Iron-Enriched Nutritional Supplements for the 2030 Pharmacy Shelves
Abstract
:1. Introduction
2. Nutritional Sources of Iron
3. Nutritional Sources that Hinder Iron Absorption
4. Nutritional Resources for Iron Management
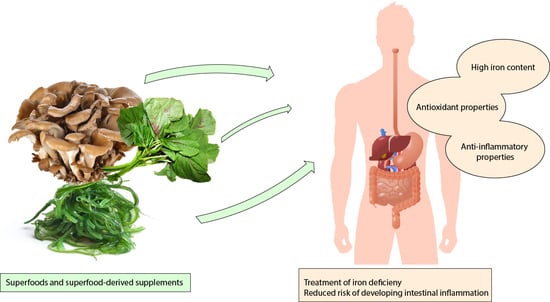
5. Superfoods as Nutritional Strategies for Iron Level Replenishment
6. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, D.C.P. Role of Iron (Fe) in Body. IOSR J. Appl. Chem. 2014, 7, 38–46. [Google Scholar] [CrossRef]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldon, J.R.; Laakso, H.A.; Heinrichs, D.E. Iron Acquisition Strategies of Bacterial Pathogens. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, B.K.; Vulpe, C.D.; Anderson, G.J. Intestinal iron absorption. J. Trace Elem. Med. Biol. 2012, 26, 115–119. [Google Scholar] [CrossRef]
- West, A.R.; Oates, P.S. Mechanisms of heme iron absorption: Current questions and controversies. World J. Gastroenterol. 2008, 14, 4101–4110. [Google Scholar] [CrossRef]
- Anderson, E.R.; Shah, Y.M. Iron homeostasis in the liver. Compr. Physiol. 2013, 3, 315–330. [Google Scholar]
- Czerwonka, M.l.; Tokarz, A. Iron in red meat–friend or foe. Meat Sci. 2017, 123, 157–165. [Google Scholar] [CrossRef]
- Alexander, D.; Ball, M.J.; Mann, J. Nutrient intake and haematological status of vegetarians and age-sex matched omnivores. Eur. J. Clin. Nutr. 1994, 48, 538–546. [Google Scholar]
- Diego Quintaes, K.; Barberá, R.; Cilla, A. Iron bioavailability in iron-fortified cereal foods: The contribution of in vitro studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 2028–2041. [Google Scholar] [CrossRef]
- Virtanen, M.A.; Svahn, C.J.; Viinikka, L.U.; Räihä, N.C.; Siimes, M.A.; Axelsson, I.E. Iron-fortified and unfortified cow’s milk: Effects on iron intakes and iron status in young children. Acta Paediatr. 2001, 90, 724–731. [Google Scholar] [CrossRef]
- Vonderheid, S.C.; Tussing-Humphreys, L.; Park, C.; Pauls, H.; OjiNjideka Hemphill, N.; LaBomascus, B.; McLeod, A.; Koenig, M.D. A Systematic Review and Meta-Analysis on the Effects of Probiotic Species on Iron Absorption and Iron Status. Nutrients 2019, 11, 2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusu, I.G.; Suharoschi, R.; Vodnar, D.C.; Pop, C.R.; Socaci, S.A.; Vulturar, R.; Istrati, M.; Moroșan, I.; Fărcaș, A.C.; Kerezsi, A.D.; et al. Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency-A Literature-Based Review. Nutrients 2020, 12, 1993. [Google Scholar] [CrossRef] [PubMed]
- Teucher, B.; Olivares, M.; Cori, H. Enhancers of iron absorption: Ascorbic acid and other organic acids. Int. J. Vitam. Nutr. Res. 2004, 74, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Calcium and iron absorption–mechanisms and public health relevance. Int. J. Vitam. Nutr. Res. 2010, 80, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 2001, 131, 568S–580S. [Google Scholar] [CrossRef]
- Haas, J.D.; Brownlie, T. Iron Deficiency and Reduced Work Capacity: A Critical Review of the Research to Determine a Causal Relationship. J. Nutr. 2001, 131, 676S–690S. [Google Scholar] [CrossRef] [Green Version]
- Reichert, C.O.; Da Cunha, J.; Levy, D.; Maselli, L.M.F.; Bydlowski, S.P.; Spada, C.; Gargallo-Puyuelo, C.J.; Alfambra, E.; García-Erce, J.A.; Gomollon, F.; et al. Iron Supplements Modulate Colon Microbiota Composition and Potentiate the Protective Effects of Probiotics in Dextran Sodium Sulfate-induced Colitis. Nutrients 2019, 10, 1–12. [Google Scholar]
- Attia, M.A.; Essa, S.A.; Nosair, N.A.; Amin, A.M.; El-Agamy, O.A. Effect of iron deficiency anemia and its treatment on cell mediated immunity. Indian J. Hematol. Blood Transfus. 2009, 25, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Verna, G.; Liso, M.; De Santis, S.; Dicarlo, M.; Cavalcanti, E.; Crovace, A.; Sila, A.; Campiglia, P.; Santino, A.; Lippolis, A.; et al. Iron overload mimicking conditions skews bone marrow dendritic cells differentiation into mhciilowcd11c+cd11b+f4/80+ cells. Int. J. Mol. Sci. 2020, 21, 1353. [Google Scholar] [CrossRef] [Green Version]
- Ganz, T.; Nemeth, E. Regulation of iron acquisition and iron distribution in mammals. Biochim. Biophys. Acta 2006, 1763, 690–699. [Google Scholar] [CrossRef] [Green Version]
- Brune, M.; Magnusson, B.; Persson, H.; Hallberg, L. Iron losses in sweat. Am. J. Clin. Nutr. 1986, 43, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron-Deficiency Anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasricha, S.-R.; Drakesmith, H.; Black, J.; Hipgrave, D.; Biggs, B.-A. Control of iron deficiency anemia in low- and middle-income countries. Blood 2013, 121, 2607–2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osazuwa, F.; Ayo, O.M.; Imade, P. A significant association between intestinal helminth infection and anaemia burden in children in rural communities of Edo state, Nigeria. N. Am. J. Med. Sci. 2011, 3, 30–34. [Google Scholar] [CrossRef]
- Casey, G.J.; Montresor, A.; Cavalli-Sforza, L.T.; Thu, H.; Phu, L.B.; Tinh, T.T.; Tien, N.T.; Phuc, T.Q.; Biggs, B.-A. Elimination of Iron Deficiency Anemia and Soil Transmitted Helminth Infection: Evidence from a Fifty-four Month Iron-Folic Acid and De-worming Program. PLoS Negl. Trop. Dis. 2013, 7, e2146. [Google Scholar] [CrossRef] [Green Version]
- Akanni, E.O.; Adefioye, O.A.; Akanni, R.A.; Taiwo, S.S. Iron deficiency anaemia associated with helminths and asymptomatic malaria infections among rural school children in Southwestern Nigeria. Asian Pac. J. Trop. Dis. 2014, 4, S590–S594. [Google Scholar] [CrossRef]
- Hossain, M.S.; Das, S.; Gazi, M.A.; Mahfuz, M.; Ahmed, T. Ascaris lumbricoides infection: Still a threat for iron deficiency anaemia in 2-year-old Bangladeshi slum-dwelling children. J. Infect. Dev. Ctries. 2019, 13, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Yip, R.; Ramakrishnan, U. Experiences and Challenges in Developing Countries. J. Nutr. 2002, 132, 827S–830S. [Google Scholar] [CrossRef] [Green Version]
- Gautam, S.; Min, H.; Kim, H.; Jeong, H.-S. Determining factors for the prevalence of anemia in women of reproductive age in Nepal: Evidence from recent national survey data. PLoS ONE 2019, 14, e0218288. [Google Scholar] [CrossRef] [Green Version]
- Galloway, R.; Dusch, E.; Elder, L.; Achadi, E.; Grajeda, R.; Hurtado, E.; Favin, M.; Kanani, S.; Marsaban, J.; Meda, N.; et al. Women’s perceptions of iron deficiency and anemia prevention and control in eight developing countries. Soc. Sci. Med. 2002, 55, 529–544. [Google Scholar] [CrossRef]
- Lutter, C.K. Iron Deficiency in Young Children in Low-Income Countries and New Approaches for Its Prevention. J. Nutr. 2008, 138, 2523–2528. [Google Scholar] [CrossRef] [PubMed]
- Gasche, C.; Lomer, M.C.E.; Cavill, I.; Weiss, G. Iron, anaemia, and inflammatory bowel diseases. Gut 2004, 53, 1190–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niepel, D.; Klag, T.; Malek, N.P.; Wehkamp, J. Practical guidance for the management of iron deficiency in patients with inflammatory bowel disease. Therap. Adv. Gastroenterol. 2018, 11, 1756284818769074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez, K.M.; Gasche, C. Management of Iron Deficiency Anaemia in Inflammatory Bowel Disease. Acta Haematol. 2019, 142, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Iiritano, E.; Roberto, G.; Teresa, S.; Federico, B. Life-Threatening Jejunal Hemorrhage as First Presentation of Crohn’s Disease. Inflamm. Bowel Dis. 2010, 16, 1277–1278. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.H.; Sachar, D.B.; Aufses, A.H., Jr.; Greenstein, A.J. Management of severe hemorrhage in ulcerative colitis. Am. J. Surg. 1990, 159, 550–555. [Google Scholar] [CrossRef]
- Farmer, R.G. Lower gastrointestinal bleeding in inflammatory bowel disease. Gastroenterol. Jpn. 1991, 26 (Suppl. 3), 93–100. [Google Scholar] [CrossRef]
- Belaiche, J.; Louis, E.; D’Haens, G.; Cabooter, M.; Naegels, S.; De Vos, M.; Fontaine, F.; Schurmans, P.; Baert, F.; De Reuck, M.; et al. Acute lower gastrointestinal bleeding in Crohn’s disease: Characteristics of a unique series of 34 patients. Belgian IBD Research Group. Am. J. Gastroenterol. 1999, 94, 2177–2181. [Google Scholar] [CrossRef]
- Stein, J.; Hartmann, F.; Dignass, A.U. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 599–610. [Google Scholar] [CrossRef]
- Ghishan, F.K.; Kiela, P.R. Vitamins and Minerals in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 797–808. [Google Scholar] [CrossRef]
- Percy, L.; Mansour, D.; Fraser, I. Iron deficiency and iron deficiency anaemia in women. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 40, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Chen, L.-W.; Heude, B.; Bernard, J.Y.; Harvey, N.C.; Duijts, L.; Mensink-Bout, S.M.; Polanska, K.; Mancano, G.; Suderman, M.; et al. Dietary Inflammatory Index and Non-Communicable Disease Risk: A Narrative Review. Nutrients 2019, 11, 1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aigner, E.; Feldman, A.; Datz, C. Obesity as an emerging risk factor for iron deficiency. Nutrients 2014, 6, 3587–3600. [Google Scholar] [CrossRef] [PubMed]
- Cepeda-Lopez, A.C.; Baye, K. Obesity, iron deficiency and anaemia: A complex relationship. Public Health Nutr. 2020, 23, 1703–1704. [Google Scholar] [CrossRef]
- Manios, Y.; Moschonis, G.; Chrousos, G.P.; Lionis, C.; Mougios, V.; Kantilafti, M.; Tzotzola, V.; Skenderi, K.P.; Petridou, A.; Tsalis, G.; et al. The double burden of obesity and iron deficiency on children and adolescents in Greece: The Healthy Growth Study. J. Hum. Nutr. Diet. 2013, 26, 470–478. [Google Scholar] [CrossRef]
- Soliman, A.T.; De Sanctis, V.; Yassin, M.; Soliman, N. Iron deficiency anemia and glucose metabolism. Acta Bio-Med. 2017, 88, 112–118. [Google Scholar]
- Broide, E.; Reifen, R.; Matalon, S.; Berkovich, Z.; Shirin, H. Expression of Duodenal Iron Transporter Proteins in Diabetic Patients with and without Iron Deficiency Anemia. J. Diabetes Res. 2018, 2018, 7494821. [Google Scholar]
- Liso, M.; De Santis, S.; Scarano, A.; Verna, G.; Dicarlo, M.; Galleggiante, V.; Campiglia, P.; Mastronardi, M.; Lippolis, A.; Vacca, M.; et al. A Bronze-Tomato Enriched Diet Affects the Intestinal Microbiome under Homeostatic and Inflammatory Conditions. Nutrients 2018, 10, 1862. [Google Scholar] [CrossRef] [Green Version]
- De Santis, S.; Clodoveo, M.L.; Cariello, M.; D’Amato, G.; Franchini, C.; Faienza, M.F.; Corbo, F. Polyphenols and obesity prevention: Critical insights on molecular regulation, bioavailability and dose in preclinical and clinical settings. Crit. Rev. Food Sci. Nutr. 2020, 1–23. [Google Scholar] [CrossRef]
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef]
- School of Public Health. Superfoods or Superhype? Available online: https://www.hsph.harvard.edu/nutritionsource/superfoods/ (accessed on 1 June 2020).
- Fernando, I.P.S.; Nah, J.W.; Jeon, Y.J. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C.; Pagani, A.; Nai, A.; Silvestri, L. The mutual control of iron and erythropoiesis. Int. J. Lab. Hematol. 2016, 38, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.T. A Review of Nutrients and Compounds, Which Promote or Inhibit Intestinal Iron Absorption: Making a Platform for Dietary Measures That Can Reduce Iron Uptake in Patients with Genetic Haemochromatosis. J. Nutr. Metab. 2020, 2020, 7373498. [Google Scholar] [CrossRef] [PubMed]
- Lombardi-Boccia, G.; Martinez-Dominguez, B.; Aguzzi, A. Total Heme and Non-heme Iron in Raw and Cooked Meats. J. Food Sci. 2002, 67, 1738–1741. [Google Scholar] [CrossRef]
- Ochoa, J.J.; Pulido-Morán, M.; Hijano, S.; Kajarabille, N.; Moreno-Fernández, J.; Díaz-Castro, J. Interactions Between Omega-3 Fatty Acids and Iron. In Omega-3 Fatty Acids: Keys to Nutritional Health; Hegde, M.V., Zanwar, A.A., Adekar, S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 293–299. [Google Scholar] [CrossRef]
- Przybyszewska, J.; Zekanowska, E. The role of hepcidin, ferroportin, HCP1, and DMT1 protein in iron absorption in the human digestive tract. Prz. Gastroenterol. 2014, 9, 208–213. [Google Scholar] [CrossRef]
- Gomme, P.T.; McCann, K.B. Transferrin: Structure, function and potential therapeutic actions. Drug Discov. Today 2005, 10, 267–273. [Google Scholar] [CrossRef]
- Pizarro, F.; Olivares, M.; Hertrampf, E.; Mazariegos, D.I.; Arredondo, M. Heme-iron absorption is saturable by heme-iron dose in women. J. Nutr. 2003, 133, 2214–2217. [Google Scholar] [CrossRef]
- Valenzuela, C.; López de Romaña, D.; Olivares, M.; Morales, M.S.; Pizarro, F. Total Iron and Heme Iron Content and their Distribution in Beef Meat and Viscera. Biol. Trace Elem. Res. 2009, 132, 103–111. [Google Scholar] [CrossRef]
- Venugopal, V.; Gopakumar, K. Shellfish: Nutritive Value, Health Benefits, and Consumer Safety. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1219–1242. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Torres, C.; Romano, E.; Layrisse, M. Effect of cysteine on iron absorption in man. Am. J. Clin. Nutr. 1981, 34, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Layrisse, M.; Cook, J.D.; Martinez, C.; Roche, M.; Kuhn, I.N.; Walker, R.B.; Finch, C.A. Food Iron Absorption: A Comparison of Vegetable and Animal Foods. Blood 1969, 33, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Dainty, J.R.; Berry, R.; Lynch, S.R.; Harvey, L.J.; Fairweather-Tait, S.J. Estimation of dietary iron bioavailability from food iron intake and iron status. PLoS ONE 2014, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skikne, B.S.; Lynch, S.R.; Cook, J.D. Role of Gastric Acid in Food Iron Absorption. Gastroenterology 1981, 81, 1068–1071. [Google Scholar] [CrossRef]
- Saunders, A.V.; Craig, W.J.; Baines, S.K.; Posen, J.S. Iron and vegetarian diets. Med. J. Aust. 2013, 199, S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J.R.; Bae, D.H.; Merlot, A.M.; Sahni, S.; Richardson, D.R. Duodenal cytochrome b (DCYTB) in Iron metabolism: An update on function and regulation. Nutrients 2015, 7, 2274–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, K.H.C.; Riddell, L.J.; Nowson, C.A.; Booth, A.O.; Szymlek-Gay, E.A. Iron and zinc nutrition in the economically-developed world: A review. Nutrients 2013, 5, 3184–3211. [Google Scholar] [CrossRef]
- Chieppa, M.; Galleggiante, V.; Serino, G.; Massaro, M.; Santino, A. Iron Chelators Dictate Immune Cells Inflammatory Ability: Potential Adjuvant Therapy for IBD. Curr. Pharm. Des. 2017, 23, 2289–2298. [Google Scholar] [CrossRef]
- Naviglio, D.; Salvatore, M.M.; Limatola, M.; Langella, C.; Faralli, S.; Ciaravolo, M.; Andolfi, A.; Salvatore, F.; Gallo, M. Iron (II) Citrate Complex as a Food Supplement: Synthesis, Characterization and Complex Stability. Nutrients 2018, 10, 1647. [Google Scholar] [CrossRef] [Green Version]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- García-Casal, M.N.; Pereira, A.C.; Leets, I.; Ramírez, J.; Quiroga, M.F. High iron content and bioavailability in humans from four species of marine algae. J. Nutr. 2007, 137, 2691–2695. [Google Scholar] [CrossRef]
- Martínez-Zavala, M.; Mora-Avilés, M.A.; Anaya-Loyola, M.A.; Guzmán-Maldonado, H.; Aguilera-Barreyro, A.; Blanco-Labra, A.; García-Gasca, T. Common Bean Leaves as a Source of Dietary Iron: Functional Test in an Iron-Deficient Rat Model. Plant Foods Hum. Nutr. 2016, 71, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Scheers, N. Regulatory effects of Cu, Zn, and Ca on Fe absorption: The intricate play between nutrient transporters. Nutrients 2013, 5, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Pizarro, F.; Gaitán, D.; Ruz, M. Acute inhibition of iron absorption by zinc. Nutr. Res. 2007, 27, 279–282. [Google Scholar] [CrossRef]
- Walczyk, T.; Muthayya, S.; Wegmüller, R.; Thankachan, P.; Sierksma, A.; Frenken, L.G.J.; Thomas, T.; Kurpad, A.; Hurrell, R.F. Inhibition of Iron Absorption by Calcium Is Modest in an Iron-Fortified, Casein- and Whey-Based Drink in Indian Children and Is Easily Compensated for by Addition of Ascorbic Acid. J. Nutr. 2014, 144, 1703–1709. [Google Scholar] [CrossRef]
- Liao, Y.; Weber, D.; Xu, W.; Durbin-Johnson, B.P.; Phinney, B.S.; Lönnerdal, B. Absolute Quantification of Human Milk Caseins and the Whey/Casein Ratio during the First Year of Lactation. J. Proteome Res. 2017, 16, 4113–4121. [Google Scholar] [CrossRef]
- Kibangou, I.B.; Bouhallab, S.; Henry, G.; Bureau, F.; Allouche, S.; Blais, A.; Guérin, P.; Arhan, P.; Bouglé, D.L. Milk proteins and iron absorption: Contrasting effects of different caseinophosphopeptides. Pediatr. Res. 2005, 58, 731–734. [Google Scholar] [CrossRef] [Green Version]
- Bondi, S.A.; Lieuw, K. Excessive Cow’s Milk Consumption and Iron Deficiency in Toddlers:Two Unusual Presentations and Review. ICAN Infant Child Adolesc. Nutr. 2009, 1, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Gross, S.; Vergis, M.; Good, A. The relationship between milk protein and iron content on hematologic values in infancy. J. Pediatrics 1968, 73, 521–530. [Google Scholar] [CrossRef]
- Kazal, L.A., Jr. Prevention of iron deficiency in infants and toddlers. Am. Fam. Physician 2002, 66, 1217–1224. [Google Scholar]
- Ziegler, E. Consumption of cow’s milk as a cause of iron deficiency in infants and toddlers. Nutr. Rev. 2011, 69 (Suppl. 1), S37–S42. [Google Scholar] [CrossRef]
- Callender, S.T.; Marney, S.R., Jr.; Warner, G.T. Eggs and iron absorption. Br. J. Haematol. 1970, 19, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Mecham, D.K.; Olcott, H.S. Phosvitin, the Principal Phosphoprotein of Egg Yolk. J. Am. Chem. Soc. 1949, 71, 3670–3679. [Google Scholar] [CrossRef]
- Ishikawa, S.I.; Tamaki, S.; Arihara, K.; Itoh, M. Egg yolk protein and egg yolk phosvitin inhibit calcium, magnesium, and iron absorptions in rats. J. Food Sci. 2007, 72, S412–S419. [Google Scholar] [CrossRef] [PubMed]
- Huntington, J.A.; Stein, P.E. Structure and properties of ovalbumin. J. Chromatogr. B Biomed. Sci. Appl. 2001, 756, 189–198. [Google Scholar] [CrossRef]
- Miller, J.; Nnanna, I. Bioavailability of iron in cooked egg yolk for maintenance of hemoglobin levels in growing rats. J. Nutr. 1983, 113, 1169–1175. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, Y.; Wakasugi, E.; Yasui, R.; Kuwahata, M.; Kido, Y. Egg Yolk Protein Delays Recovery while Ovalbumin Is Useful in Recovery from Iron Deficiency Anemia. Nutrients 2015, 7, 4792–4803. [Google Scholar] [CrossRef] [Green Version]
- Weinborn, V.; Pizarro, F.; Olivares, M.; Brito, A.; Arredondo, M.; Flores, S.; Valenzuela, C. The Effect of Plant Proteins Derived from Cereals and Legumes on Heme Iron Absorption. Nutrients 2015, 7, 8977–8986. [Google Scholar] [CrossRef]
- Zhou, Y.; Alekel, D.L.; Dixon, P.M.; Messina, M.; Reddy, M.B. The effect of soy food intake on mineral status in premenopausal women. J. Womens Health 2011, 20, 771–780. [Google Scholar] [CrossRef] [Green Version]
- Lorencová, E.; Vltavská, P.; Budinský, P.; Koutný, M. Antibacterial effect of phosphates and polyphosphates with different chain length. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2012, 47, 2241–2245. [Google Scholar] [CrossRef]
- Long, N.; Gál, R.; BuHka, F.J.A.J.o.B. Use of phosphates in meat products. Afr. J. Biotechnol. 2011, 10, 19874–19882. [Google Scholar]
- Sabbagh, Y.; Giral, H.; Caldas, Y.; Levi, M.; Schiavi, S.C. Intestinal phosphate transport. Adv. Chronic Kidney Dis. 2011, 18, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Hernando, N.; Wagner, C.A. Mechanisms and Regulation of Intestinal Phosphate Absorption. Compr. Physiol. 2018, 8, 1065–1090. [Google Scholar]
- Ritz, E.; Hahn, K.; Ketteler, M.; Kuhlmann, M.K.; Mann, J. Phosphate additives in food--a health risk. Dtsch. Arztebl. Int. 2012, 109, 49–55. [Google Scholar] [PubMed]
- Watanabe, M.T.; Barretti, P.; Caramori, J.C.T. Attention to Food Phosphate and Nutrition Labeling. J. Ren. Nutr. 2018, 28, e29–e31. [Google Scholar] [CrossRef] [Green Version]
- Tran, L.; Batech, M.; Rhee, C.M.; Streja, E.; Kalantar-Zadeh, K.; Jacobsen, S.J.; Sim, J.J. Serum phosphorus and association with anemia among a large diverse population with and without chronic kidney disease. Nephrol. Dial. Transplant. 2016, 31, 636–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojcicki, J.M. Hyperphosphatemia is associated with anemia in adults without chronic kidney disease: Results from the National Health and Nutrition Examination Survey (NHANES): 2005–2010. BMC Nephrol. 2013, 14, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaufay, F.; Quarles, E.; Franz, A.; Katamanin, O.; Wholey, W.-Y.; Jakob, U. Polyphosphate Functions In Vivo as an Iron Chelator and Fenton Reaction Inhibitor. mBio 2020, 11, e01017–e01020. [Google Scholar] [CrossRef]
- Cunningham, S.E.; Czaya, B.E.; Faul, C. Elevated Phosphate Levels Induce Markers of Systemic Inflammation and Anemia in Murine Hepatocytes. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Czaya, B.; Richter, B.; Yanucil, C.; Campos, I.; Heitman, K.; Faul, C. Hyperphosphatemia Contributes to Inflammation and Iron Dysregulation in Models of Normal and Impaired Renal Function. Blood 2019, 134, 2238. [Google Scholar] [CrossRef]
- Lewis, J.B.; Sika, M.; Koury, M.J.; Chuang, P.; Schulman, G.; Smith, M.T.; Whittier, F.C.; Linfert, D.R.; Galphin, C.M.; Athreya, B.P.; et al. Ferric Citrate Controls Phosphorus and Delivers Iron in Patients on Dialysis. J. Am. Soc. Nephrol. 2015, 26, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.; Noh, Y.; Shin, S. Ferric citrate in the management of hyperphosphatemia and iron deficiency anemia: A meta-analysis in patients with chronic kidney disease. Br. J. Clin. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Delvecchio, F.R.; Vadrucci, E.; Cavalcanti, E.; De Santis, S.; Kunde, D.; Vacca, M.; Myers, J.; Allen, F.; Bianco, G.; Huang, A.Y.; et al. Polyphenol administration impairs T-cell proliferation by imprinting a distinct dendritic cell maturational profile. Eur. J. Immunol. 2015, 45, 2638–2649. [Google Scholar] [CrossRef]
- De Santis, S.; Kunde, D.; Serino, G.; Galleggiante, V.; Caruso, M.L.; Mastronardi, M.; Cavalcanti, E.; Ranson, N.; Pinto, A.; Campiglia, P.; et al. Secretory leukoprotease inhibitor is required for efficient quercetin-mediated suppression of TNFβ secretion. Oncotarget 2016, 7, 75800–75809. [Google Scholar] [CrossRef] [Green Version]
- Santino, A.; Scarano, A.; De Santis, S.; De Benedictis, M.; Giovinazzo, G.; Chieppa, M. Gut Microbiota Modulation and Anti-Inflammatory Properties of Dietary Polyphenols in IBD: New and Consolidated Perspectives. Curr. Pharm. Des. 2017, 23, 2344–2351. [Google Scholar] [CrossRef]
- Galleggiante, V.; De Santis, S.; Cavalcanti, E.; Scarano, A.; De Benedictis, M.; Serino, G.; Caruso, M.L.; Mastronardi, M.; Pinto, A.; Campiglia, P.; et al. Dendritic Cells Modulate Iron Homeostasis and Inflammatory Abilities Following Quercetin Exposure. Curr. Pharm. Des. 2017, 23, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- De Santis, S.; Galleggiante, V.; Scandiffio, L.; Liso, M.; Sommella, E.; Sobolewski, A.; Spilotro, V.; Pinto, A.; Campiglia, P.; Serino, G.; et al. Secretory Leukoprotease Inhibitor (Slpi) Expression Is Required for Educating Murine Dendritic Cells Inflammatory Response Following Quercetin Exposure. Nutrients 2017, 9, 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachodimitropoulou, E.; Sharp, P.; Naftalin, R. Quercetin-iron chelates are transported via glucose transporters. Free Radic. Biol. Med. 2011, 50, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Luo, G.; Tang, Y.; Yao, P. Quercetin and iron metabolism: What we know and what we need to know. Food Chem. Toxicol. 2018, 114, 190–203. [Google Scholar] [CrossRef]
- Lesjak, M.; KS Srai, S. Role of Dietary Flavonoids in Iron Homeostasis. Pharmaceuticals 2019, 12, 119. [Google Scholar] [CrossRef] [Green Version]
- Muckenthaler, M.U.; Galy, B.; Hentze, M.W. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 2008, 28, 197–213. [Google Scholar] [CrossRef]
- Basseri, R.J.; Nemeth, E.; Vassilaki, M.E.; Basseri, B.; Enayati, P.; Shaye, O.; Bourikas, L.A.; Ganz, T.; Papadakis, K.A. Hepcidin is a key mediator of anemia of inflammation in Crohn’s disease. J. Crohns Colitis 2013, 7, e286–e291. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, S.; Bhandari, S. Oral iron: Properties and current place in the treatment of anaemia. Prescriber 2012, 23, 12–18. [Google Scholar] [CrossRef]
- Jin, X.; He, X.; Cao, X.; Xu, P.; Xing, Y.; Sui, S.; Wang, L.; Meng, J.; Lu, W.; Cui, R.; et al. Iron overload impairs normal hematopoietic stem and progenitor cells through reactive oxygen species and shortens survival in myelodysplastic syndrome mice. Haematologica 2018, 103, 1627–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajji, H.E.; Nkhili, E.; Tomao, V.; Dangles, O. Interactions of quercetin with iron and copper ions: Complexation and autoxidation. Free Radic. Res. 2006, 40, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Perez, C.; Wei, Y.; Rapoza, E.; Su, G.; Bou-Abdallah, F.; Chasteen, N.D. Iron-binding properties of plant phenolics and cranberry’s bio-effects. Dalton Trans. 2007, 43, 4951–4961. [Google Scholar] [CrossRef] [Green Version]
- Piccinin, E.; Cariello, M.; De Santis, S.; Ducheix, S.; Sabbà, C.; Ntambi, J.M.; Moschetta, A. Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients 2019, 11, 2283. [Google Scholar] [CrossRef] [Green Version]
- Cariello, M.; Contursi, A.; Gadaleta, R.M.; Piccinin, E.; De Santis, S.; Piglionica, M.; Spaziante, A.F.; Sabbà, C.; Villani, G.; Moschetta, A. Extra-Virgin Olive Oil from Apulian Cultivars and Intestinal Inflammation. Nutrients 2020, 12, 1084. [Google Scholar] [CrossRef] [Green Version]
- De Santis, S.; Cariello, M.; Piccinin, E.; Sabbà, C.; Moschetta, A. Extra Virgin Olive Oil: Lesson from Nutrigenomics. Nutrients 2019, 11, 2085. [Google Scholar] [CrossRef] [Green Version]
- Shotton, A.D.; Droke, E.A. Iron utilization and liver mineral concentrations in rats fed safflower oil, flaxseed oil, olive oil, or beef tallow in combination with different concentrations of dietary iron. Biol. Trace Elem. Res. 2004, 97, 265–278. [Google Scholar] [CrossRef]
- Gibson, R.S.; Raboy, V.; King, J.C. Implications of phytate in plant-based foods for iron and zinc bioavailability, setting dietary requirements, and formulating programs and policies. Nutr. Rev. 2018, 76, 793–804. [Google Scholar] [CrossRef]
- Hertrampf, E.; Cayazzo, M.; Pizarro, F.; Stekel, A. Bioavailability of iron in soy-based formula and its effect on iron nutriture in infancy. Pediatrics 1986, 78, 640–645. [Google Scholar] [PubMed]
- Silva, M.L.; Speridião, P.G.L.; Marciano, R.; Amâncio, O.; Morais, T.; Morais, M. Intestinal absorption of iron and calcium from soy and cow’s milk-based infant formulas in weanling rats pups. Rev. Nutr. 2017, 30, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Govindaraj, T.; KrishnaRau, L.; Prakash, J. In vitro bioavailability of iron and sensory qualities of iron-fortified wheat biscuits. Food Nutr. Bull. 2007, 28, 299–306. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Jefferds, M.E.D.; Peña-Rosas, J.P. Point-of-use fortification of foods with micronutrient powders containing iron in children of preschool and school-age. Cochrane Database Syst. Rev. 2017, 11, CD009666. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huffman, S.L. Review of fortified food and beverage products for pregnant and lactating women and their impact on nutritional status. Matern. Child Nutr. 2011, 7, 19–43. [Google Scholar] [CrossRef]
- Salovaara, S.; Sandberg, A.-S.; Andlid, T. Combined Impact of pH and Organic Acids on Iron Uptake by Caco-2 Cells. J. Agric. Food Chem. 2003, 51, 7820–7824. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, W.M.; Howarth, L.; Omori, M. Concentrations of Tartaric Acid and Malic Acids and Their Salts in Vitis Vinifera Grapes. Am. J. Enol. Vitic. 1967, 18, 42. [Google Scholar]
- Ma, B.; Yuan, Y.; Gao, M.; Li, C.; Ogutu, C.; Li, M.; Ma, F. Determination of Predominant Organic Acid Components in Malus Species: Correlation with Apple Domestication. Metabolites 2018, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Fan-Chiang, H.J.; Wrolstad, R.E. Sugar and nonvolatile acid composition of blackberries. J. AOAC Int. 2010, 93, 956–965. [Google Scholar] [CrossRef] [Green Version]
- Forney, C.F.; Kalt, W.; Jordan, M.A.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.E. Blueberry and cranberry fruit composition during development. J. Berry Res. 2012, 2, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhou, L.; Wang, Q.; He, Y. Determination of organic acids for quality evaluation in Coptis herbs by ion chromatography. 3 Biotech 2018, 8, 285. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, D.; Vrancheva, R.; Petkova, N.; Ognyanov, M.; Desseva, I.; Ivanov, I.; Popova, M.; Popova, A. Carotenoids, tocopherols, organic acids, charbohydrate and mineral content in different medicinal plant extracts. Z. fur Nat. C 2018, 73, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Z.H.; Das Bhowmik, S.S.; Hoang, T.M.L.; Karbaschi, M.R.; Johnson, A.A.T.; Williams, B.; Mundree, S.G. Finger on the Pulse: Pumping Iron into Chickpea. Front. Plant Sci. 2017, 8, 1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junqueira-Franco, M.V.M.; Dutra de Oliveira, J.E.; Nutti, M.R.; Pereira, H.S.; Carvalho, J.L.V.; Abrams, S.A.; Brandão, C.F.C.; Marchini, J.S. Iron absorption from beans with different contents of iron, evaluated by stable isotopes. Clin. Nutr. ESPEN 2018, 25, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Delimont, N.M.; Haub, M.D.; Lindshield, B.L. The Impact of Tannin Consumption on Iron Bioavailability and Status: A Narrative Review. Curr. Dev. Nutr. 2017, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.; Russo, C.; Takata, Y.; Bobe, G. Legume Consumption Patterns in US Adults: National Health and Nutrition Examination Survey (NHANES) 2011-2014 and Beans, Lentils, Peas (BLP) 2017 Survey. Nutrients 2020, 12, 1237. [Google Scholar] [CrossRef]
- Figueira, N.; Curtain, F.; Beck, E.; Grafenauer, S. Consumer Understanding and Culinary Use of Legumes in Australia. Nutrients 2019, 11, 1575. [Google Scholar] [CrossRef] [Green Version]
- Doumani, N.; Severin, I.; Dahbi, L.; Bou-Maroun, E.; Tueni, M.; Sok, N.; Chagnon, M.C.; Maalouly, J.; Cayot, P. Lemon Juice, Sesame Paste, and Autoclaving Influence Iron Bioavailability of Hummus: Assessment by an In Vitro Digestion/Caco-2 Cell Model. Foods 2020, 9, 474. [Google Scholar] [CrossRef]
- Engelmann, M.D.M.; Davidsson, L.; Sandström, B.; Walczyk, T.; Hurrell, R.F.; Michaelsen, K.F. The Influence of Meat on Nonheme Iron Absorption in Infants. Pediatr. Res. 1998, 43, 768–773. [Google Scholar] [CrossRef] [Green Version]
- Brazaca, S.G.C.; da Silva, F.C. Enhancers and inhibitors of iron availability in legumes. Plant Foods Hum. Nutr. 2003, 58, 1–8. [Google Scholar] [CrossRef]
- Baech, S.B.; Hansen, M.; Bukhave, K.; Jensen, M.; Sørensen, S.S.; Kristensen, L.; Purslow, P.P.; Skibsted, L.H.; Sandström, B. Nonheme-iron absorption from a phytate-rich meal is increased by the addition of small amounts of pork meat. Am. J. Clin. Nutr. 2003, 77, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- University of California. What Makes Superfood So Super? Available online: https://www.ucdavis.edu/food/what-makes-superfood-so-super/#:~:text=The%20origin%20of%20the%20term,of%20cheap%2C%20easily%20digestible%20nutrition (accessed on 1 May 2020).
- Proestos, C. Superfoods: Recent Data on their Role in the Prevention of Diseases. Curr. Res. Nutr. Food Sci. J. 2018, 6, 576–593. [Google Scholar] [CrossRef]
- Daugherty, B. Superfoods: The Healthiest Foods on the Planet. J. Nutr. Educ. Behav. 2011, 43, 207.e7. [Google Scholar] [CrossRef]
- Khalesi, M.; Salami, M.; Moslehishad, M.; Winterburn, J.; Moosavi-Movahedi, A.A. Biomolecular content of camel milk: A traditional superfood towards future healthcare industry. Trends Food Sci. Technol. 2017, 62, 49–58. [Google Scholar] [CrossRef]
- Coscia, A.; Bertino, E.; Tonetto, P.; Peila, C.; Cresi, F.; Arslanoglu, S.; Moro, G.E.; Spada, E.; Milani, S.; Giribaldi, M.; et al. Nutritional adequacy of a novel human milk fortifier from donkey milk in feeding preterm infants: Study protocol of a randomized controlled clinical trial. Nutr. J. 2018, 17, 6. [Google Scholar] [CrossRef] [Green Version]
- Spada, V.; Ferranti, P.; Chianese, L.; Salimei, E.; Addeo, F.; Picariello, G. Antibacterial potential of donkey’s milk disclosed by untargeted proteomics. J. Proteom. 2021, 231, 104007. [Google Scholar] [CrossRef]
- Hancock, R.; McDougall, G.; Stewart, D. Berry fruit as ‘superfood’: Hope or hype? Biologist 2007, 54, 73–79. [Google Scholar]
- Conti, M.V.; Campanaro, A.; Coccetti, P.; De Giuseppe, R.; Galimberti, A.; Labra, M.; Cena, H. Potential role of neglected and underutilized plant species in improving women’s empowerment and nutrition in areas of sub-Saharan Africa. Nutr. Rev. 2019, 77, 817–828. [Google Scholar] [CrossRef]
- Scarano, A.; Butelli, E.; De Santis, S.; Cavalcanti, E.; Hill, L.; De Angelis, M.; Giovinazzo, G.; Chieppa, M.; Martin, C.; Santino, A. Combined Dietary Anthocyanins, Flavonols, and Stilbenoids Alleviate Inflammatory Bowel Disease Symptoms in Mice. Front. Nutr. 2018, 4, 75. [Google Scholar] [CrossRef] [Green Version]
- Lentjes, M.A.H. The balance between food and dietary supplements in the general population. Proc. Nutr. Soc. 2019, 78, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Johnson-Wimbley, T.D.; Graham, D.Y. Diagnosis and management of iron deficiency anemia in the 21st century. Therap. Adv. Gastroenterol. 2011, 4, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.A.; Powell, J.J. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: A systematic review and meta-analysis. PLoS ONE 2015, 10, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.W.; Tsai, F.P. Comparison of the Therapeutic Effects and Side Effects of Oral Iron Supplements in Iron Deficiency Anemia. Drug Res. 2016, 66, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Düzen Oflas, N.; Demircioğlu, S.; Yıldırım Doğan, N.; Eker, E.; Kutlucan, A.; Doğan, A.; Aslan, M.; Demir, C. Comparison of the effects of oral iron treatment every day and every other day in female patients with iron deficiency anaemia. Intern. Med. J. 2020, 50, 854–858. [Google Scholar] [CrossRef]
- Hoppe, M.; Önning, G.; Hulthén, L. Freeze-dried Lactobacillus plantarum 299v increases iron absorption in young females—Double isotope sequential single-blind studies in menstruating women. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Rosen, G.M.; Morrissette, S.; Larson, A.; Stading, P.; Griffin, K.H.; Barnes, T.L. Use of a Probiotic to Enhance Iron Absorption in a Randomized Trial of Pediatric Patients Presenting with Iron Deficiency. J. Pediatr. 2019, 207, 192–197.e1. [Google Scholar] [CrossRef]
- Skrypnik, K.; Bogdański, P.l.; Schmidt, M.; Suliburska, J. The Effect of Multispecies Probiotic Supplementation on Iron Status in Rats. Biol. Trace Elem. Res. 2019. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, M.; Önning, G.; Berggren, A.; Hulthén, L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: A double-isotope cross-over single-blind study in women of reproductive age. Br. J. Nutr. 2015, 114, 1195–1202. [Google Scholar] [CrossRef] [Green Version]
- Appenroth, K.-J.; Sree, K.S.; Bog, M.; Ecker, J.; Seeliger, C.; Böhm, V.; Lorkowski, S.; Sommer, K.; Vetter, W.; Tolzin-Banasch, K.; et al. Nutritional Value of the Duckweed Species of the Genus Wolffia (Lemnaceae) as Human Food. Front. Chem. 2018, 6, 483. [Google Scholar] [CrossRef] [Green Version]
- Yaskolka Meir, A.; Tsaban, G.; Zelicha, H.; Rinott, E.; Kaplan, A.; Youngster, I.; Rudich, A.; Shelef, I.; Tirosh, A.; Brikner, D.; et al. A Green-Mediterranean Diet, Supplemented with Mankai Duckweed, Preserves Iron-Homeostasis in Humans and Is Efficient in Reversal of Anemia in Rats. J. Nutr. 2019, 149, 1004–1011. [Google Scholar] [CrossRef]
- Cabrita, A.R.J.; Maia, M.R.G.; Oliveira, H.M.; Sousa-Pinto, I.; Almeida, A.A.; Pinto, E.; Fonseca, A.J.M. Tracing seaweeds as mineral sources for farm-animals. J. Appl. Phycol. 2016, 28, 3135–3150. [Google Scholar] [CrossRef]
- Davis, M.R.; Hester, K.K.; Shawron, K.M.; Lucas, E.A.; Smith, B.J.; Clarke, S.L. Comparisons of the iron deficient metabolic response in rats fed either an AIN-76 or AIN-93 based diet. Nutr. Metab. 2012, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Qu, H.; Gao, Z.; Zeng, D.; Wang, J.; Baranenko, D.; Li, Y.; Lu, W. Protective effects of Ulva pertusa polysaccharide and polysaccharide-iron (III) complex on cyclophosphamide induced immunosuppression in mice. Int. J. Biol. Macromol. 2019, 133, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Pereira, H.; Silva, J.; Santos, T.; Gangadhar, K.N.; Raposo, A.; Nunes, C.; Coimbra, M.A.; Gouveia, L.; Barreira, L.; Varela, J. Nutritional potential and toxicological evaluation of Tetraselmis sp. CtP4 microalgal biomass produced in industrial photobioreactors. Molecules 2019, 24, 3192. [Google Scholar] [CrossRef] [Green Version]
- Shreadah, M.A.; Abd El Moneam, N.M.; El-Assar, S.A.; Nabil-Adam, A. Marine Algae of the Genus Gracilaria as a Multi Products Source for different Biotechnological and Medical Applications. Recent Pat. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Robertson, R.C.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.B.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. The Anti-Inflammatory Effect of Algae-Derived Lipid Extracts on Lipopolysaccharide (LPS)-Stimulated Human THP-1 Macrophages. Mar. Drugs 2015, 13, 5402–5424. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Park, S.Y.; Thapa, D.; Choi, M.K.; Chung, I.M.; Park, Y.J.; Yong, C.S.; Choi, H.G.; Kim, J.A. Grifola frondosa water extract alleviates intestinal inflammation by suppressing TNF-alpha production and its signaling. Exp. Mol. Med. 2010, 42, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Mills, G.L.; Nair, M.G. Cyclooxygenase Inhibitory and Antioxidant Compounds from the Mycelia of the Edible Mushroom Grifola frondosa. J. Agric. Food Chem. 2002, 50, 7581–7585. [Google Scholar] [CrossRef]
- Xu, L.; Meng, Y.; Liu, Y.; Meng, Q.; Zhang, Z.; Li, J.; Lu, Q. A novel iron supplements preparation from Grifola frondosa polysaccharide and assessment of antioxidant, lymphocyte proliferation and complement fixing activities. Int. J. Biol. Macromol. 2018, 108, 1148–1157. [Google Scholar] [CrossRef]
- Wang, D.; Sun, S.-Q.; Wu, W.-Z.; Yang, S.-L.; Tan, J.-M. Characterization of a water-soluble polysaccharide from Boletus edulis and its antitumor and immunomodulatory activities on renal cancer in mice. Carbohydr. Polym. 2014, 105, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, T.; Liu, H.; Fan, H.; Chen, B.; Wang, D.; Zhang, Y.; Sun, F. Preparation and Characterization of a Novel Polysaccharide-Iron(III) Complex in Auricularia auricula Potentially Used as an Iron Supplement. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, M.B.; Armah, S.M. Impact of Iron-Enriched Aspergillus oryzae on Iron Bioavailability, Safety, and Gut Microbiota in Rats. J. Agric. Food Chem. 2018, 66, 6213–6218. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.B.; Armah, S.M.; Stewart, J.W.; O’Brien, K.O. Iron Absorption from Iron-Enriched Aspergillus oryzae Is Similar to Ferrous Sulfate in Healthy Female Subjects. Curr. Dev. Nutr. 2018, 2, nzy004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, U.; Hossain, M.M.; Oba, S. Nutritional and antioxidant components and antioxidant capacity in green morph Amaranthus leafy vegetable. Sci. Rep. 2020, 10, 1336. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Protein, dietary fiber, minerals, antioxidant pigments and phytochemicals, and antioxidant activity in selected red morph Amaranthus leafy vegetable. PLoS ONE 2019, 14, e0222517. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.; Kumar, A.; Tomer, V.; Kumar, V.; Saini, M. Potential of Colocasia leaves in human nutrition: Review on nutritional and phytochemical properties. J. Food Biochem. 2019, 43, e12878. [Google Scholar] [CrossRef]
- Owade, J.O.; Abong, G.; Okoth, M.; Mwang’ombe, A.W. A review of the contribution of cowpea leaves to food and nutrition security in East Africa. Food Sci. Nutr. 2019, 8, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Murray-Kolb, L.E.; Welch, R.; Theil, E.C.; Beard, J.L. Women with low iron stores absorb iron from soybeans. Am. J. Clin. Nutr. 2003, 77, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Tako, E.; Reed, S.; Budiman, J.; Hart, J.; Glahn, R. Higher iron pearl millet (Pennisetum glaucum L.) provides more absorbable iron that is limited by increased polyphenolic content. Nutr. J. 2015, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, Y.; Slamet-Loedin, I.H. Genetic Biofortification to Enrich Rice and Wheat Grain Iron: From Genes to Product. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Khalid, H.; Ayub, M.; Zia-ur-Rehman, M.; Rizwan, M.; Rasul, A.; Anwar-ul-Haq, M. Biofortification of Cereals with Zinc and Iron: Recent Advances and Future Perspectives. Resour. Use Effic. Agric. 2020, 615–646. [Google Scholar] [CrossRef]
- Sant’ Ana, C.T.; Antunes, P.T.; Reis, T.C.D.; Váz-Tostes, M.D.G.; Meira, E.F.; Costa, N.M.B. Bioaccessibility and bioavailability of iron in biofortified germinated cowpea. J. Sci. Food Agric. 2019, 99, 6287–6295. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.; Beyene, G.; Chauhan, R.D.; Gaitán-Solís, E.; Gehan, J.; Butts, P.; Siritunga, D.; Okwuonu, I.; Woll, A.; Jiménez-Aguilar, D.M.; et al. Biofortification of field-grown cassava by engineering expression of an iron transporter and ferritin. Nat. Biotechnol. 2019, 37, 144–151. [Google Scholar] [CrossRef]
- Hurrell, R.F. Iron Biofortified Potatoes; Every Little Bit Helps. J. Nutr. 2020, 150, 3051–3052. [Google Scholar] [CrossRef]
- Jongstra, R.; Mwangi, M.N.; Burgos, G.; Zeder, C.; Low, J.W.; Mzembe, G.; Liria, R.; Penny, M.; Andrade, M.I.; Fairweather-Tait, S.; et al. Iron Absorption from Iron-Biofortified Sweetpotato Is Higher Than Regular Sweetpotato in Malawian Women while Iron Absorption from Regular and Iron-Biofortified Potatoes Is High in Peruvian Women. J. Nutr. 2020, 150, 3094–3102. [Google Scholar] [CrossRef]
- Schultz, M.; Veltkamp, C.; Dieleman, L.A.; Grenther, W.B.; Wyrick, P.B.; Tonkonogy, S.L.; Sartor, R.B. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflamm. Bowel Dis. 2002, 8, 71–80. [Google Scholar] [CrossRef]
- Keubler, L.M.; Buettner, M.; Häger, C.; Bleich, A. A Multihit Model: Colitis Lessons from the Interleukin-10-deficient Mouse. Inflamm. Bowel Dis. 2015, 21, 1967–1975. [Google Scholar] [CrossRef]
- Chacón-Lee, T.L.; González-Mariño, G.E. Microalgae for “Healthy” Foods—Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Niksic, M.; Van, G.; Vrvic, M.; Jakovljevic, D. Polysaccharides of higher fungi: Biological role, structure, and antioxidative activity. Hem. Ind. 2014, 68, 305–320. [Google Scholar] [CrossRef]
- He, H.; Huang, Q.; Liu, C.; Jia, S.; Wang, Y.; An, F.; Song, H. Effectiveness of AOS-iron on iron deficiency anemia in rats. RSC Adv. 2019, 9, 5053–5063. [Google Scholar] [CrossRef] [Green Version]
- Wachtel-Galor, S.; Yuen, J.; Buswell, J.A.; Benzie, I.F.F. Ganoderma lucidum (Lingzhi or Reishi): A Medicinal Mushroom. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Ahmed, H.; Aslam, M. Effect of Ganoderma lucidum (Reishi) on Hematological Parameters in Wistar Rats. Int. J. Med. Res. Health Sci. 2018, 7, 151–157. [Google Scholar]
- Bissinger, R.; Bhuyan, A.A.M.; Qadri, S.M.; Lang, F. Oxidative stress, eryptosis and anemia: A pivotal mechanistic nexus in systemic diseases. FEBS J. 2019, 286, 826–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abate, M.; Pepe, G.; Randino, R.; Pisanti, S.; Basilicata, M.G.; Covelli, V.; Bifulco, M.; Cabri, W.; D’Ursi, A.M.; Campiglia, P.; et al. Ganoderma lucidum Ethanol Extracts Enhance Re-Epithelialization and Prevent Keratinocytes from Free-Radical Injury. Pharmaceuticals 2020, 13, 224. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef] [Green Version]
- Hefferon, K.L. Nutritionally enhanced food crops; progress and perspectives. Int. J. Mol. Sci. 2015, 16, 3895–3914. [Google Scholar] [CrossRef]
- Ang, P.O.J.; Wang, H. Chinese traditional uses of algae in medicine and food. In Proceedings of the Asia-Pacific Conference on Algal Biotechnology, Hong Kong, China, 12–15 October 2006. [Google Scholar]
- Kandale, A.; Meena, A.; Meda, M.; Panda, P.; Babu, A.K. Marine algae: An Introduction, Food value and Medicinal uses. J. Pharm. Res. 2011, 4, 219. [Google Scholar]
- Zhang, J.-J.; Li, Y.; Zhou, T.; Xu, D.; Zhang, P.; Li, S.; Li, H.-B. Bioactivities and Health Benefits of Mushrooms Mainly from China. Molecules 2016, 21, 938. [Google Scholar] [CrossRef] [Green Version]
- Detzel, P.; Wieser, S. Food Fortification for Addressing Iron Deficiency in Filipino Children: Benefits and Cost-Effectiveness. Ann. Nutr. Metab. 2015, 66 (Suppl. 2), 35–42. [Google Scholar] [CrossRef]
- Thi Le, H.; Brouwer, I.D.; Burema, J.; Nguyen, K.C.; Kok, F.J. Efficacy of iron fortification compared to iron supplementation among Vietnamese schoolchildren. Nutr. J. 2006, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Sommella, E.; Ismail, O.H.; Pagano, F.; Pepe, G.; Ostacolo, C.; Mazzoccanti, G.; Russo, M.; Novellino, E.; Gasparrini, F.; Campiglia, P. Development of an improved online comprehensive hydrophilic interaction chromatography × reversed-phase ultra-high-pressure liquid chromatography platform for complex multiclass polyphenolic sample analysis. J. Sep. Sci. 2017, 40, 2188–2197. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Salviati, E.; Merciai, F.; Manfra, M.; Bertamino, A.; Gasparrini, F.; Novellino, E.; Campiglia, P. Online comprehensive hydrophilic interaction chromatography × reversed phase liquid chromatography coupled to mass spectrometry for in depth peptidomic profile of microalgae gastro-intestinal digests. J. Pharm. Biomed. Anal. 2019, 175, 112783. [Google Scholar] [CrossRef]
- Cepeda-Lopez, A.C.; Allende-Labastida, J.; Melse-Boonstra, A.; Osendarp, S.J.; Herter-Aeberli, I.; Moretti, D.; Rodriguez-Lastra, R.; Gonzalez-Salazar, F.; Villalpando, S.; Zimmermann, M.B. The effects of fat loss after bariatric surgery on inflammation, serum hepcidin, and iron absorption: A prospective 6-mo iron stable isotope study. Am. J. Clin. Nutr. 2016, 104, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Santoro, N.; Calabrò, P.; Grandone, A.; Swinkels, D.W.; Perrone, L.; del Giudice, E.M. Effect of body mass index reduction on serum hepcidin levels and iron status in obese children. Int. J. Obes. 2010, 34, 1772–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, K.-X.; Gao, T.-L.; Xu, R.; Cai, J.; Zhang, H.-Q.; Sun, Y.-Y.; Zhong, F.; Ma, A.-G. Quantifying the Effect of Supplementation with Algae and Its Extracts on Glycolipid Metabolism: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 1712. [Google Scholar] [CrossRef] [PubMed]
- Qudus, B.; Aroyehun, A.; Abdul Razak, S.; Palaniveloo, K.; Nagappan, T.; Suraiza Nabila Rahmah, N.; Wee Jin, G.; Chellappan, D.K.; Chellian, J.; Kunnath, A.P. Bioprospecting Cultivated Tropical Green Algae, Caulerpa racemosa: A Perspective on Nutritional Properties, Antioxidative Capacity and Anti-Diabetic Potential. Foods 2020, 9, 1313. [Google Scholar] [CrossRef]
- Lee, S.H.; Min, K.H.; Han, J.S.; Lee, D.H.; Park, D.B.; Jung, W.K.; Park, P.J.; Jeon, B.T.; Kim, S.K.; Jeon, Y.J. Effects of brown alga, Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem. Toxicol. 2012, 50, 575–582. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [Green Version]
- Horton, S. The economics of food fortification. J. Nutr. 2006, 136, 1068–1071. [Google Scholar] [CrossRef]

| Category of Superfood | Beneficial Effects | References |
|---|---|---|
| Probiotics | Increase of iron absorption rates | [158,159,160,161] |
| Algae | Increase of iron absorption rates Increase of hemoglobin levels Antioxidant and anti-inflammatory | [162,163,164,165,166,167,168,169,170] |
| Mushrooms | Easy-to-absorb iron Anti-inflammatory Immunomodulatory | [171,172,173,174,175,176,177] |
| Vegetable leaves | High iron content Anti-inflammatory | [178,179,180,181,182] |
| Fortified crops | Increased iron content Increase of iron absorption rates Anti-inflammatory | [183,184,185,186,187,188,189] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verna, G.; Sila, A.; Liso, M.; Mastronardi, M.; Chieppa, M.; Cena, H.; Campiglia, P. Iron-Enriched Nutritional Supplements for the 2030 Pharmacy Shelves. Nutrients 2021, 13, 378. https://doi.org/10.3390/nu13020378
Verna G, Sila A, Liso M, Mastronardi M, Chieppa M, Cena H, Campiglia P. Iron-Enriched Nutritional Supplements for the 2030 Pharmacy Shelves. Nutrients. 2021; 13(2):378. https://doi.org/10.3390/nu13020378
Chicago/Turabian StyleVerna, Giulio, Annamaria Sila, Marina Liso, Mauro Mastronardi, Marcello Chieppa, Hellas Cena, and Pietro Campiglia. 2021. "Iron-Enriched Nutritional Supplements for the 2030 Pharmacy Shelves" Nutrients 13, no. 2: 378. https://doi.org/10.3390/nu13020378
APA StyleVerna, G., Sila, A., Liso, M., Mastronardi, M., Chieppa, M., Cena, H., & Campiglia, P. (2021). Iron-Enriched Nutritional Supplements for the 2030 Pharmacy Shelves. Nutrients, 13(2), 378. https://doi.org/10.3390/nu13020378