Most Effective Combination of Nutraceuticals for Improved Memory and Cognitive Performance in the House Cricket, Acheta domesticus
Abstract
1. Introduction
2. Effect of Nutraceuticals
2.1. Polyphenols
2.2. Probiotics
2.3. Multivitamins
2.4. Omega-3 Polyunsaturated Fatty Acids
2.5. Zinc
3. Materials and Methods
3.1. Animals
3.2. Nutrient Treatments
3.2.1. Multivitamins
3.2.2. Zinc
3.2.3. Polyphenols
3.2.4. Omega-3 Fatty Acids
3.2.5. Probiotics
3.3. Groups
3.4. Spatial Memory Testing
3.4.1. Habituation and Practice Sessions
3.4.2. Alternation Test
3.4.3. Recognition Memory Test
3.5. Potential Bias
3.6. Statistical Analysis
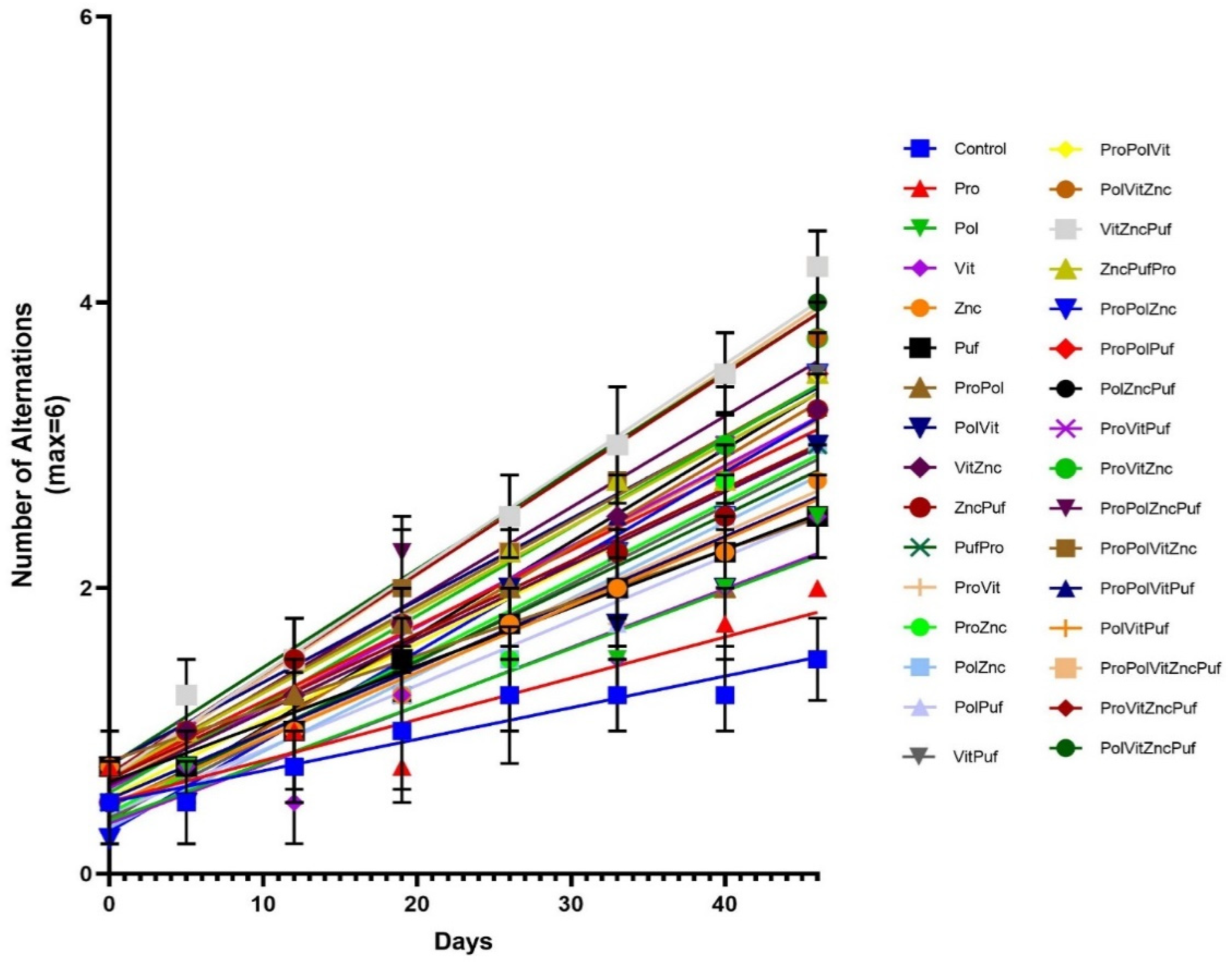
4. Results
5. Discussion
6. Implications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gundersen, C.; Ziliak, J.P. Food insecurity and health outcomes. Health Aff. 2015, 34, 1830–1839. [Google Scholar] [CrossRef]
- Shankar, P.; Chung, R.; Frank, D.A. Association of food insecurity with children’s behavioral, emotional, and academic outcomes: A systematic review. J. Dev. Behav. Pediatr. 2017, 38, 135–150. [Google Scholar] [CrossRef]
- Coleman-Jensen, A.; Rabbitt, M.P.; Gregory, C.A.; Singh, A. Household Food Security in the United States in 2016; Economic Research Report–237; Economic Research Service, U.S. Department of Agriculture: Washington, DC, USA, 2017. Available online: https://www.ers.usda.gov/webdocs/publications/84973/err-237.pdf (accessed on 25 January 2021).
- Gregory, C.A.; Coleman-Jensen, A. Food insecurity, Chronic Disease, and Health among Working-Age Adults; Economic Research Report–235; Economic Research Service, U.S. Department of Agriculture: Washington, DC, USA, 2017. Available online: https://www.ers.usda.gov/webdocs/publications/84467/err-235.pdf (accessed on 25 January 2021).
- Madireddy, S.; Madireddy, S. The role of diet in maintaining strong brain health by taking the advantage of the gut-brain axis. J. Food Nutr. Res. 2019, 7, 41–50. [Google Scholar]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; dos Santos, C.N. Polyphenols beyond barriers: A glimpse into the brain. Curr. Neuropharmacol. 2017, 15, 562–594. [Google Scholar] [CrossRef]
- Grima, N.A.; Pase, M.P.; Macpherson, H.; Pipingas, A. The effects of multivitamins on cognitive performance: A systematic review and meta-analysis. J. Alzheimers Dis. 2012, 29, 561–569. [Google Scholar] [CrossRef]
- Lange, K.W.; Nakamura, Y.; Chen, N.; Guo, J.; Kanaya, S.; Lange, K.M.; Li, S. Diet and medical foods in Parkinson’s disease. Food Sci. Hum. Wellness 2019, 8, 83–95. [Google Scholar] [CrossRef]
- Papalini, S.; Michels, F.; Kohn, N.; Wegman, J.; van Hemert, S.; Roelofs, K.; Arias-Vasquez, A.; Aarts, E. Stress matters: Randomized controlled trial on the effect of probiotics on neurocognition. Neurobiol. Stress 2019, 10, 100141. [Google Scholar] [CrossRef]
- Piechal, A.; Blecharz-Klin, K.; Pyrzanowska, J.; Widy-Tyszkiewicz, E. Influence of long-term zinc administration on spatial learning and exploratory activity in rats. Biol. Trace Elem. Res. 2016, 172, 408–418. [Google Scholar] [CrossRef]
- Rosli, H.; Shahar, S.; Din, N.C.; Haron, H.; Rajab, N.F. Prevalence of poor mental health and cognitive status among middle-aged adults and its predictors in relation to polyphenols intake. Malays. J. Med. Sci. 2019, 26, 72–89. [Google Scholar] [CrossRef]
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.F.; Barberger-Gateau, P. Flavonoid intake and cognitive decline over a 10-year period. Am. J. Epidemiol. 2007, 165, 1364–1371. [Google Scholar] [CrossRef]
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef]
- Madireddy, S.; Madireddy, S. Protection from the pathogenesis of neurodegenerative disorders, including Alzheimer’s disease, Amyotrophic Lateral Sclerosis, Huntington’s disease, and Parkinson’s diseases, through the mitigation of reactive oxygen species. J. Neurosci. Neurol. Disord. 2019, 3, 148–161. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Hassan, W.; Silva, C.E.B.; Mohammadzai, I.U.; da Rocha, J.B.T.; Landeira-Fernandez, J. Association of oxidative stress to the genesis of anxiety: Implications for possible therapeutic interventions. Curr. Neuropharmacol. 2014, 12, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Taysi, S.; Tascan, A.S.; Ugur, M.G.; Demir, M. Radicals, oxidative/nitrosative stress and preeclampsia. Mini Rev. Med. Chem. 2019, 19, 178–193. [Google Scholar] [CrossRef]
- Akbar, M.; Essa, M.M.; Daradkeh, G.; Abdelmegeed, M.A.; Choi, Y.; Mahmood, L.; Song, B.J. Mitochondrial dysfunction and cell death in neurodegenerative diseases through nitroxidative stress. Brain Res. 2016, 1637, 34–55. [Google Scholar] [CrossRef]
- Madireddy, S.; Madireddy, S. Regulation of reactive oxygen species-mediated damage in the pathogenesis of schizophrenia. Brain Sci. 2020, 10, 742. [Google Scholar] [CrossRef]
- Joseph, J.A.; Shukitt-Hale, B.; Casadesus, G. Reversing the deleterious effects of aging on neuronal communication and behavior: Beneficial properties of fruit polyphenolic compounds. Am. J. Clin. Nutr. 2005, 81, 313S–316S. [Google Scholar] [CrossRef]
- Lange, K.W. Red wine, resveratrol, and Alzheimer’s disease. Mov. Nutr. Health Dis. 2018, 2, 31–38. [Google Scholar]
- Lange, K.W.; Li, S. Resveratrol, pterostilbene and dementia. BioFactors 2018, 44, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic compounds prevent Alzheimer’s pathology through different effects on the Amyloid-β aggregation pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hamaguchi, T.; Naiki, H.; Yamada, M. Anti-amyloidogenic effects of antioxidants: Implications for the prevention and therapeutics of Alzheimer’s disease. Biochim. Biophys. Acta 2006, 1762, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Ramassamy, C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: A review of their intracellular targets. Eur. J. Pharmacol. 2006, 545, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Carroll, N.J. Dietary modulation of oxidative stress in Alzheimer’s disease. Int. J. Mol. Sci. 2017, 18, 1583. [Google Scholar] [CrossRef]
- Romo-Araiza, A.; Ibarra, A. Prebiotics and probiotics as potential therapy for cognitive impairment. Med. Hypotheses 2019, 134, 109410. [Google Scholar] [CrossRef] [PubMed]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behavior. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Miki, T.; Eguchi, M.; Kurotani, K.; Kochi, T.; Kuwahara, K.; Ito, R.; Kimura, Y.; Tsuruoka, H.; Akter, S.; Kashino, I.; et al. Dietary fiber intake and depressive symptoms in Japanese employees: The furukawa nutrition and health study. Nutrition 2016, 32, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, D.; Fogliano, V. Food design to feed the human gut microbiota. J. Agric. Food Chem. 2018, 66, 3754–3758. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, M.; Hamidi, G.; Salami, M. Probiotic supplementation improves the cognitive function and the anxiety-like behaviors in the stressed rats. Iran. J. Basic Med. Sci. 2019, 22, 506–514. [Google Scholar]
- Romo-Araiza, A.; Gutiérrez-Salmeán, G.; Galván, E.J.; Hernández-Frausto, M.; Herrera-López, G.; Romo-Parra, H.; García-Contreras, V.; Fernández-Presas, A.M.; Jasso-Chávez, R.; Borlongan, C.V.; et al. Probiotics and, prebiotics as a therapeutic strategy to improve memory in a model of middle-aged rats. Front. Aging Neurosci. 2018, 10, 416. [Google Scholar] [CrossRef]
- Bahrami, A.; Bahrami-Taghanaki, H.; Khorasanchi, Z.; Tayefi, M.; Ferns, G.A.; Sadeghnia, H.R.; Ghayour-Mobarhan, M. The association between neuropsychological function with serum vitamins A, D, and E and hs-CRP concentrations. J. Mol. Neurosci. 2019, 68, 243–250. [Google Scholar] [CrossRef]
- Gordstein, F.; O’Brien, J.; Kang, J.H.; Dushkes, R.; Cook, N.R.; Okereke, O.; Manson, J.E.; Glynn, R.J.; Buring, J.E.; Gaziano, M.; et al. Long-term multivitamin supplementation and cognitive function in men: A randomized trial. Ann. Intern. Med. 2013, 159, 806–814. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Denton, D.A.; Di Nisio, M.; Chong, L.Y.; Abraham, R.P.; Al-Assaf, A.S.; Anderson, J.L.; Malik, M.A.; Vernooij, R.W.; Martínez, G.; et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst. Rev. 2018, 12, CD011906. [Google Scholar] [CrossRef] [PubMed]
- Manders, M.; de Groot, L.C.P.G.M.; van Staveren, W.A.; Wouters-Wesseling, W.; Mulders, A.J.M.J.; Schols, W.J.M.G.A.; Hoefnagels, W.H.L. Effectiveness of nutritional supplements on cognitive functioning in elderly persons: A systematic review. J. Gerontol. Biol. Sci. Med. Sci. 2004, 59, 1041–1049. [Google Scholar] [CrossRef]
- McCleery, J.; Abraham, R.P.; Denton, D.A.; Rutjes, A.W.; Chong, L.Y.; Al-Assaf, A.S.; Griffith, D.J.; Rafeeq, S.; Yaman, H.; Malik, M.A.; et al. Vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2018, 11, CD011905. [Google Scholar] [CrossRef]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; De Lutiis, M.A.; Patruno, A.; Grilli, A.; Felaco, M.; Speranza, L. Astaxanthin treatment confers protection against oxidative stress in U937 cells stimulated with lipopolysaccharide reducing O2-production. PLoS ONE 2014, 9, e88359. [Google Scholar] [CrossRef]
- Olivera-Pueyo, J.; Carmelo Pelegrín-Valero, C. Dietary supplements for cognitive impairment. Actas Esp. Psiquiatr. 2017, 45, 37–47. [Google Scholar] [PubMed]
- May, J. Vitamin C transport and its role in the central nervous system. Subcell. Biochem. 2012, 56, 85–103. [Google Scholar] [PubMed]
- Almeida, O.P.; Ford, A.H.; Hirani, V.; Singh, V.; van Bockxmeer, F.M.; McCaul, K. B vitamins to enhance treatment response to antidepressants in middle-aged and older adults: Results from the B-VITAGE randomised, double-blind, placebo-controlled trial. Br. J. Psychiatry 2014, 205, 450–457. [Google Scholar] [CrossRef]
- Clarke, R.; Bennett, D.; Parish, S.; Lewington, S.; Skeaff, M.; Eussen, S.J.P.M.; Lewerin, C.; Stott, D.J.; Armitage, J.; Hankey, G.J.; et al. Effects of homocysteine lowering with B vitamins on cognitive aging: Meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am. J. Clin. Nutr. 2014, 100, 657–666. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H. Homocysteine, B vitamins, and cognitive impairment. Annu. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef]
- Venkatramanan, S.; Armata, I.E.; Strupp, B.J.; Finkelstein, J.L. Vitamin B-12 and cognition in children. Adv. Nutr. 2016, 7, 879–888. [Google Scholar] [CrossRef]
- Suh, S.W.; Kim, H.S.; Han, J.H.; Bae, J.B.; Oh, D.J.; Han, J.W.; Kim, K.W. Efficacy of vitamins on cognitive function of non-demented people: A systematic review and meta-analysis. Nutrients 2020, 12, 1168. [Google Scholar] [CrossRef]
- Maddock, J.; Zhou, A.; Cavadino, A.; Kuźma, E.; Bao, Y.; Smart, M.; Saum, K.; Schöttker, B.; Engmann, J.; Kjærgaard, M.; et al. Vitamin D and cognitive function: A mendelian randomisation study. Sci. Rep. 2017, 7, 13230. [Google Scholar] [CrossRef]
- Mokry, L.E.; Ross, S.; Morris, J.A.; Manousaki, D.; Forgetta, V.; Richards, J.B. Genetically decreased vitamin D and risk of Alzheimer disease. Neurology 2016, 87, 2567–2574. [Google Scholar] [CrossRef]
- Pettersen, J.A. Does high dose vitamin D supplementation enhance cognition? A randomized trial in healthy adults. Exp. Gerontol. 2017, 90, 90–97. [Google Scholar] [CrossRef]
- Farghali, M.; Ruga, S.; Morsanuto, V.; Uberti, F. Can Brain Health Be Supported by Vitamin D-Based Supplements? A Critical Review. Brain Sci. 2020, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Alex, A.; Abbott, K.A.; McEvoy, M.; Schofield, P.W.; Garg, M.L. Long-chain omega-3 polyunsaturated fatty acids and cognitive decline in non-demented adults: A systematic review and meta-analysis. Nutr. Rev. 2020, 78, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Madireddy, S.; Madireddy, S. The role of diet in maintaining strong brain health by taking the advantage of the gut-brain axis. In Advances in Food Science, 1st ed.; Hien, P.P., Ed.; Vide Leaf: Hyderabad, India, 2020. [Google Scholar]
- Birberg-Thornberg, U.; Karlsson, T.; Gustafsson, P.A.; Duche, K. Nutrition and theory of mind—The role of polyunsaturated fatty acids (PUFA) in the development of theory of mind. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kuszewski, J.C.; Wong, R.H.X.; Howe, P.R.C. Effects of long-chain omega-3 polyunsaturated fatty acids on endothelial vasodilator function and cognition—Are they interrelated? Nutrients 2017, 9, 487. [Google Scholar] [CrossRef] [PubMed]
- Baym, C.L.; Khan, N.A.; Monti, J.M.; Raine, L.B.; Drollette, E.S.; Moore, R.D.; Scudder, M.R.; Kramer, A.F.; Hillman, C.H.; Cohen, N.J. Dietary lipids are differentially associated with hippocampal-dependent relational memory in prepubescent children. Am. J. Clin. Nutr. 2014, 99, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Gomez-Pinilla, F. ‘Metabolic syndrome’ in the brain: Deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J. Physiol. 2012, 590, 2485–2499. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.S.; Agrawal, R.; Sharma, S.; Huo, Y.-X.; Ying, Z.; Gomez-Pinilla, F. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PLoS ONE 2011, 6, e28451. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef]
- Innis, S.M. Dietary (n23) fatty acids and brain development. J. Nutr. 2007, 137, 855–859. [Google Scholar] [CrossRef]
- Ciappolino, V.; Mazzocchi, A.; Enrico, P.; Syrén, M.L.; Delvecchio, G.; Agostoni, C.; Brambilla, P. N-3 polyunsatured fatty acids in menopausal transition: A systematic review of depressive and cognitive disorders with accompanying vasomotor symptoms. Int. J. Mol. Sci. 2018, 19, 1849. [Google Scholar] [CrossRef]
- Mudd, A.T.; Fil, J.E.; Knight, L.C.; Dilger, R.N. Dietary iron repletion following early-life dietary iron deficiency does not correct regional volumetric or diffusion tensor changes in the developing pig brain. Front. Neurol. 2018, 8, 735. [Google Scholar] [CrossRef] [PubMed]
- Gow, R.V.; Hibbeln, J.R. Omega-3 fatty acid and nutrient deficits in adverse neurodevelopment and childhood behaviors. Child Adolesc. Psychiatr. Clin. 2014, 23, 555–590. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Schmitt, F.; Loeffler, J.P.; Gonzalez de Aguilar, J.L. Fatting the brain: A brief of recent research. Front. Cell. Neurosci. 2013, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, V.K.; Huang, B.X.; Desai, A.; Kevala, K.; Kim, H.Y. Role of DHA in aging-related changes in mouse brain synaptic plasma membrane proteome. Neurobiol. Aging 2016, 41, 73–85. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, K.J. The interaction of zinc and the blood-brain barrier under physiological and ischemic conditions. Toxicol. Appl. Pharmacol. 2019, 364, 114–119. [Google Scholar] [CrossRef]
- Grabrucker, A.M.; Knight, M.J.; Proepper, C.; Bockmann, J.; Joubert, M.; Rowan, M.; Nienhaus, G.U.; Garner, C.C.; Bowie, J.U.; Kreutz, M.R.; et al. Concerted action of zinc and ProSAP/Shank in synaptogenesis and synapse maturation. EMBO J. 2011, 30, 569–581. [Google Scholar] [CrossRef]
- Jung, A.; Spira, D.; Steinhagen-Thiessen, E.; Demuth, I.; Norman, K. Zinc deficiency is associated with depressive symptoms-results from the Berlin aging study II. J. Gerontol. Biol. Sci. Med. Sci. 2017, 72, 1149–1154. [Google Scholar] [CrossRef]
- Vela, G.; Stark, P.; Socha, M.; Sauer, A.K.; Hagmeyer, S.; Grabrucker, A.M. Zinc in gut-brain interaction in autism and neurological disorders. Neural Plast. 2015, 972791. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Nakamura, M.; Fujii, H.; Tamano, H. Synaptic Zn2+ homeostasis and its significance. Metallomics 2013, 5, 417–423. [Google Scholar] [CrossRef]
- De Moura, J.E.; de Moura, E.N.; Alves, C.X.; Vale, S.H.; Dantas, M.M.; Silva, A.D.; Almeida, M.G.; Leite, L.D.; Brandão-Neto, J. Oral zinc supplementation may improve cognitive function in schoolchildren. Biol. Trace Elem. Res. 2013, 155, 23–28. [Google Scholar] [CrossRef]
- Takeda, A.; Tamano, H. Significance of the degree of synaptic Zn2+ signaling in cognition. Biometals 2016, 29, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Zheng, Y.; Shen, Z.; Li, Y.; Tian, X.; Dou, X.; Qian, J.; Shen, H. Psychological stress-induced lower serum zinc and zinc redistribution in rats. Biol. Trace Elem. Res. 2013, 155, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Sandusky-Beltran, L.A.; Manchester, B.L.; McNay, E.C. Supplementation with zinc in rats enhances memory and reverses an age-dependent increase in plasma copper. Behav. Brain Res. 2017, 333, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jing, X.-P.; Zhang, S.-P.; Gu, R.-X.; Tang, F.-X.; Wang, X.-L.; Xiong, Y.; Qiu, M.; Sun, X.-Y.; Ke, D.; et al. High dose zinc supplementation induces hippocampal zinc deficiency and memory impairment with inhibition of BDNF signaling. PLoS ONE 2013, 8, e55384. [Google Scholar] [CrossRef] [PubMed]
- Tamano, H.; Koike, Y.; Nakada, H.; Shakushi, Y.; Takeda, A. Significance of synaptic Zn2+ signaling in zincergic and non-zincergic synapses in the hippocampus in cognition. J. Trace Elem. Med. Biol. 2016, 38, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.G.; Foucaud, J.; Mery, F. Costs of memory: Lessons from ‘mini’ brains. Proc. R. Soc. B 2011, 278, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Mallory, H.S.; Howard, A.F.; Weiss, M.R. Timing of Environmental Enrichment Affects Memory in the House Cricket, Acheta domesticus. PLoS ONE 2016, 11, e0152245. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Malaterre, J.; Strambi, C.; Chiang, A.; Aouane, A.; Strambi, A.; Cayre, M. Development of cricket mushroom bodies. J. Comp. Neurol. 2002, 452, 215–227. [Google Scholar] [CrossRef]
- Scotto-Lomassese, S.; Strambi, C.; Aouane, A.; Strambi, A.; Cayre, M. Sensory inputs stimulate progenitor cell proliferation in an adult insect brain. Curr. Biol. 2002, 12, 1001–1005. [Google Scholar] [CrossRef][Green Version]
- Cayre, M.; Malaterre, J.; Scotto-Lomassese, S.; Strambi, C.; Strambi, A. The common properties of neurogenesis in the adult brain: From invertebrates to vertebrates. Comp. Biochem. Phys. B 2002, 132, 1–15. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Mizunami, M. Lifetime olfactory memory in the cricket Gryllus bimaculatus. J. Comp. Physiol. A 2002, 188, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Córdoba-Aguilar, A.; Nava-Sánchez, A.; González-Tokman, D.M.; Munguía-Steyer, R.; Gutiérrez-Cabrera, A.E. Immune priming, fat reserves, muscle mass and body weight of the house cricket is affected by diet composition. Neotrop. Entomol. 2016, 45, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Rapkin, J.; Jensen, K.; Lane, S.M.; House, C.M.; Sakaluk, S.K. Macronutrient intake regulates sexual conflict in decorated crickets. J. Evol. Biol. 2016, 29, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Erregger, B.; Kovac, H.; Stabentheiner, A.; Hartbauer, M.; Römer, H.; Schmidt, A.K.D. Cranking up the heat: Relationships between energetically costly song features and the increase in thorax temperature in male crickets and katydids. J. Exp. Biol. 2017, 220, 2635–2644. [Google Scholar] [CrossRef] [PubMed]
- Gabel, E.; Gray, D.A.; Hennig, R.M. How females of chirping and trilling field crickets integrate the ‘what’ and ‘where’ of male acoustic signals during decision making. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2016, 202, 823–837. [Google Scholar] [CrossRef]
- Adamo, S.A.; Kovalko, I.; Mosher, B. The behavioural effects of predator-induced stress responses in the cricket (Gryllus texensis): The upside of the stress response. J. Exp. Biol. 2013, 216, 4608–4614. [Google Scholar] [CrossRef]
- Erregger, B.; Hennig, R.M.; Römer, H. The ‘hot male’ hypothesis: Do female crickets prefer males with increased body temperature in mate choice scenarios? Anim. Behav. 2018, 138, 75–84. [Google Scholar] [CrossRef]
- Boroujeni, S.T.; Naghdi, N.; Shahbazi, M.; Bagherzadeh, F.; Kazemnejad, A.; Javadian, M. The effect of severe zinc deficiency and zinc supplement on spatial learning and memory. Biol. Trace Elem. Res. 2009, 130, 48–61. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Zhang, H.B.; Cheng, Q.; Zhu, Y.M.; Xia, C.H.; Zhu, Y.H.; Zhang, Y. Zinc-enriched yeast improves learning and memory impairments in zinc-deficient rats. Biol. Trace Elem. Res. 2019, 189, 180–185. [Google Scholar] [CrossRef]
- Rico, J.A.; Kordas, K.; López, P.; Rosado, J.L.; Vargas, G.G.; Ronquillo, D.; Stoltzfus, R.J. Efficacy of iron and/or zinc supplementation on cognitive performance of lead-exposed Mexican schoolchildren: A randomized, placebo-Controlled Trial. Pediatrics 2006, 117, e518–e527. [Google Scholar] [CrossRef] [PubMed]
- Arzi, A.; Hemmati, A.A.; Razian, A. Effect of vitamins C and E on cognitive function in mouse. Pharmacol. Res. 2004, 49, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Kim, S.Y.; Sok, S.R. Effects of multivitamin supplements on cognitive function, serum homocysteine level, and depression of Korean older adults with mild cognitive impairment in care facilities. J. Nurs. Scholarsh. 2016, 48, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Kong, H.; Pang, W.; Yang, H.; Lu, H.; Huang, C.; Jiang, Y. B vitamin supplementation improves cognitive function in the middle aged and elderly with hyperhomocysteinemia. Nutr. Neurosci. 2016, 19, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Madireddy, S. A winning combination among polyphenols, probiotics, vitamins for improved memory and cognitive performance. Int. J. Med. Res. Health Sci. 2020, 9, 27–31. [Google Scholar]
- Privitera, G.J.; Zavala, A.R.; Federico, S.; Sotak, K.L. High fat diet intake during pre and periadolescence impairs learning of a conditioned place preference in adulthood. Behav. Brain Funct. 2011, 7, 21. [Google Scholar] [CrossRef]
- Hajjar, T.; Meng, G.Y.; Rajion, M.A.; Vidyadaran, S.; Othman, F.; Farjam, A.S.; Li, T.A.; Ebrahimi, M. Omega 3 polyunsaturated fatty acid improves spatial learning and hippocampal peroxisome proliferator activated receptors (PPARα and PPARγ) gene expression in rats. BMC Neurosci. 2012, 13, 109. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Kiliaan, A.J. Fatty acids, lipid metabolism and Alzheimer pathology. Eur. J. Pharmacol. 2008, 585, 176–196. [Google Scholar] [CrossRef] [PubMed]
- Lapillonne, A.; Clarke, S.D.; Heird, W.C. Polyunsaturated fatty acids and gene expression. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 151–156. [Google Scholar] [CrossRef]
- Su, H.M. Mechanisms of n-3 fatty acid-mediated development and maintenance of learning memory performance. J. Nutr. Biochem. 2010, 21, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Mizunami, M.; Matsumoto, Y.; Watanabe, H.; Nishino, H. Chapter 41: Olfactory and visual learning in cockroaches and crickets. In Invertebrate Learning and Memory; Menzel, R., Benjamin, P.R., Eds.; Springer: Berlin, Germany, 2013; pp. 547–558. [Google Scholar]
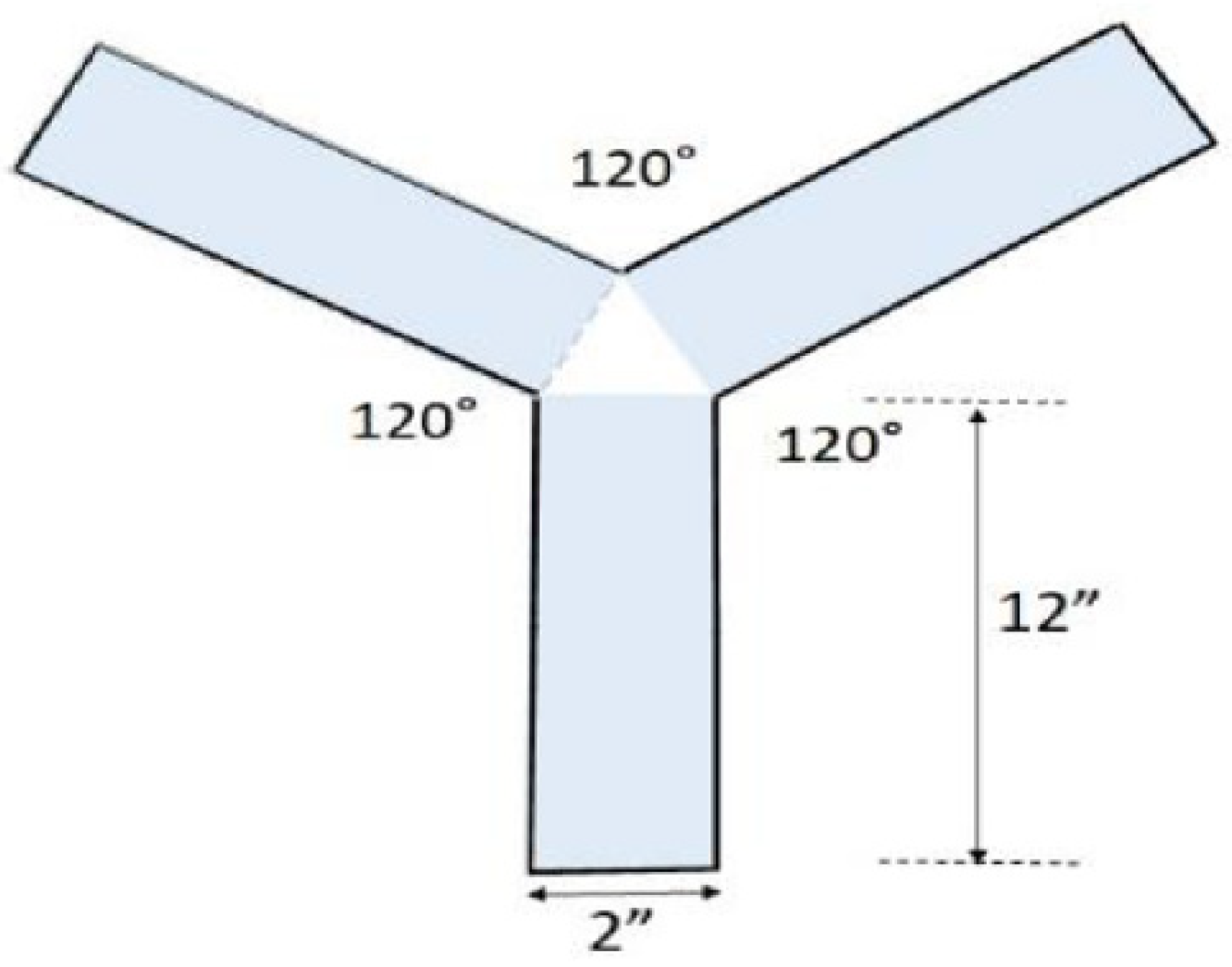
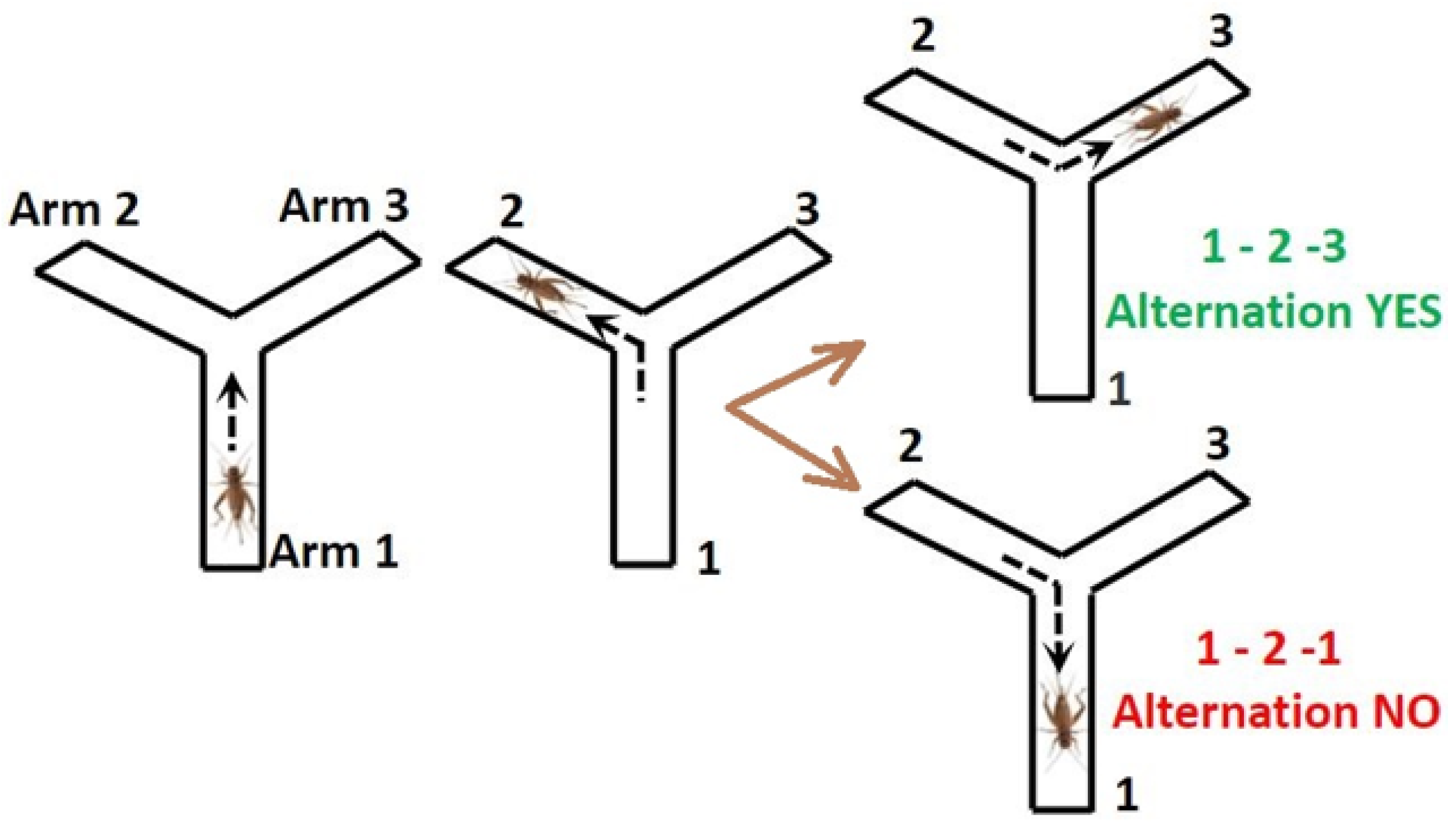

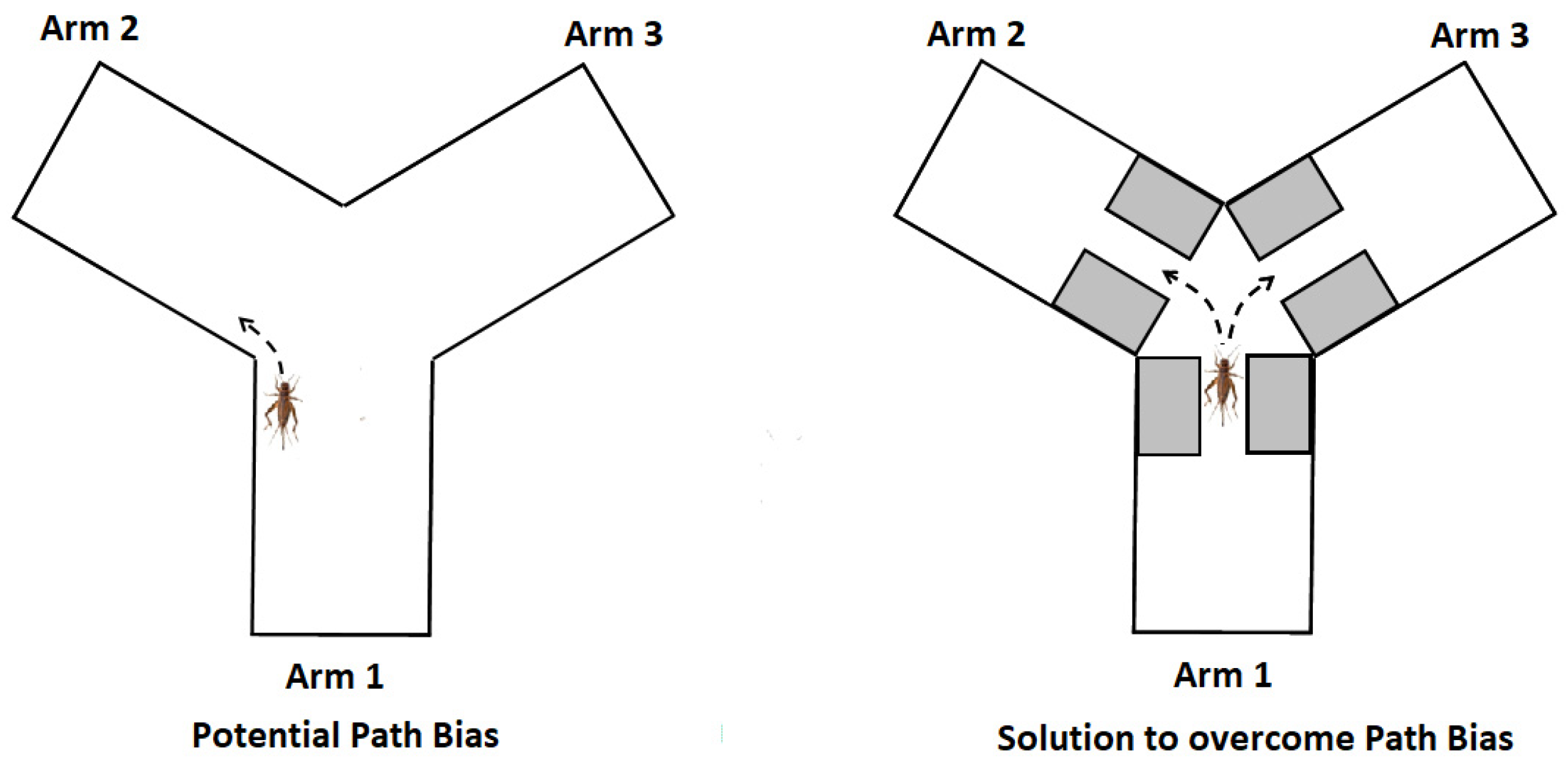


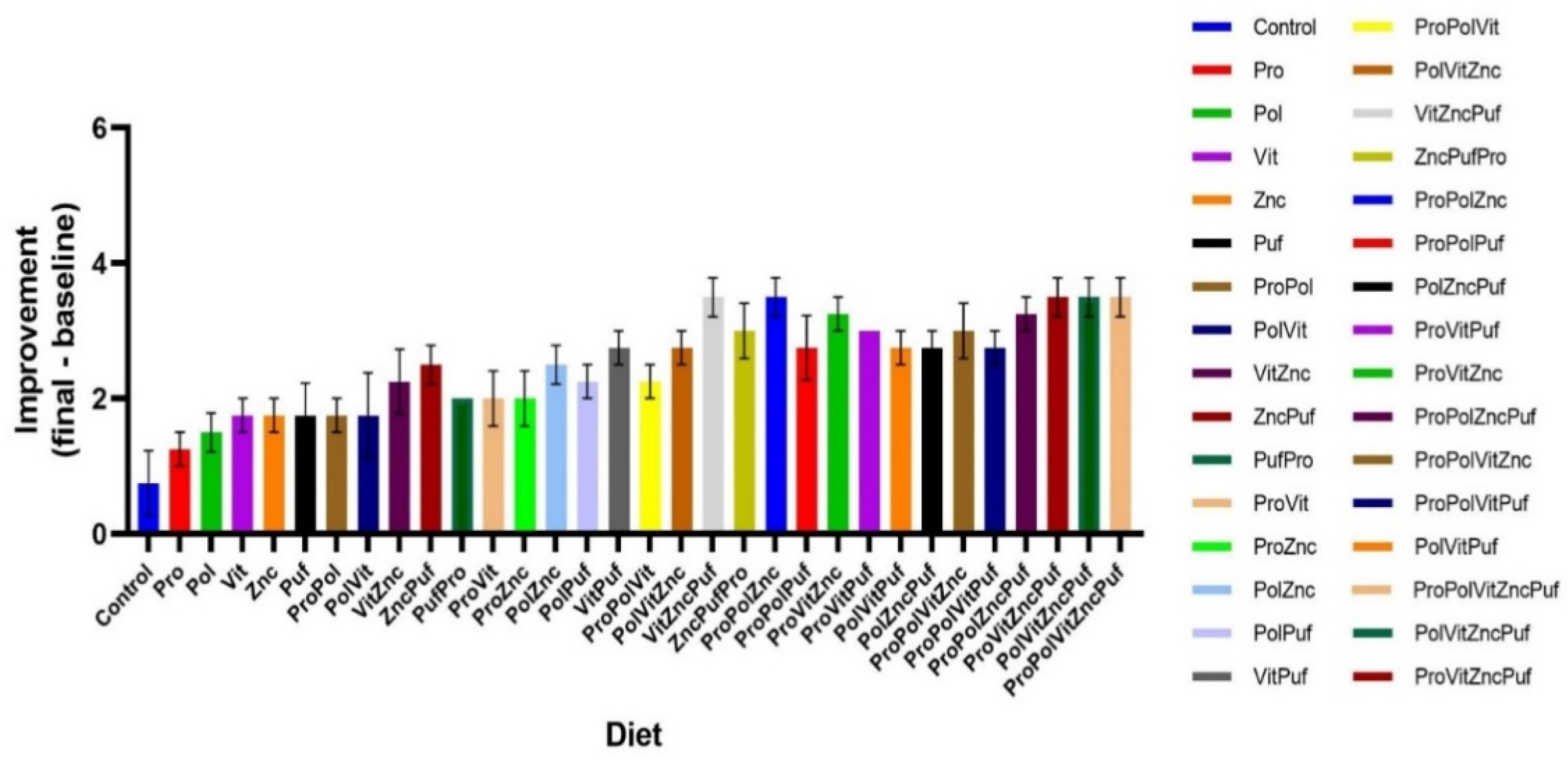
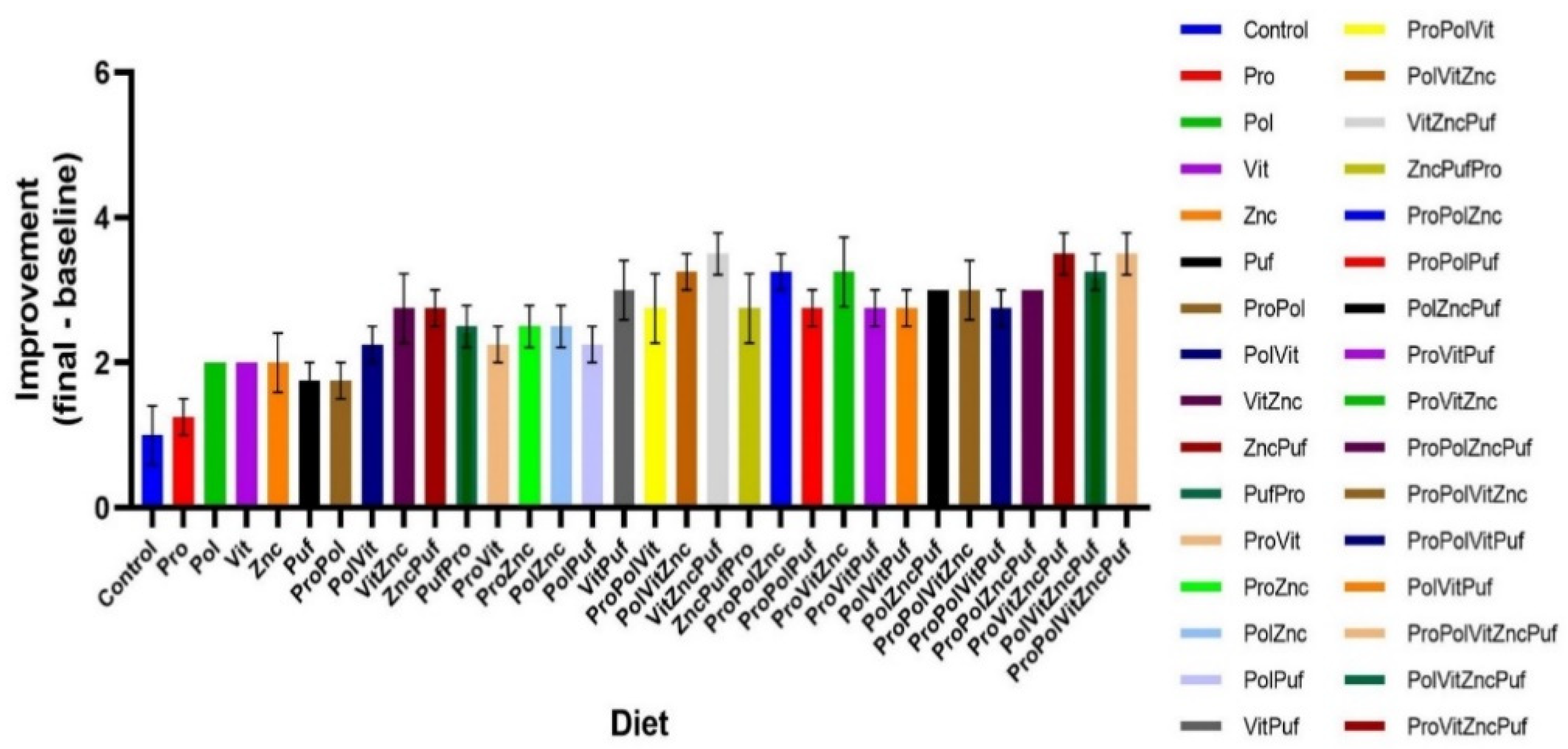
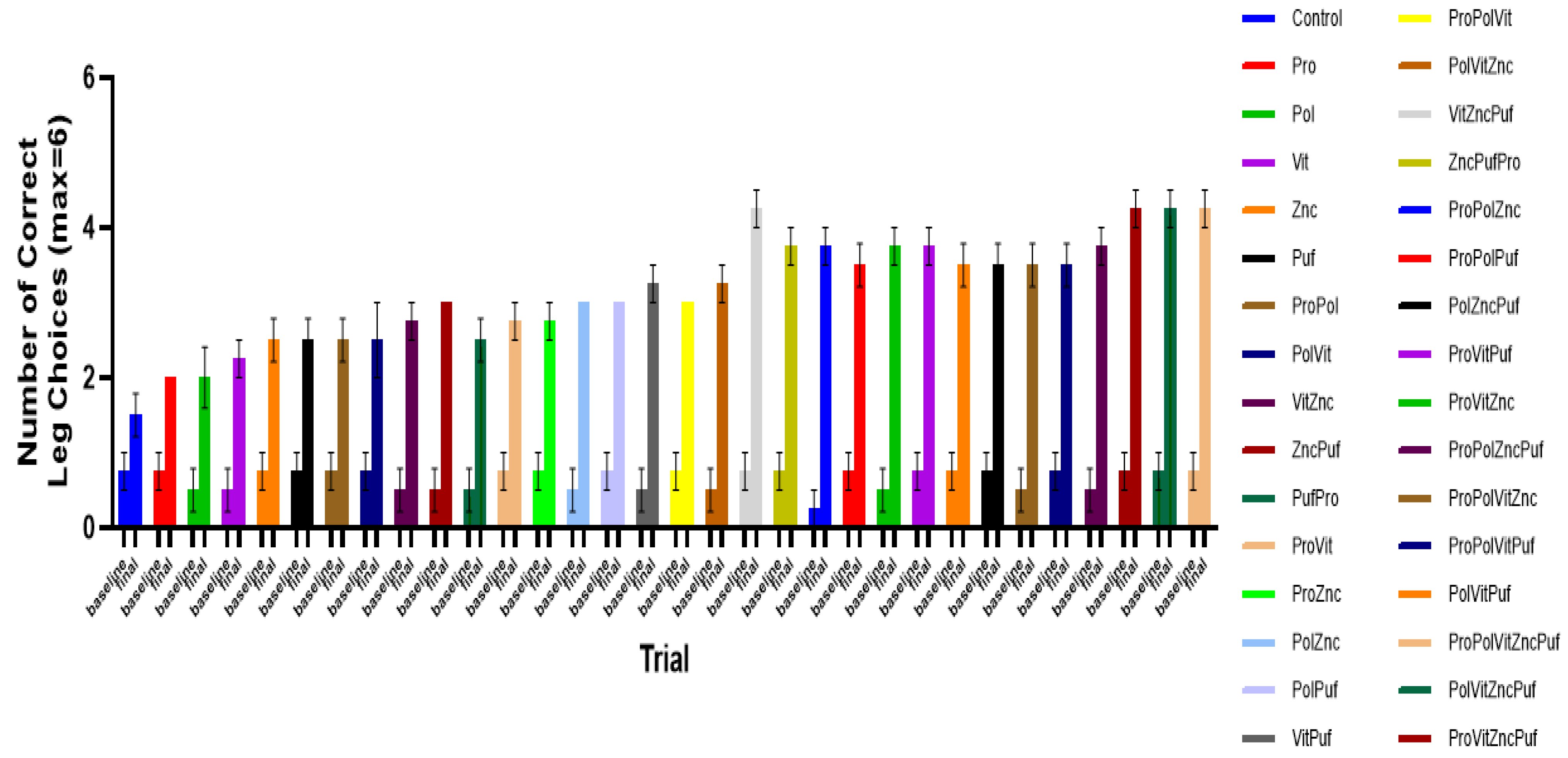
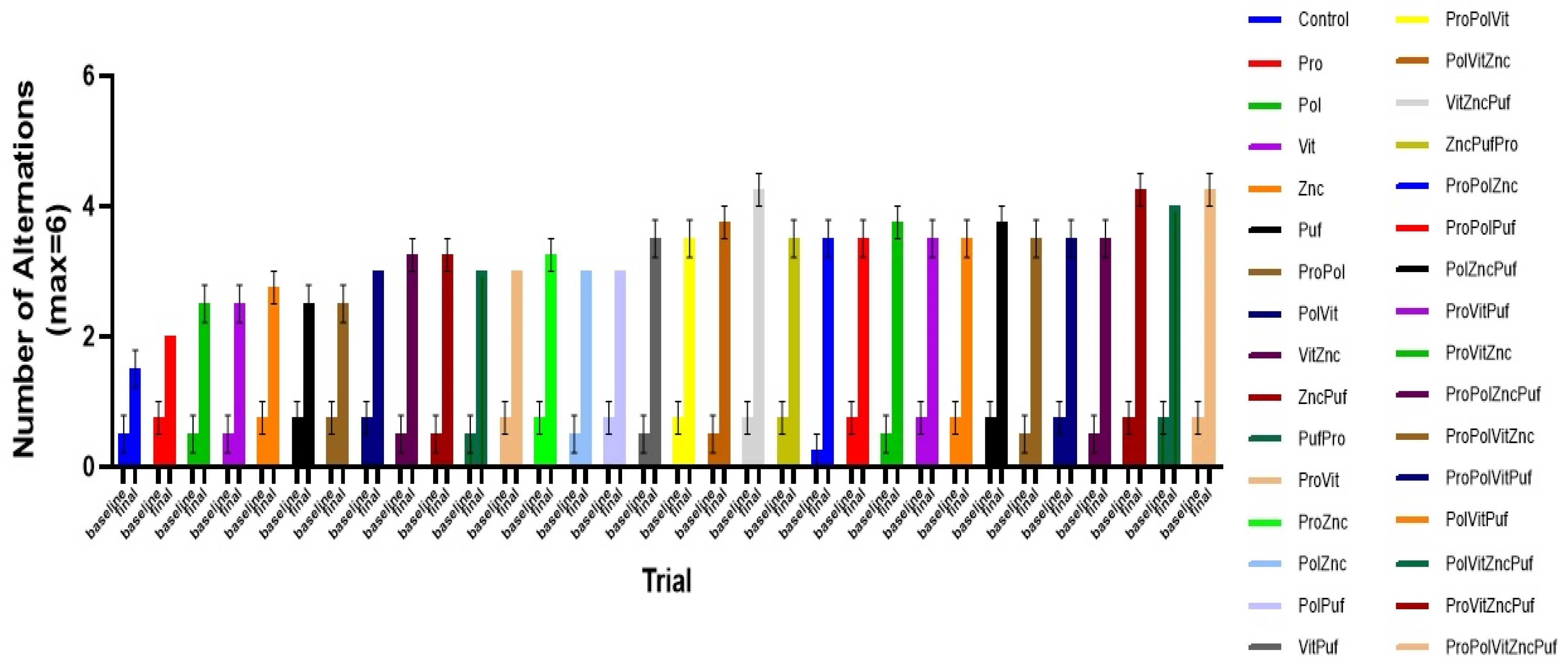
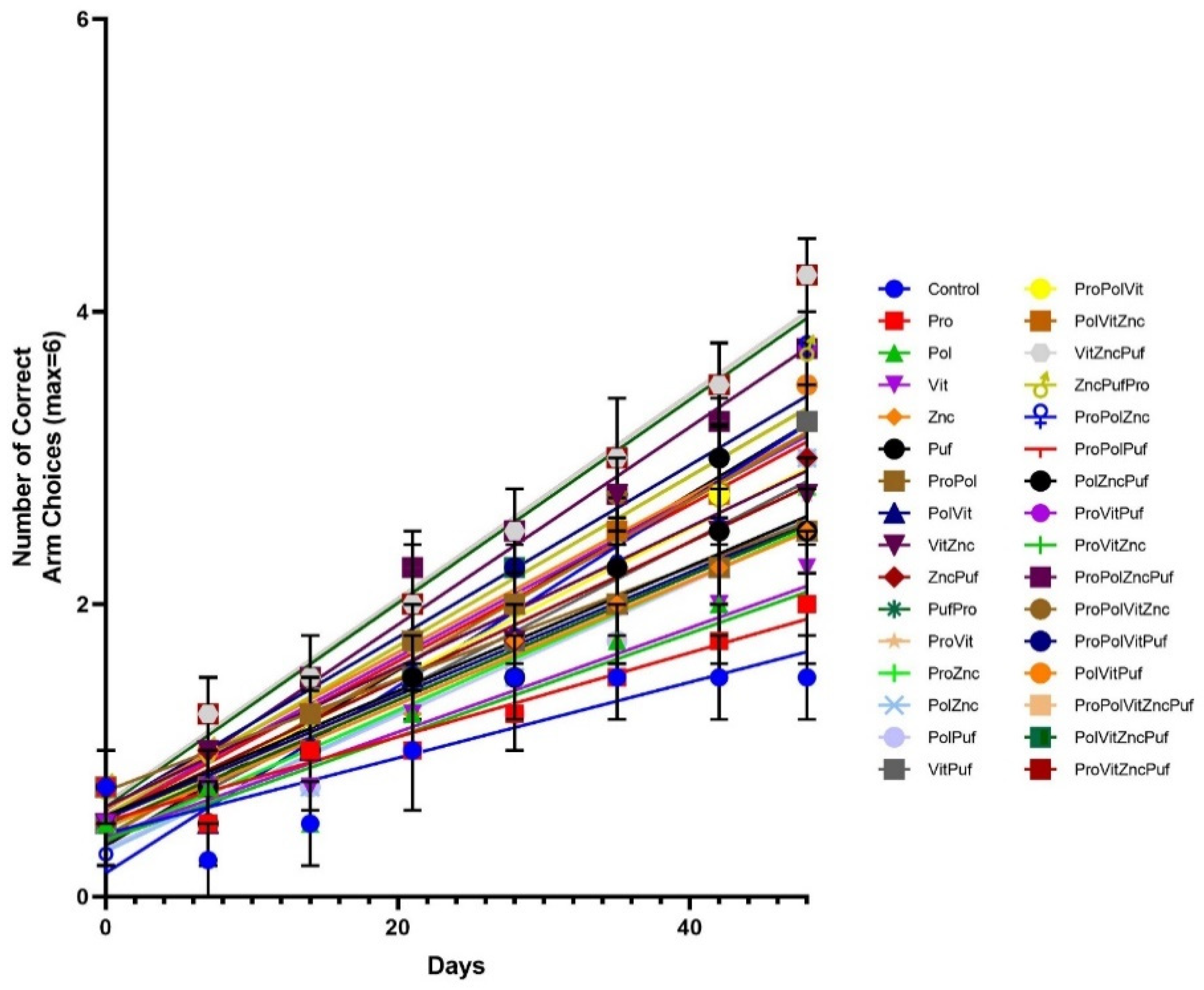
| Group | Group Label | Diet |
|---|---|---|
| Group 1 | Control | Normal diet |
| Group 2 | Pro | Probiotics |
| Group 3 | Pol | Polyphenols |
| Group 4 | Vit | Multivitamins |
| Group 5 | Znc | Zinc |
| Group 6 | Puf | Omega-3 PUFAs |
| Group 7 | ProPol | Probiotics and Polyphenols |
| Group 8 | PolVit | Polyphenols and Multivitamins |
| Group 9 | VitZnc | Multivitamins and Zinc |
| Group 10 | ZncPuf | Zinc and Omega-3 PUFAs |
| Group 11 | PufPro | Omega-3 PUFAs and Probiotics |
| Group 12 | ProVit | Probiotics and Multivitamins |
| Group 13 | ProZnc | Probiotics and Zinc |
| Group 14 | PolZnc | Polyphenols and Zinc |
| Group 15 | PolPuf | Polyphenols and Omega-3 PUFAs |
| Group 16 | VitPuf | Multivitamins and Omega-3 PUFAs |
| Group 17 | ProPolVit | Probiotics, Polyphenols, and Multivitamins |
| Group 18 | PolVitZnc | Polyphenols, Multivitamins, and Zinc |
| Group 19 | VitZncPuf | Multivitamins, Zinc, and Omega-3 PUFAs |
| Group 20 | ZncPufPro | Zinc, Omega-3 PUFAs, Probiotics |
| Group 21 | ProPolZnc | Probiotics, Polyphenols, and Zinc |
| Group 22 | ProPolPuf | Probiotics, Polyphenols, and Omega-3 PUFAs |
| Group 23 | ProVitZnc | Probiotics, Multivitamins, and Zinc |
| Group 24 | ProVitPuf | Probiotics, Multivitamins, and Omega-3 PUFAs |
| Group 25 | PolVitPuf | Polyphenols, Multivitamins, and Omega-3 PUFAs |
| Group 26 | PolZncPuf | Polyphenols, Zinc, and Omega-3 PUFAs |
| Group 27 | ProPolVitZnc | Probiotics, Polyphenols, Multivitamins, and Zinc |
| Group 28 | ProPolVitPuf | Probiotics, Polyphenols, Multivitamins, and Omega-3 PUFAs |
| Group 29 | ProPolZncPuf | Probiotics, Polyphenols, Zinc, and Omega-3 PUFAs |
| Group 30 | ProVitZncPuf | Probiotics, Multivitamins, Zinc, and Omega-3 PUFAs |
| Group 31 | PolVitZncPuf | Polyphenols, Multivitamins, Zinc, and Omega-3 PUFAs |
| Group 32 | ProPolVitZncPuf | Probiotics, Polyphenols, Multivitamins, Zinc, and Omega-3 PUFAs |
| (a) | (b) | ||
|---|---|---|---|
| Ranking | Combination | Ranking | Combination |
| 1 | VitZncPuf | 1 | VitZncPuf ProVitZncPuf ProPolVitZncPuf |
| 2 | ProPolVitZncPuf | 2 | PolVitZncPuf |
| 3 | ProVitZncPuf | 3 | ProPolZncPuf |
| 4 | PolVitZncPuf | 4 | ProPolZnc |
| 5 | PolZncPuf | 5 | PolZncPuf |
| 6 | ProPolZncPuf | 6 | ProPolVitPuf |
| 7 | PolVitZnc | 7 | ProPolVitZnc |
| 8 | ProPolZnc | 8 | ZncPufPro |
| 9 | ProVitZnc | 9 | PolVitZnc |
| 10 | ProPolVitZnc | 10 | ProVitZnc |
| 11 | ZncPufPro | 11 | ProVitPuf |
| 12 | ProPolVitPuf | 12 | PolVitPuf |
| 13 | ProVitPuf | 13 | ProPolPuf |
| 14 | PolVitPuf | 14 | VitPuf |
| 15 | VitPuf | 15 | ProPolVit |
| 16 | ProZnc | 16 | VitZnc |
| 17 | ProPolVit | 17 | PolZnc |
| 18 | PolZnc | 18 | ZncPuf |
| 19 | ProPolPuf | 19 | PolPuf |
| 20 | VitZnc | 20 | ProZnc |
| 21 | PufPro | 21 | Puf |
| 22 | ZncPuf | 22 | PufPro |
| 23 | ProVit | 23 | PolVit |
| 24 | Znc | 24 | Znc |
| 25 | PolVit | 25 | ProVit |
| 26 | PolPuf | 26 | ProPol |
| 27 | Vit | 27 | Vit |
| 28 | Puf | 28 | Pol |
| 29 | Pol | 29 | Pro |
| 30 | ProPol | 30 | Control |
| 31 | Pro | ||
| 32 | Control | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madireddy, S.; Madireddy, S. Most Effective Combination of Nutraceuticals for Improved Memory and Cognitive Performance in the House Cricket, Acheta domesticus. Nutrients 2021, 13, 362. https://doi.org/10.3390/nu13020362
Madireddy S, Madireddy S. Most Effective Combination of Nutraceuticals for Improved Memory and Cognitive Performance in the House Cricket, Acheta domesticus. Nutrients. 2021; 13(2):362. https://doi.org/10.3390/nu13020362
Chicago/Turabian StyleMadireddy, Samskruthi, and Sahithi Madireddy. 2021. "Most Effective Combination of Nutraceuticals for Improved Memory and Cognitive Performance in the House Cricket, Acheta domesticus" Nutrients 13, no. 2: 362. https://doi.org/10.3390/nu13020362
APA StyleMadireddy, S., & Madireddy, S. (2021). Most Effective Combination of Nutraceuticals for Improved Memory and Cognitive Performance in the House Cricket, Acheta domesticus. Nutrients, 13(2), 362. https://doi.org/10.3390/nu13020362




