Different Blood Metabolomics Profiles in Infants Consuming a Meat- or Dairy-Based Complementary Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Analysis
2.3. Statistical Approach
3. Results
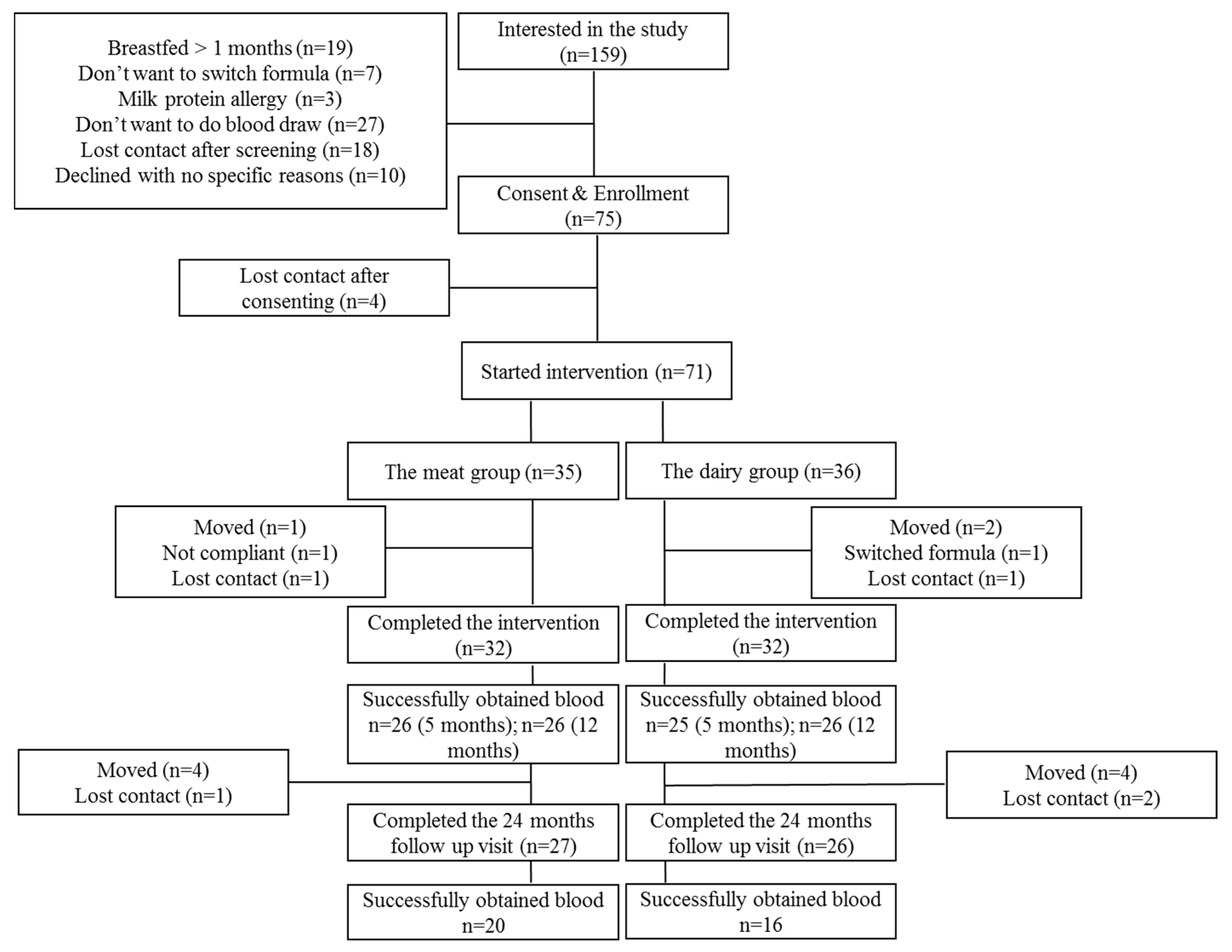
3.1. Subjects
3.2. Essential Amino Acids
3.3. Acylcarnitines & TMAO (Quantitative)
3.4. Untargeted Metabolomics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brisbois, T.D.; Farmer, A.P.; McCargar, L.J. Early markers of adult obesity: A review. Obes. Rev. 2012, 13, 347–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewey, K.G.; Heinig, M.J.; Nommsen, L.A.; Peerson, J.M.; Lonnerdal, B. Growth of breast-fed and formula-fed infants from 0 to 18 months: The DARLING Study. Pediatrics 1992, 89, 1035–1041. [Google Scholar] [PubMed]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; Dobrzanska, A.; et al. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [PubMed] [Green Version]
- Turck, D.; Grillon, C.; Lachambre, E.; Robiliard, P.; Beck, L.; Maurin, J.L.; Kempf, C.; Bernet, J.P.; Marx, J.; Lebrun, F.; et al. Adequacy and safety of an infant formula with a protein/energy ratio of 1.8 g/100 kcal and enhanced protein efficiency for term infants during the first 4 months of life. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 364–371. [Google Scholar] [CrossRef]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellof, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef]
- Roess, A.A.; Jacquier, E.F.; Catellier, D.J.; Carvalho, R.; Lutes, A.C.; Anater, A.S.; Dietz, W.H. Food Consumption Patterns of Infants and Toddlers: Findings from the Feeding Infants and Toddlers Study (FITS) 2016. J. Nutr. 2018, 148, 1525S–1535S. [Google Scholar] [CrossRef] [Green Version]
- Turner, K.M.; Keogh, J.B.; Clifton, P.M. Red meat, dairy, and insulin sensitivity: A randomized crossover intervention study. Am. J. Clin. Nutr. 2015, 101, 1173–1179. [Google Scholar] [CrossRef] [Green Version]
- Rohrmann, S.; Platz, E.A.; Kavanaugh, C.J.; Thuita, L.; Hoffman, S.C.; Helzlsouer, K.J. Meat and dairy consumption and subsequent risk of prostate cancer in a US cohort study. Cancer Causes Control 2007, 18, 41–50. [Google Scholar] [CrossRef]
- Tang, M.; O’Connor, L.E.; Campbell, W.W. Diet-induced weight loss: The effect of dietary protein on bone. J. Acad. Nutr. Diet. 2014, 114, 72–85. [Google Scholar] [CrossRef]
- Tang, M.; Hendricks, A.E.; Krebs, N.F. A meat- or dairy-based complementary diet leads to distinct growth patterns in formula-fed infants: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 107, 734–742. [Google Scholar] [CrossRef]
- Tang, M.; Andersen, V.; Hendricks, A.E.; Krebs, N.F. Different Growth Patterns Persist at 24 Months of Age in Formula-Fed Infants Randomized to Consume a Meat- or Dairy-Based Complementary Diet from 5 to 12 Months of Age. J. Pediatr. 2019, 206, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Kirchberg, F.F.; Harder, U.; Weber, M.; Grote, V.; Demmelmair, H.; Peissner, W.; Rzehak, P.; Xhonneux, A.; Carlier, C.; Ferre, N.; et al. Dietary protein intake affects amino acid and acylcarnitine metabolism in infants aged 6 months. J. Clin. Endocrinol. Metab. 2015, 100, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehrke, S.; Rice, S.; Stefanoni, D.; Wilkerson, R.B.; Nemkov, T.; Reisz, J.A.; Hansen, K.C.; Lucas, A.; Cabrales, P.; Drew, K.; et al. Red Blood Cell Metabolic Responses to Torpor and Arousal in the Hibernator Arctic Ground Squirrel. J. Proteome Res. 2019, 18, 1827–1841. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; D’Alessandro, A.; Hansen, K.C. Three-minute method for amino acid analysis by UHPLC and high-resolution quadrupole orbitrap mass spectrometry. Amino Acids 2015, 47, 2345–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCurdy, C.E.; Schenk, S.; Hetrick, B.; Houck, J.; Drew, B.G.; Kaye, S.; Lashbrook, M.; Bergman, B.C.; Takahashi, D.L.; Dean, T.A.; et al. Maternal obesity reduces oxidative capacity in fetal skeletal muscle of Japanese macaques. JCI Insight 2016, 1, e86612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Klebach, M.; Visser, M.; Hofman, Z. Amino Acid Availability of a Dairy and Vegetable Protein Blend Compared to Single Casein, Whey, Soy, and Pea Proteins: A Double-Blind, Cross-Over Trial. Nutrients 2019, 11, 2613. [Google Scholar] [CrossRef] [Green Version]
- Markova, M.; Pivovarova, O.; Hornemann, S.; Sucher, S.; Frahnow, T.; Wegner, K.; Machann, J.; Petzke, K.J.; Hierholzer, J.; Lichtinghagen, R.; et al. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals With Type 2 Diabetes. Gastroenterology 2017, 152, 571–585. [Google Scholar] [CrossRef] [Green Version]
- Socha, P.; Grote, V.; Gruszfeld, D.; Janas, R.; Demmelmair, H.; Closa-Monasterolo, R.; Subias, J.E.; Scaglioni, S.; Verduci, E.; Dain, E.; et al. Milk protein intake, the metabolic-endocrine response, and growth in infancy: Data from a randomized clinical trial. Am. J. Clin. Nutr. 2011, 94, 1776S–1784S. [Google Scholar]
- Luque, V.; Closa-Monasterolo, R.; Escribano, J.; Ferre, N. Early Programming by Protein Intake: The Effect of Protein on Adiposity Development and the Growth and Functionality of Vital Organs. Nutr. Metab. Insights 2015, 8, 49–56. [Google Scholar] [CrossRef]
- Shimomura, Y.; Yamamoto, Y.; Bajotto, G.; Sato, J.; Murakami, T.; Shimomura, N.; Kobayashi, H.; Mawatari, K. Nutraceutical effects of branched-chain amino acids on skeletal muscle. J. Nutr. 2006, 136, 529S–532S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, A.; MacGregor, A.; Spector, T.; Cassidy, A. Amino Acid Intakes Are Associated With Bone Mineral Density and Prevalence of Low Bone Mass in Women: Evidence from Discordant Monozygotic Twins. J. Bone Miner. Res. 2016, 31, 326–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammann, P.; Laib, A.; Bonjour, J.P.; Meyer, J.M.; Ruegsegger, P.; Rizzoli, R. Dietary essential amino acid supplements increase bone strength by influencing bone mass and bone microarchitecture in ovariectomized adult rats fed an isocaloric low-protein diet. J. Bone Miner. Res. 2002, 17, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Kaimila, Y.; Divala, O.; Agapova, S.E.; Stephenson, K.B.; Thakwalakwa, C.; Trehan, I.; Manary, M.J.; Maleta, K.M. Consumption of Animal-Source Protein is Associated with Improved Height-for-Age z Scores in Rural Malawian Children Aged 12(-)36 Months. Nutrients 2019, 11, 480. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Sheng, X.Y.; Krebs, N.F.; Hambidge, K.M. Meat as complementary food for older breastfed infants and toddlers: A randomized, controlled trial in rural China. Food Nutr. Bull. 2014, 35, S188–S192. [Google Scholar] [CrossRef] [Green Version]
- Millward, D.J.; Layman, D.K.; Tome, D.; Schaafsma, G. Protein quality assessment: Impact of expanding understanding of protein and amino acid needs for optimal health. Am. J. Clin. Nutr. 2008, 87, 1576S–1581S. [Google Scholar] [CrossRef]
- Rousseau, M.; Guenard, F.; Garneau, V.; Allam-Ndoul, B.; Lemieux, S.; Perusse, L.; Vohl, M.C. Associations Between Dietary Protein Sources, Plasma BCAA and Short-Chain Acylcarnitine Levels in Adults. Nutrients 2019, 11, 173. [Google Scholar] [CrossRef] [Green Version]
- Blanton, L.V.; Charbonneau, M.R.; Salih, T.; Barratt, M.J.; Venkatesh, S.; Ilkaveya, O.; Subramanian, S.; Manary, M.J.; Trehan, I.; Jorgensen, J.M.; et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016, 351, 6275. [Google Scholar] [CrossRef] [Green Version]
- Jaeggi, T.; Kortman, G.A.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [Green Version]
- Cheung, W.; Keski-Rahkonen, P.; Assi, N.; Ferrari, P.; Freisling, H.; Rinaldi, S.; Slimani, N.; Zamora-Ros, R.; Rundle, M.; Frost, G.; et al. A metabolomic study of biomarkers of meat and fish intake. Am. J. Clin. Nutr. 2017, 105, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.; DeLany, J.P. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef] [PubMed]
| Meat Group | Dairy Group | p-Value | |
|---|---|---|---|
| Birth weight (kg) | 3.30 ± 0.41 | 3.32 ± 0.39 | 0.45 |
| Maternal BMI 2 | 28 ± 6 | 28 ± 7 | 0.88 |
| 5 months LAZ | −0.19 ± 0.86 | −0.30 ± 1.02 | 0.63 |
| 5 months WAZ | −0.03 ± 0.69 | −0.14 ± 0.82 | 0.59 |
| 5 months WLZ | 0.08 ± 0.79 | 0.04 ± 0.66 | 0.80 |
| 12 months LAZ | 0.14 ± 0.90 | −0.60 ± 0.91 | 0.002 |
| 12 months WAZ | 0.40 ± 0.74 | 0.39 ± 0.78 | 0.99 |
| 12 months WLZ | 0.95 ± 0.90 | 0.33 ± 0.48 | 0.04 |
| 24 months LAZ | 0.19 ± 0.62 | −0.37 ± 0.60 | 0.01 |
| 24 months WAZ | 0.40 ± 0.56 | 0.31 ± 0.89 | 0.65 |
| 24 months WLZ | 0.55 ± 0.99 | 0.34 ± 0.70 | 0.47 |
| Meat Vs. Dairy 2 p-Value | Change over Time 2 p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Amino Acids | Meat 5 m | Dairy 5 m | Meat 12 m | Dairy 12 m | Meat 24 m | Dairy 24 m | 12–5 m Model | 24–12 m Model | 12–5 m Model | 24–12 m Model |
| L_Histidine | 86.7 ± 21.3 | 76.5 ± 12.3 | 94.8 ± 17.02 | 89.4 ± 13.4 | 94.3 ± 13.1 | 81.7 ± 13.3 | 0.8596 | 0.6570 | 0.0500 | 0.0901 |
| L_Leucine | 83.2 ± 33.8 | 74.5 ± 24.2 | 93.4 ± 32.4 | 101.8 ± 47.5 | 66.2 ± 20.0 | 65.0 ± 20.8 | 0.8596 | 0.9979 | 0.1202 | 0.0819 |
| L_Isoleucine | 124.6 ± 44.1 | 113.8 ± 33.2 | 149.7 ± 55.2 | 161.5 ± 68.7 | 111.5 ± 32.8 | 114.5 ± 36.7 | 0.8596 | 0.9979 | 0.0467 | 0.1621 |
| L_Lysine | 169.4 ± 36.1 | 160.3 ± 28.8 | 202.1 ± 61.7 | 200.5 ± 48.4 | 176.8 ± 39.7 | 159.3 ± 36.6 | 0.8596 | 0.9979 | 0.0903 | 0.1133 |
| L_Methionine | 31.9 ± 7.8 | 28.5 ± 5.8 | 32.9 ± 10.1 | 33.3 ± 7.6 | 30.7 ± 9.2 | 27.6 ± 8.6 | 0.8769 | 0.9979 | 0.2812 | 0.2696 |
| L_Phenylalanine | 63.7 ± 12.2 | 57.3 ± 9.5 | 81.6 ± 21.2 | 76.9 ± 15.3 | 84.9 ± 41.7 | 68.4 ± 13.4 | 0.8596 | 0.9979 | 0.0015 | 0.4377 |
| L_Threonine | 164.7 ± 56.4 | 166.6 ± 39.5 | 142.8 ± 46.6 | 147. ± 38.1 | 134.2 ± 39.7 | 120.5 ± 30.1 | 0.7546 | 0.9979 | 0.0098 | 0.2607 |
| Meat Vs. Dairy Change over Time 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acyl Carnitines | Meat 5 m | Dairy 5 m | Meat 12 m | Dairy 12 m | Meat 24 m | Dairy 24 m | 12–5 | 24–12 | 12–5 | 24–12 |
| TMAO | 0.79± 0.99 | 1.46 ± 0.91 | 1.80 ± 1.70 | 1.42 ± 1.15 | 1.36 ± 1.18 | 1.21 ± 0.65 | 0.7546 | 0.9979 | 0.4147 | 0.8000 |
| TMAO (meat only) | 0.0431 | 0.2882 | ||||||||
| Acyl-C8:1 | 0.34 ± 0.14 | 0.31 ± 0.16 | 0.24 ± 0.16 | 0.33 ± 0.15 | 0.14 ± 0.08 | 0.13 ± 0.11 | 0.9064 | 0.9979 | 0.3122 | 0.0901 |
| Acyl-C8 | 0.14 ± 0.06 | 0.12 ± 0.05 | 0.10 ± 0.06 | 0.12 ± 0.07 | 0.07 ± 0.05 | 0.07 ± 0.04 | 0.8596 | 0.9979 | 0.5238 | 0.2112 |
| Acyl- C6 | 0.06 ± 0.02 | 0.05 ± 0.02 | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.8596 | 0.9979 | 0.0903 | 0.0912 |
| Acyl-C5:1 | 0.006 ± 0.003 | 0.006± 0.003 | 0.009± 0.005 | 0.012± 0.005 | 0.011± 0.003 | 0.008 ± 0.01 | 0.8596 | 0.9979 | 0.0040 | 0.5416 |
| Acyl-C5 | 0.13 ± 0.06 | 0.11 ± 0.05 | 0.14 ± 0.10 | 0.16 ± 0.10 | 0.16 ± 0.09 | 0.11 ± 0.05 | 0.8596 | 0.9979 | 0.0136 | 0.1850 |
| Acyl-C4 | 0.21 ± 0.11 | 0.22 ± 0.07 | 0.22 ± 0.11 | 0.24 ± 0.13 | 0.22 ± 0.06 | 0.15 ± 0.05 | 0.7546 | 0.8726 | 0.0467 | 0.0045 |
| Aycl-C3 | 0.59 ± 0.18 | 0.61 ± 0.20 | 0.62 ± 0.26 | 0.68 ± 0.32 | 0.56 ± 0.17 | 0.46 ± 0.17 | 0.9990 | 0.9979 | 0.3122 | 0.1137 |
| Acyl-C2 | 11.92 ± 4.06 | 12.92 ± 3.01 | 9.76 ± 5.76 | 11.41 ± 4.36 | 7.34 ± 2.06 | 7.29 ± 2.20 | 0.8596 | 0.9979 | 0.1427 | 0.0819 |
| Acyl-C10:1 | 0.11 ± 0.06 | 0.12 ± 0.05 | 0.09 ± 0.06 | 0.13 ± 0.07 | 0.04 ± 0.03 | 0.06 ± 0.04 | 0.8596 | 0.9979 | 0.9111 | 0.0986 |
| Acyl-C10 | 0.10 ± 0.09 | 0.12 ± 0.06 | 0.08 ± 0.08 | 0.11 ± 0.10 | 0.05 ± 0.06 | 0.06 ± 0.06 | 0.8596 | 0.9979 | 0.9111 | 0.3996 |
| Acyl-C12 | 0.12 ± 0.13 | 0.15 ± 0.07 | 0.09 ± 0.08 | 0.14 ± 0.11 | 0.06 ± 0.07 | 0.09 ± 0.11 | 0.8596 | 0.9979 | 0.5238 | 0.7337 |
| Acyl-C12:1 | 0.05 ± 0.07 | 0.07 ± 0.05 | 0.05 ± 0.06 | 0.07 ± 0.07 | 0.06 ± 0.06 | 0.08 ± 0.05 | 0.9723 | 0.9979 | 0.9111 | 0.8219 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Weaver, N.E.; Berman, L.M.; Brown, L.D.; Hendricks, A.E.; Krebs, N.F. Different Blood Metabolomics Profiles in Infants Consuming a Meat- or Dairy-Based Complementary Diet. Nutrients 2021, 13, 388. https://doi.org/10.3390/nu13020388
Tang M, Weaver NE, Berman LM, Brown LD, Hendricks AE, Krebs NF. Different Blood Metabolomics Profiles in Infants Consuming a Meat- or Dairy-Based Complementary Diet. Nutrients. 2021; 13(2):388. https://doi.org/10.3390/nu13020388
Chicago/Turabian StyleTang, Minghua, Nicholas E. Weaver, Lillian M. Berman, Laura D. Brown, Audrey E. Hendricks, and Nancy F. Krebs. 2021. "Different Blood Metabolomics Profiles in Infants Consuming a Meat- or Dairy-Based Complementary Diet" Nutrients 13, no. 2: 388. https://doi.org/10.3390/nu13020388
APA StyleTang, M., Weaver, N. E., Berman, L. M., Brown, L. D., Hendricks, A. E., & Krebs, N. F. (2021). Different Blood Metabolomics Profiles in Infants Consuming a Meat- or Dairy-Based Complementary Diet. Nutrients, 13(2), 388. https://doi.org/10.3390/nu13020388






