Green Salad Intake Is Associated with Improved Oral Cancer Survival and Lower Soluble CD44 Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
2.2. Questionnaire
2.3. Oral Rinse Assays
2.4. HPV and CD44 Status
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Association of Soluble CD44 and Protein with Nutrition
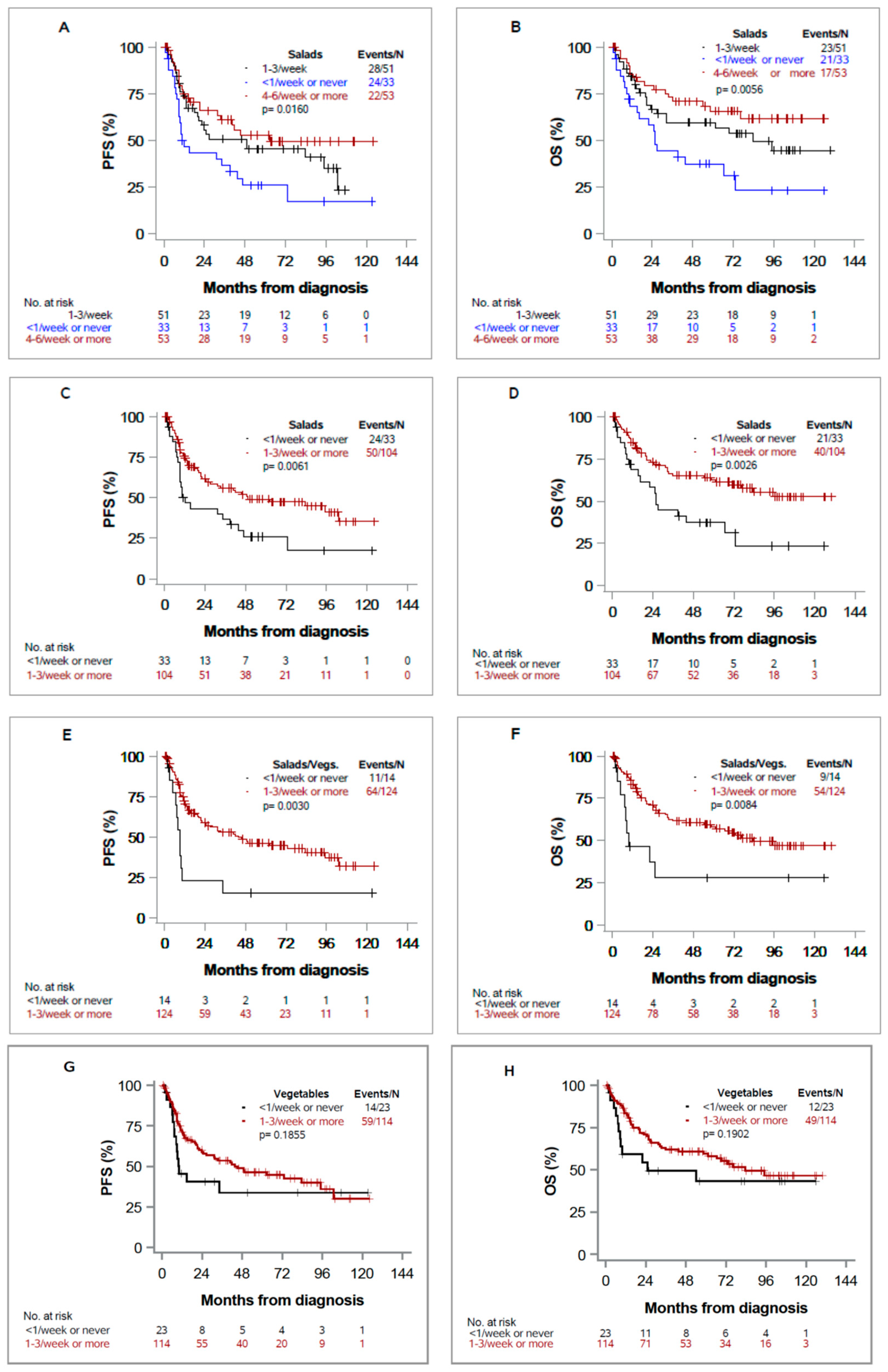
3.3. Progression-Free Survival (PFS) and Overall Survival (OS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA A Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef]
- Weisburger, J.H. Antimutagens, anticarcinogens, and effective worldwide cancer prevention. J. Environ. Pathol. Toxicol. Oncol. Off. Organ Int. Soc. Environ. Toxicol. Cancer 1999, 18, 85–93. [Google Scholar]
- Key, T.J.; Schatzkin, A.; Willett, W.C.; Allen, N.E.; Spencer, E.A.; Travis, R.C. Diet, nutrition and the prevention of cancer. Public Health Nutr. 2004, 7, 187–200. [Google Scholar] [CrossRef]
- Freedman, N.D.; Park, Y.; Subar, A.F.; Hollenbeck, A.R.; Leitzmann, M.F.; Schatzkin, A.; Abnet, C.C. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int. J. Cancer 2008, 122, 2330–2336. [Google Scholar] [CrossRef]
- Duffy, S.A.; Ronis, D.L.; McLean, S.; Fowler, K.E.; Gruber, S.B.; Wolf, G.T.; Terrell, J.E. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 1969–1975. [Google Scholar] [CrossRef]
- Boeing, H.; Dietrich, T.; Hoffmann, K.; Pischon, T.; Ferrari, P.; Lahmann, P.H.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Allen, N.; Key, T.; et al. Intake of fruits and vegetables and risk of cancer of the upper aero-digestive tract: The prospective EPIC-study. Cancer Causes Control CCC 2006, 17, 957–969. [Google Scholar] [CrossRef]
- Sandoval, M.; Font, R.; Mañós, M.; Dicenta, M.; Quintana, M.J.; Bosch, F.X.; Castellsagué, X. The role of vegetable and fruit consumption and other habits on survival following the diagnosis of oral cancer: A prospective study in Spain. Int. J. Oral Maxillofac. Surg. 2009, 38, 31–39. [Google Scholar] [CrossRef]
- Fund, W.C.R. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; American Institute for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- Trapasso, S.; Allegra, E. Role of CD44 as a marker of cancer stem cells in head and neck cancer. Biol. Targets Ther. 2012, 6, 379–383. [Google Scholar] [CrossRef]
- Franzmann, E.J.; Reategui, E.P.; Pedroso, F.; Pernas, F.G.; Karakullukcu, B.M.; Carraway, K.L.; Hamilton, K.; Singal, R.; Goodwin, W.J. Soluble CD44 is a potential marker for the early detection of head and neck cancer. Cancer Epidemiol. Biomark. 2007, 16, 1348–1355. [Google Scholar] [CrossRef]
- Franzmann, E.J.; Reategui, E.P.; Pereira, L.H.; Pedroso, F.; Joseph, D.; Allen, G.O.; Hamilton, K.; Reis, I.; Duncan, R.; Goodwin, W.J.; et al. Salivary protein and solCD44 levels as a potential screening tool for early detection of head and neck squamous cell carcinoma. Head Neck 2012, 34, 687–695. [Google Scholar] [CrossRef]
- Hardisson, D. Molecular pathogenesis of head and neck squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2003, 260, 502–508. [Google Scholar] [CrossRef]
- Assimakopoulos, D.; Kolettas, E.; Patrikakos, G.; Evangelou, A. The role of CD44 in the development and prognosis of head and neck squamous cell carcinomas. Histol. Histopathol. 2002, 17, 1269–1281. [Google Scholar] [CrossRef]
- Krump, M.; Ehrmann, J. Differences in CD44s expression in HNSCC tumours of different areas within the oral cavity. Biomed. Pap. Med Fac. Univ. Palacky Olomouc Czechoslov. 2013, 157, 280–283. [Google Scholar] [CrossRef]
- Takamune, Y.; Ikebe, T.; Nagano, O.; Nakayama, H.; Ota, K.; Obayashi, T.; Saya, H.; Shinohara, M. ADAM-17 associated with CD44 cleavage and metastasis in oral squamous cell carcinoma. Virchows Archiv An Int. J. Pathol. 2007, 450, 169–177. [Google Scholar] [CrossRef]
- Klement, J.D.; Paschall, A.V.; Redd, P.S.; Ibrahim, M.L.; Lu, C.; Yang, D.; Celis, E.; Abrams, S.I.; Ozato, K.; Liu, K. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J. Clin. Investig. 2018, 128, 5549–5560. [Google Scholar] [CrossRef]
- Franzmann, E.J.; Reategui, E.P.; Carraway, K.L.; Hamilton, K.L.; Weed, D.T.; Goodwin, W.J. Salivary soluble CD44: A potential molecular marker for head and neck cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 735–739. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Lu, J.; Xiong, H.; Shi, X.; Gong, L. Significance of CD44 expression in head and neck cancer: A systemic review and meta-analysis. BMC Cancer 2014, 14, 15. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Pereira, L.H.; Adebisi, I.N.; Perez, A.; Wiebel, M.; Reis, I.; Duncan, R.; Goodwin, W.J.; Hu, J.J.; Lokeshwar, V.B.; Franzmann, E.J. Salivary markers and risk factor data: A multivariate modeling approach for head and neck squamous cell carcinoma detection. Cancer Biomark. Sect. A Dis. Markers 2011, 10, 241–249. [Google Scholar] [CrossRef]
- Klussmann, J.P.; Gültekin, E.; Weissenborn, S.J.; Wieland, U.; Dries, V.; Dienes, H.P.; Eckel, H.E.; Pfister, H.J.; Fuchs, P.G. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am. J. Pathol. 2003, 162, 747–753. [Google Scholar] [CrossRef]
- Hafkamp, H.C.; Manni, J.J.; Haesevoets, A.; Voogd, A.C.; Schepers, M.; Bot, F.J.; Hopman, A.H.; Ramaekers, F.C.; Speel, E.J. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int. J. Cancer 2008, 122, 2656–2664. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Westra, W.H. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: A guide for interpretative relevance and consistency. Head Neck 2012, 34, 459–461. [Google Scholar] [CrossRef]
- Cohen, E.R.; Reis, I.M.; Gomez-Fernandez, C.; Smith, D.; Pereira, L.; Freiser, M.E.; Marotta, G.; Thomas, G.R.; Sargi, Z.B.; Franzmann, E.J. CD44 and associated markers in oral rinses and tissues from oral and oropharyngeal cancer patients. Oral Oncol. 2020, 106, 104720. [Google Scholar] [CrossRef]
- Pereira, L.H.; Reis, I.M.; Reategui, E.P.; Gordon, C.; Saint-Victor, S.; Duncan, R.; Gomez, C.; Bayers, S.; Fisher, P.; Perez, A.; et al. Risk Stratification System for Oral Cancer Screening. Cancer Prev. Res. 2016, 9, 445–455. [Google Scholar] [CrossRef]
- Allegra, E.; Trapasso, S.; Sacco, A.; Aragona, T.; Belfiore, A.; Garozzo, A. Elisa Detection of Salivary Levels of Cd44sol as a Diagnostic Test for Laryngeal Carcinomas. J. Cancer Sci. Ther. 2012, 4, 330334. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer Science and Business Media: New York, NY, USA, 2013. [Google Scholar]
- Meyers, J.P.; Mandrekar, J.N. Cutpoint Determination Methods in Survival Analysis Using SAS®: Updated %FINDCUT Macro. Available online: https://support.sas.com/resources/papers/proceedings15/3249-2015.pdf (accessed on 14 January 2021).
- Zhang, Y.; Wang, R.; Miao, L.; Zhu, L.; Jiang, H.; Yuan, H. Different levels in alcohol and tobacco consumption in head and neck cancer patients from 1957 to 2013. PLoS ONE 2015, 10, e0124045. [Google Scholar] [CrossRef]
- Tumban, E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses 2019, 11, 922. [Google Scholar] [CrossRef]
- Kawano, T.; Yanoma, S.; Nakamura, Y.; Shiono, O.; Kokatu, T.; Kubota, A.; Furukawa, M.; Tsukuda, M. Evaluation of soluble adhesion molecules CD44 (CD44st, CD44v5, CD44v6), ICAM-1, and VCAM-1 as tumor markers in head and neck cancer. Am. J. Otolaryngol. 2005, 26, 308–313. [Google Scholar] [CrossRef]
- Chuang, S.C.; Jenab, M.; Heck, J.E.; Bosetti, C.; Talamini, R.; Matsuo, K.; Castellsague, X.; Franceschi, S.; Herrero, R.; Winn, D.M.; et al. Diet and the risk of head and neck cancer: A pooled analysis in the INHANCE consortium. Cancer Causes Control CCC 2012, 23, 69–88. [Google Scholar] [CrossRef]
- Maasland, D.H.; van den Brandt, P.A.; Kremer, B.; Goldbohm, R.A.; Schouten, L.J. Consumption of vegetables and fruits and risk of subtypes of head-neck cancer in the Netherlands Cohort Study. Int. J. Cancer 2015, 136, E396–E409. [Google Scholar] [CrossRef]
- Pavia, M.; Pileggi, C.; Nobile, C.G.; Angelillo, I.F. Association between fruit and vegetable consumption and oral cancer: A meta-analysis of observational studies. Am. J. Clin. Nutr. 2006, 83, 1126–1134. [Google Scholar] [CrossRef]

| Variable/Category | Cases (n = 150) | Controls (n = 150) | P | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age, years | |||||
| <60 | 84 | 56.0 | 85 | 56.7 | 0.907 |
| ≥60 | 66 | 44.0 | 65 | 43.3 | |
| Gender | |||||
| Male | 121 | 80.7 | 118 | 78.7 | 0.667 |
| Female | 29 | 19.3 | 32 | 21.3 | |
| Race | |||||
| White | 123 | 82.6 | 118 | 79.7 | 0.534 |
| Black | 26 | 17.4 | 30 | 20.3 | |
| Asian/Other/Missing (1 case Other, 1 control Asian, and 1 control missing) | 1 | 2 | |||
| Ethnicity | |||||
| Hispanic | 77 | 51.3 | 93 | 62.0 | 0.062 |
| Non-Hispanic | 73 | 48.7 | 57 | 38.0 | |
| Education | |||||
| ≤Grade 12 or GED | 77 | 52.0 | 57 | 38.0 | 0.015 |
| Some college or college graduate | 71 | 48.0 | 93 | 62.0 | |
| Refused/Missing | 2 | NA | 0 | NA | |
| Employment | |||||
| Out-of/unable-to work | 61 | 40.9 | 45 | 30.0 | 0.048 |
| Occupation with some income | 88 | 59.1 | 105 | 70.0 | |
| Refused/Missing | 1 | NA | 0 | NA | |
| Income | |||||
| $25,000 or less | 86 | 67.2 | 80 | 58.4 | 0.139 |
| >$25,000 | 42 | 32.8 | 57 | 41.6 | |
| Refused/Missing | 22 | NA | 13 | NA | |
| SES1 | |||||
| Low | 100 | 66.7 | 90 | 60.0 | 0.231 |
| High | 50 | 33.3 | 60 | 40.0 | |
| Oral health score | |||||
| Poor/Fair | 80 | 64.0 | 87 | 58.0 | 0.310 |
| Good | 45 | 36.0 | 63 | 42.0 | |
| Missing | 25 | NA | 0 | NA | |
| Teeth removed | |||||
| None/1 to 5 | 86 | 58.9 | 92 | 63.0 | 0.472 |
| 6 or more but not al/All | 60 | 41.1 | 54 | 37.0 | |
| Gargle | |||||
| Poor/Fair | 38 | 27.5 | 12 | 8.4 | <0.0001 |
| Good | 100 | 72.5 | 131 | 91.6 | |
| Missing | 12 | NA | 7 | NA | |
| Smoking history 2 | |||||
| Never | 33 | 22.0 | 32 | 21.3 | 0.889 |
| Ever | 117 | 78.0 | 118 | 78.7 | |
| Drinking habits 3 | |||||
| Non-drinker/Mild | 78 | 52.0 | 85 | 56.7 | 0.417 |
| Moderate/Heavy | 72 | 48.0 | 65 | 43.3 | |
| Variable | Univariable Models | Multivariable Model 1 (74 Events in 137 Patients) | Multivariable Model 2 (75 Events in 138 Patients) | ||||
|---|---|---|---|---|---|---|---|
| Events/N | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| Servings of 1–3/week or more vs. <1/week or never | |||||||
| Salads | 74/137 | 0.51 (0.31, 0.83) | 0.007 | 0.72 (0.42, 1.23) | 0.229 | -- | |
| In P16− | 29/45 | 0.98 (0.43, 2.24) | 0.971 | -- | -- | ||
| In P16+ | 8/27 | 0.27 (0.06, 1.16) | 0.079 | -- | -- | ||
| In p16 = NA | 37/65 | 0.41 (0.21, 0.82) | 0.011 | -- | -- | ||
| Other Vegetables | 73/137 | 0.68 (0.38, 1.21) | 0.188 | -- | -- | ||
| Salads or other vegetables | 75/138 | 0.39 (0.20, 0.74) | 0.004 | -- | 0.39 (0.19, 0.83) | 0.014 | |
| Carrots | 72/135 | 1.03 (0.65, 1.65) | 0.893 | -- | -- | ||
| Potatoes | 73/136 | 1.06 (0.54, 2.07) | 0.866 | -- | -- | ||
| Fruits | 78/142 | 0.88 (0.53, 1.47) | 0.627 | -- | -- | ||
| Juices | 75/137 | 1.54 (0.88, 2.67) | 0.129 | -- | -- | ||
| HPV status P16− vs. P16+ | 84/149 | 2.39 (1.20, 4.78) | 0.013 | 1.87 (0.78, 4.49) | 0.162 | 2.05 (0.85, 4.95) | 0.112 |
| NA vs. P16+ | 1.87 (0.96, 3.64) | 0.064 | 2.91 (1.26, 6.72) | 0.013 | 3.25 (1.41, 7.49) | 0.006 | |
| T Stage: T3-T4 vs. T1-T2 | 84/149 | 2.56 (1.60, 4.11) | <0.0001 | 2.02 (1.10, 3.70) | 0.024 | 1.88 (1.00, 3.51) | 0.049 |
| solCD44 ≥8.1 vs. <8.1 * | 84/149 | 4.57 (2.71, 7.71) | <0.0001 | 2.66 (1.28, 5.55) | 0.009 | 2.41 (1.18, 4.90) | 0.015 |
| Protein ≥1.05 vs. < 1.05 * | 84/149 | 2.64 (1.70, 4.11) | <0.0001 | 1.32 (0.73, 2.39) | 0.360 | 1.58 (0.86, 2.89) | 0.141 |
| Age: ≥60 vs. < 60 years-old | 84/149 | 2.18 (1.41, 3.37) | <0.001 | 1.76 (1.02, 3.03) | 0.042 | 1.86 (1.08, 3.22) | 0.026 |
| Race: Black vs. non-Black | 84/149 | 2.48 (1.49, 4.12) | <0.001 | 1.60 (0.79, 3.21) | 0.191 | 1.45 (0.70, 3.03) | 0.320 |
| Ethnicity: Non-Hispanic vs. Hispanic | 84/149 | 1.37 (0.89, 2.11) | 0.151 | 1.82 (0.97, 3.44) | 0.063 | 1.82 (0.96, 3.45) | 0.066 |
| Gender: Female vs. Male | 84/149 | 0.94 (0.55, 1.6) | 0.822 | 1.03 (0.54, 1.95) | 0.940 | 1.14 (0.60, 2.18) | 0.692 |
| Smoking History: Ever vs. Never | 84/149 | 1.64 (0.93, 2.92) | 0.090 | 0.88 (0.44, 1.76) | 0.717 | 1.16 (0.59, 2.29) | 0.671 |
| Drinking Habits: Mod/Heavy vs. Mild/Non-Drinker | 84/149 | 1.58 (1.03, 2.43) | 0.037 | 1.39 (0.79, 2.47) | 0.256 | 1.34 (0.76, 2.37) | 0.315 |
| SES: Low vs. High | 84/149 | 1.58 (0.99, 2.53) | 0.056 | 1.27 (0.58, 2.80) | 0.553 | 1.20 (0.54, 2.63) | 0.656 |
| Oral heath score: poor/fair/missing vs. good. | 84/149 | 2.10 (1.23, 3.58) | 0.006 | 1.57 (0.81, 3.06) | 0.183 | 1.67 (0.87, 3.19) | 0.122 |
| Teeth removed: 6 or more/All/missing vs. 5 or less | 84/149 | 1.82 (1.18, 2.81) | 0.007 | 1.25 (0.68, 2.29) | 0.472 | 1.11 (0.60, 2.04) | 0.747 |
| Gargle: Poor/Fair/Missing vs. Good | 84/149 | 2.10 (1.36, 3.25) | <0.001 | 1.31 (0.76, 2.25) | 0.338 | 1.30 (0.75, 2.24) | 0.353 |
| Variable | Univariable Models | Multivariable Model 1 (61 Deaths in 137 Patients) | Multivariable Model 2 (63 Deaths in 138 Patients) | ||||
|---|---|---|---|---|---|---|---|
| Deaths/N | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| Servings of 1–3/week or more vs. <1/week or never | |||||||
| Salads | 61/137 | 0.45 (0.26, 0.77) | 0.003 | 0.64 (0.35, 1.16) | 0.141 | -- | |
| In P16− | 23/45 | 0.67 (0.27, 1.66) | 0.381 | -- | -- | ||
| In P16+ | 7/27 | 0.25 (0.05, 1.12) | 0.069 | -- | -- | ||
| In p16 = NA | 31/65 | 0.43 (0.20, 0.89) | 0.024 | -- | -- | ||
| Other Vegetables | 61/137 | 0.66 (0.35, 1.24) | 0.194 | -- | -- | ||
| Salads or Other Vegetables | 63/138 | 0.40 (0.20, 0.81) | 0.011 | -- | 0.40 (0.17, 0.91) | 0.029 | |
| Potatoes | 60/136 | 1.31 (0.6, 2.89) | 0.501 | -- | -- | ||
| Carrots | 61/135 | 0.83 (0.5, 1.38) | 0.480 | -- | -- | ||
| Fruits | 65/142 | 0.98 (0.55, 1.75) | 0.956 | -- | -- | ||
| Juices | 63/137 | 1.28 (0.71, 2.32) | 0.417 | -- | -- | ||
| HPV status: P16− vs. P16+ | 71/149 | 1.85 (0.89, 3.86) | 0.099 | 1.71 (0.64, 4.59) | 0.289 | 1.89 (0.70, 5.16) | 0.212 |
| NA vs. P16+ | 1.77 (0.88, 3.58) | 0.109 | 3.70 (1.41, 9.70) | 0.008 | 4.48 (1.71, 11.72) | 0.002 | |
| T stage: T3-T4 vs. T1-T2 | 71/149 | 2.55 (1.52, 4.29) | <0.001 | 1.85 (0.93, 3.67) | 0.081 | 1.68 (0.84, 3.37) | 0.145 |
| solCD44 ≥8.1 vs. <8.1 * | 71/149 | 5.48 (3.14, 9.55) | <0.0001 | 3.17 (1.43, 7.03) | 0.004 | 3.12 (1.45, 6.69) | 0.004 |
| Protein ≥1.05 vs. < 1.05 * | 71/149 | 2.79 (1.74, 4.47) | <0.0001 | 1.36 (0.72, 2.60) | 0.347 | 1.61 (0.84, 3.10) | 0.151 |
| Age: ≥60 vs. <60 years-old | 71/149 | 2.18 (1.36, 3.50) | 0.001 | 2.15 (1.15, 4.03) | 0.017 | 2.14 (1.15, 3.97) | 0.016 |
| Race: Black vs. non-Black | 71/149 | 2.97 (1.74, 5.05) | <0.0001 | 1.62 (0.77, 3.44) | 0.205 | 1.68 (0.78, 3.61) | 0.188 |
| Ethnicity: Non-Hispanic vs. Hispanic | 71/149 | 1.88 (1.17, 3.02) | 0.009 | 3.04 (1.51, 6.10) | 0.002 | 2.54 (1.28, 5.01) | 0.007 |
| Gender: Female vs. Male | 71/149 | 1.22 (0.70, 2.14) | 0.477 | 1.55 (0.77, 3.13) | 0.221 | 1.65 (0.82, 3.35) | 0.163 |
| Drinking Habits: Mod/Heavy vs. Mild/Non-Drinker | 71/149 | 1.72 (1.08, 2.75) | 0.023 | 0.85 (0.39, 1.84) | 0.675 | 1.25 (0.58, 2.67) | 0.566 |
| Smoking History: Ever vs. Never | 71/149 | 1.64 (0.86, 3.13) | 0.130 | 2.04 (1.06, 3.92) | 0.032 | 1.87 (0.99, 3.54) | 0.053 |
| SES: Low vs. High | 71/149 | 1.54 (0.92, 2.58) | 0.104 | 1.94 (0.81, 4.64) | 0.135 | 1.60 (0.68, 3.78) | 0.286 |
| Oral heath score: poor/fair/missing vs. good. | 71/149 | 1.89 (1.07, 3.35) | 0.028 | 1.31 (0.63, 2.75) | 0.470 | 1.48 (0.73, 2.99) | 0.279 |
| Teeth removed: 6 or more/All/missing vs. 5 or less | 71/149 | 1.45 (0.91, 2.32) | 0.117 | 0.97 (0.49, 1.90) | 0.925 | 0.95 (0.47, 1.90) | 0.884 |
| Gargle: Poor/Fair/Missing vs. Good | 71/149 | 2.45 (1.53, 3.92) | <0.001 | 1.35 (0.72, 2.53) | 0.355 | 1.25 (0.68, 2.31) | 0.476 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bell, E.B.; Reis, I.M.; Cohen, E.R.; Almuhaimid, T.; Smith, D.H.; Alotaibi, F.; Gordon, C.; Gomez-Fernandez, C.; Goodwin, W.J.; Franzmann, E.J. Green Salad Intake Is Associated with Improved Oral Cancer Survival and Lower Soluble CD44 Levels. Nutrients 2021, 13, 372. https://doi.org/10.3390/nu13020372
Bell EB, Reis IM, Cohen ER, Almuhaimid T, Smith DH, Alotaibi F, Gordon C, Gomez-Fernandez C, Goodwin WJ, Franzmann EJ. Green Salad Intake Is Associated with Improved Oral Cancer Survival and Lower Soluble CD44 Levels. Nutrients. 2021; 13(2):372. https://doi.org/10.3390/nu13020372
Chicago/Turabian StyleBell, Elizabeth Bradford, Isildinha M. Reis, Erin R. Cohen, Turki Almuhaimid, Drew H. Smith, Faisal Alotaibi, Claudia Gordon, Carmen Gomez-Fernandez, W. Jarrard Goodwin, and Elizabeth J. Franzmann. 2021. "Green Salad Intake Is Associated with Improved Oral Cancer Survival and Lower Soluble CD44 Levels" Nutrients 13, no. 2: 372. https://doi.org/10.3390/nu13020372
APA StyleBell, E. B., Reis, I. M., Cohen, E. R., Almuhaimid, T., Smith, D. H., Alotaibi, F., Gordon, C., Gomez-Fernandez, C., Goodwin, W. J., & Franzmann, E. J. (2021). Green Salad Intake Is Associated with Improved Oral Cancer Survival and Lower Soluble CD44 Levels. Nutrients, 13(2), 372. https://doi.org/10.3390/nu13020372






