001 | <0.0001 | 0.57 |
| Abdominal circumference at 8 weeks, cm | 16.1 ± 0.2 b | 16.6 ± 0.2 b | 20.3 ± 0.6 a | 20.2 ± 0.5 a | <0.0001 | 0.62 | 0.53 |
| Abdominal circumference at 16 weeks, cm | 18.5 ± 0.4 b | 16.2 ± 0.2 c | 22.6 ± 0.3 a | 17.9 ± 0.3 b | <0.0001 | <0.0001 | 0.0003 |
| Whole-body lean mass at 8 weeks, g | 291 ± 9 a | 302 ± 6 a | 308 ± 4 a | 310 ± 5 a | 0.06 | 0.33 | 0.59 |
| Whole-body lean mass at 16 weeks, g | 293 ± 11 ab | 283 ± 4 bc | 297 ± 7 ab | 311 ± 5 a | 0.031 | 0.79 | 0.10 |
| Whole-body fat mass at 8 weeks, g | 55 ± 9 b | 50 ± 4 b | 96 ± 8 a | 84 ± 6 a | <0.0001 | 0.26 | 0.69 |
| Whole-body fat mass at 16 weeks, g | 95 ± 9 b | 42 ± 3 c | 211 ± 13 a | 102 ± 8 b | <0.0001 | <0.0001 | 0.004 |
| Bone mineral content at 8 weeks, g | 11.0 ± 0.4 b | 10.1 ± 0.2 b | 12.4 ± 0.3 a | 11.9 ± 0.2 a | <0.0001 | 0.053 | 0.62 |
| Bone mineral content at 16 weeks, g | 10.9 ± 0.3 c | 12.4 ± 0.4 b | 16.4 ± 0.5 a | 12.5 ± 0.4 b | <0.0001 | 0.015 | <0.0001 |
| Bone mineral density at 8 weeks, g/cm2 | 0.160 ± 0.003 a | 0.158 ± 0.003 a | 0.170 ± 0.002 a | 0.163 ± 0.004 a | 0.026 | 0.17 | 0.52 |
| Bone mineral density at 16 weeks, g/cm2 | 0.175 ± 0.003 b | 0.162 ± 0.003 c | 0.185 ± 0.003 a | 0.166 ± 0.003 bc | 0.029 | <0.0001 | 0.32 |
| Body mass index at 16 weeks, g/cm2 | 0.63 ± 0.01 bc | 0.60 ± 0.01 c | 0.78 ± 0.02 a | 0.67 ± 0.02 b | <0.0001 | <0.0001 | 0.015 |
| Retroperitoneal fat, mg/mm tibial length | 250 ± 21 b | 109 ± 8 c | 516 ± 35 a | 258 ± 25 b | <0.0001 | <0.0001 | 0.020 |
| Epididymal fat, mg/mm tibial length | 97 ± 16 b | 59± 8 c | 169 ± 17 a | 88 ± 10 b | 0.0003 | <0.0001 | 0.10 |
| Omental fat, mg/mm tibial length | 156 ± 13 b | 76 ± 4 c | 267 ± 13 a | 145 ± 11 b | <0.0001 | <0.0001 | 0.051 |
| Total abdominal fat, mg/mm tibial length | 502 ± 43 b | 244 ± 12 c | 952 ± 54 a | 492 ± 39 b | <0.0001 | <0.0001 | 0.014 |
| Visceral adiposity index, % | 5.72 ± 0.40 b | 3.23 ± 0.16 d | 8.79 ± 0.38 a | 5.40 ± 0.34 b | <0.0001 | <0.0001 | 0.18 |
| Heat production, 16 week, kcal/h | 3.98 ± 0.31 a | 2.66 ± 0.24 b | 4.48 ± 0.24 a | 3.61 ± 0.23 a | 0.015 | 0.001 | 0.40 |
| Heat production area under the curve | 2864 ± 151 ab | 1919 ± 149 c | 3235 ± 80 a | 2606± 111 b | <0.0001 | <0.0001 | 0.08 |
| RER, 16 week | 0.984 ± 0.02 a | 0.958 ± 0.01 a | 0.852 ± 0.02 b | 0.869 ± 0.02 b | <0.0001 | 0.98 | 0.37 |
| RER area under the curve, 16 week | 708 ± 10 a | 696 ± 6 a | 614 ± 7 b | 626 ± 7 b | <0.0001 | 0.79 | 0.64 |
| Mean liver fat vacuole area, µm2 | 14.1 ± 0.7 d | 37.4 ± 2.3 c | 142.1 ± 13.8 a | 59.9 ± 4.4 b | <0.0001 | <0.0001 | <0.0001 |
| Mean retroperitoneal adipocyte area, µm2 | 4333 ± 109 c | 2935 ± 213 d | 9587 ± 482 a | 5173 ± 487 b | <0.0001 | <0.0001 | <0.0001 |
| Faecal lipid content, mg/g | 20.8 ± 1.5 b | 16.5 ± 0.6 b | 40.6 ± 1.5 a | 43.4 ± 2.8 a | <0.0001 | 0.66 | 0.048 |
| Plasma biochemistry | |||||||
| Alanine transaminase activity, U/L | 28.9 ± 3.0 c | 44.1 ± 3.5 b | 42.7 ± 5.0 b | 62.2 ± 6.0 a | 0.002 | 0.0006 | 0.64 |
| Aspartate transaminase activity, U/L | 86 ± 8 b | 87 ± 4 b | 177 ± 22 a | 112 ± 12 b | 0.002 | 0.07 | 0.06 |
| Total cholesterol, mmol/L | 1.49 ± 0.06 b | 0.89 ± 0.07 c | 1.69 ± 0.09 a | 1.44 ± 0.07 a | <0.0001 | <0.0001 | 0.038 |
| Triglycerides, mmol/L | 0.47 ± 0.05 b | 0.56 ± 0.10 b | 1.15 ± 0.15 a | 1.23 ± 0.26 a | <0.0001 | 0.55 | 0.97 |
| Non-esterified fatty acids, mmol/L | 0.87 ± 0.16 c | 1.40 ± 0.25 c | 3.27 ± 0.16 a | 2.36 ± 0.29 b | <0.0001 | 0.39 | 0.002 |
| Basal blood glucose at 8 weeks, mmol/L | 2.4 ± 0.1 b | 2.5 ± 0.2 b | 3.1 ± 0.1 a | 3.0 ± 0.1 a | <0.0001 | 1.00 | 0.33 |
| Basal blood glucose at 16 weeks, mmol/L | 2.7 ± 0.1 ab | 2.5 ± 0.2 b | 3.0 ± 0.1 a | 2.6 ± 0.1 ab | 0.024 | 0.08 | 0.56 |
| Blood glucose area under the curve at 8 weeks, mmol/L×minutes | 495 ± 11 b | 483 ± 10 b | 580 ± 9 a | 572 ± 9 a | <0.0001 | 0.32 | 0.84 |
| Blood glucose area under the curve at 16 weeks, mmol/L×minutes | 471 ± 17 c | 461 ± 13 c | 611 ± 33 a | 546 ± 15 b | <0.0001 | 0.016 | 0.15 |
| Liver wet weight, mg/mm tibial length | 234 ± 8 b | 246 ± 7 b | 355 ± 12 a | 358 ± 9 a | <0.0001 | 0.44 | 0.61 |
| Liver glycogen, mg/g | 12.42 ± 0.60 a | 10.82 ± 1.58 a | 13.58 ± 0.51 a | 4.76 ± 0.57 b | 0.007 | <0.0001 | 0.0002 |
| Catalase activity, kU/L | 39.1 ± 3.7 b | 26.6 ± 4.7 b | 55.2 ± 5.8 a | 42.4 ± 4.7 ab | 0.033 | 0.008 | 0.98 |
| C-reactive protein, µg/mL | 432 ± 12 b | 360 ± 19 c | 508 ± 8 a | 295 ± 12 d | <0.0001 | <0.0001 | <0.0001 |
| Cardiovascular parameters | |||||||
| Systolic blood pressure at 8 weeks, mmHg | 115 ± 3 b | 117 ± 2 b | 136 ± 4 a | 132 ± 3 a | <0.0001 | 0.69 | 0.37 |
| Systolic blood pressure at 16 weeks, mmHg | 117 ± 2 b | 105 ± 5 b | 131 ± 2 a | 109 ± 5 b | 0.020 | <0.0001 | 0.16 |
| Left ventricle + septum wet weight, mg/mm tibial length | 23.1 ± 1.3 a | 19.6 ± 0.8 b | 23.2 ± 1.2 a | 21.2 ± 0.6 ab | 0.41 | 0.006 | 0.38 |
| Right ventricle, mg/mm tibial length | 4.04 ± 0.18 ab | 3.67 ± 0.14 b | 4.61 ± 0.17 a | 4.05 ± 0.15 ab | 0.006 | 0.006 | 0.56 |
| Diastolic stiffness constant (κ) | 21.1 ± 0.4 bc | 20.4 ± 0.3 c | 26.8 ± 0.8 a | 22.2 ± 0.9 b | <0.0001 | 0.0002 | 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, O.D.; Mouatt, P.; Panchal, S.K.; Brown, L. Rind from Purple Mangosteen (Garcinia mangostana) Attenuates Diet-Induced Physiological and Metabolic Changes in Obese Rats. Nutrients 2021, 13, 319. https://doi.org/10.3390/nu13020319
John OD, Mouatt P, Panchal SK, Brown L. Rind from Purple Mangosteen (Garcinia mangostana) Attenuates Diet-Induced Physiological and Metabolic Changes in Obese Rats. Nutrients. 2021; 13(2):319. https://doi.org/10.3390/nu13020319
Chicago/Turabian StyleJohn, Oliver D., Peter Mouatt, Sunil K. Panchal, and Lindsay Brown. 2021. "Rind from Purple Mangosteen (Garcinia mangostana) Attenuates Diet-Induced Physiological and Metabolic Changes in Obese Rats" Nutrients 13, no. 2: 319. https://doi.org/10.3390/nu13020319






