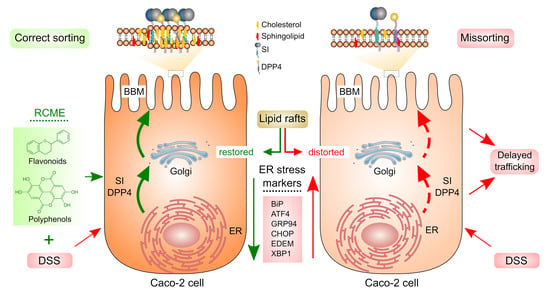
Rosa canina L. Can Restore Endoplasmic Reticulum Alterations, Protein Trafficking and Membrane Integrity in a Dextran Sulfate Sodium-Induced Inflammatory Bowel Disease Phenotype
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. RCME Preparation
2.3. Cytotoxicity Assay
2.4. Total RNA Isolation and Reverse Transcription
2.5. Biosynthetic Labeling, Immunoprecipitation and Deglycosylation of Sucrase-Isomaltase (SI) and Dipeptidyl Peptidase 4 (DDP4)
2.6. Brush Border Membrane (BBM) Preparation
2.7. Preparation of Detergent-Resistant Membranes
2.8. SDS-PAGE, Western Blotting and Fluorography
2.9. Enzymatic Assay
2.10. Statistical Analysis
3. Results
3.1. DSS-Induced ER Stress in Caco-2 Cells Is Corrected by RCME
3.2. DSS-Induced Delayed Trafficking of Intestinal Proteins from the ER to the Golgi Apparatus Is Restored by RCME
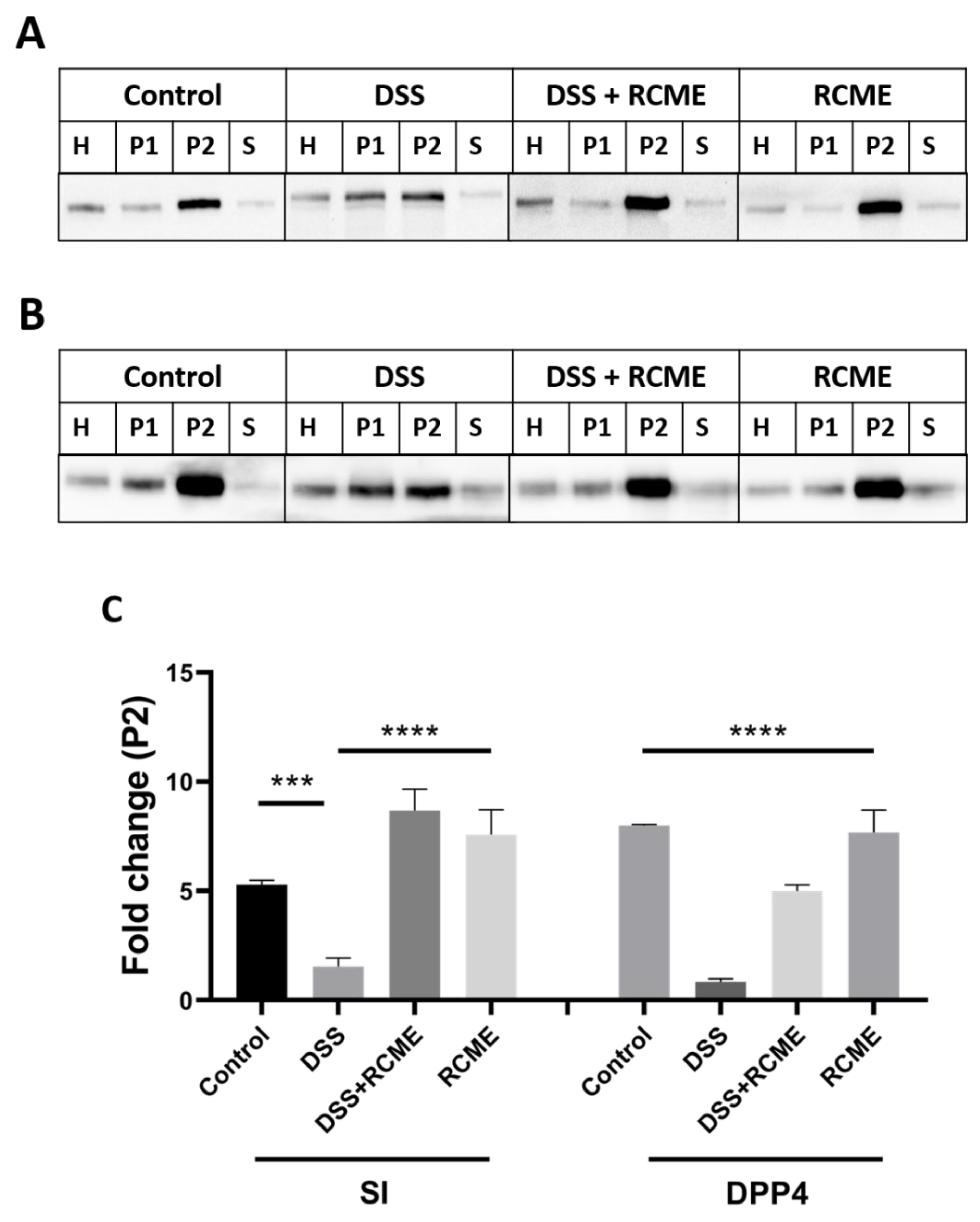
3.3. The Effect of DSS on the Sorting of Intestinal Proteins to the Brush Border Membrane Is Compromised by RCME
3.4. The Distribution of Flotillin 2 in Lipid Rafts Is Restored by RCME
3.5. The Enzymatic Activities of Sucrase-Isomaltase Are Substantially Increased in RCME-Treated Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kilby, K.; Mathias, H.; Boisvenue, L.; Heisler, C.; Jones, J.L. Micronutrient Absorption and Related Outcomes in People with Inflammatory Bowel Disease: A Review. Nutrients 2019, 11, 1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113. [Google Scholar] [PubMed]
- Cao, S.S. Epithelial ER stress in Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2016, 22, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Cao, S.S. Endoplasmic reticulum stress in intestinal epithelial cell function and inflammatory bowel disease. Gastroenterol. Res. Pract. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfalah, M.; Jacob, R.; Preuss, U.; Zimmer, K.-P.; Naim, H.; Naim, H.Y. O-linked glycans mediate apical sorting of human intestinal sucrase-isomaltase through association with lipid rafts. Curr. Biol. 1999, 9, 593–596. [Google Scholar] [CrossRef] [Green Version]
- Kaser, A.; Lee, A.-H.; Franke, A.; Glickman, J.N.; Zeissig, S.; Tilg, H.; Nieuwenhuis, E.E.; Higgins, D.E.; Schreiber, S.; Glimcher, L.H. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008, 134, 743–756. [Google Scholar] [CrossRef] [Green Version]
- Shkoda, A.; Ruiz, P.A.; Daniel, H.; Kim, S.C.; Rogler, G.; Sartor, R.B.; Haller, D. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: Impact on chronic inflammation. Gastroenterology 2007, 132, 190–207. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016. [Google Scholar] [PubMed]
- Toutounji, M.; Wanes, D.; El-Harakeh, M.; El-Sabban, M.; Rizk, S.; Naim, H.Y. Dextran Sodium Sulfate-Induced Impairment of Protein Trafficking and Alterations in Membrane Composition in Intestinal Caco-2 Cell Line. Int. J. Mol. Sci. 2020, 21, 2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutgeerts, P.; Vermeire, S.; Van Assche, G. Biological therapies for inflammatory bowel diseases. Gastroenterology 2009, 136, 1182–1197. [Google Scholar] [CrossRef]
- Jw, M.; Wang, Y.; Dj, T.; Jk, M.; Bg, F. Methotrexate for induction of remission in refractory Crohn’s disease. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [Green Version]
- Sartor, R.B. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: Antibiotics, probiotics, and prebiotics. Gastroenterology 2004, 126, 1620–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, L.; de Chambrun, G.P.; De Vroey, B.; Lavogiez, C.; Delaporte, E.; Colombel, J.-F.; Cortot, A. Stevens–Johnson syndrome with sulfasalazine treatment: Report of two cases. J. Crohns Colitis 2011, 5, 457–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantinidou, V.; Covas, M.-I.; Muñοz-Aguayo, D.; Khymenets, O.; de la Torre, R.; Saez, G.; Tormos, M.d.C.; Toledo, E.; Marti, A.; Ruiz-Gutiérrez, V. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: A randomized controlled trial. FASEB J. 2010, 24, 2546–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Fidalgo, S.; Cárdeno, A.; Sánchez-Hidalgo, M.; Aparicio-Soto, M.; de la Lastra, C.A. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J. Nutr. Biochem. 2013, 24, 1401–1413. [Google Scholar] [CrossRef]
- Wanes, D.; Jabri, M.-A.; Tounsi, H.; Rtibi, K.; Zouari, N.; Hajji, N.; Jridi, M.; Abdellaoui, A.; Sebai, H. Chemical Characterization of Bioactive Components of Rosa canina Extract and Its Protective Effect on Dextran Sulfate Sodium-Induced Intestinal Bowel Disease in a Mouse Model. J. Med. Food 2020, 23, 1109–1119. [Google Scholar] [CrossRef]
- Ercisli, S. Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem. 2007, 104, 1379–1384. [Google Scholar] [CrossRef]
- Wissemann, V.; Gallenmüller, F.; Ritz, C.; Steinbrecher, T.; Speck, T. Inheritance of growth form and mechanical characters in reciprocal polyploid hybrids of Rosa section Caninae—implications for the ecological niche differentiation and radiation process of hybrid offspring. Trees 2006, 20, 340–347. [Google Scholar] [CrossRef]
- Chrubasik, C.; Roufogalis, B.D.; Müller-Ladner, U.; Chrubasik, S. A systematic review on the Rosa canina effect and efficacy profiles. Phytother. Res. Int. J. 2008, 22, 725–733. [Google Scholar]
- Orhan, D.D.; Hartevioğlu, A.; Küpeli, E.; Yesilada, E. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits. J. Ethnopharmacol. 2007, 112, 394–400. [Google Scholar] [CrossRef]
- Gürbüz, I.; Üstün, O.; Yesilada, E.; Sezik, E.; Kutsal, O. Anti-ulcerogenic activity of some plants used as folk remedy in Turkey. J. Ethnopharmacol. 2003, 88, 93–97. [Google Scholar] [CrossRef]
- Mandade, R.J.; Choudhury, A.; Harsulkar, A.; Wakade, R. Role of the Rosa canina L. leaf extract as an antidiarrheal drug in rodents. Indian J. Pharmacol. 2011, 43, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Javanmard, M.; Asadi-Gharneh, H.A.; Nikneshan, P. Characterization of biochemical traits of dog rose (Rosa canina L.) ecotypes in the central part of Iran. Nat. Prod. Res. 2017, 32, 1738–1743. [Google Scholar] [CrossRef] [PubMed]
- Marone, M.; Mozzetti, S.; De Ritis, D.; Pierelli, L.; Scambia, G. Semiquantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biol. Proced. Online 2001, 3, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauri, H.P.; Sterchi, E.; Bienz, D.; Fransen, J.A.; Marxer, A. Expression and Intracellular Transport of Microvillus Hydrolases in Human Intestinal Epithelial Cells. J. Cell Biol. 1985, 101, 838–851. [Google Scholar] [CrossRef] [Green Version]
- Naim, H.Y.; Sterchi, E.; Lentze, M. Biosynthesis of the human sucrase-isomaltase complex. Differential O-glycosylation of the sucrase subunit correlates with its position within the enzyme complex. J. Biol. Chem. 1988, 263, 7242–7253. [Google Scholar] [CrossRef]
- Naim, H.; Sterchi, E.; Lentze, M. Biosynthesis and maturation of lactase-phlorizin hydrolase in the human small intestinal epithelial cells. Biochem. J. 1987, 241, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, J.; Preiser, H.; Maestracci, D.; Ghosh, B.; Cerda, J.; Crane, R. Purification of the human intestinal brush border membrane. Acta BBA Biomembr. 1973, 323, 98–112. [Google Scholar] [CrossRef]
- Sterchi, E.E.; Woodley, J.F. Peptide hydrolases of the human small intestinal mucosa: Identification of six distinct enzymes in the brush border membrane. Clin. Chim. Acta 1980, 102, 57–65. [Google Scholar] [CrossRef]
- Shimada, Y.; Inomata, M.; Suzuki, H.; Hayashi, M.; Abdul Waheed, A.; Ohno-Iwashita, Y. Separation of a cholesterol-enriched microdomain involved in T-cell signal transduction. FEBS J. 2005, 272, 5454–5463. [Google Scholar] [CrossRef]
- Alfalah, M.; Wetzel, G.; Fischer, I.; Busche, R.; Sterchi, E.E.; Zimmer, K.-P.; Sallmann, H.-P.; Naim, H.Y. A novel type of detergent-resistant membranes may contribute to an early protein sorting event in epithelial cells. J. Biol. Chem. 2005, 280, 42636–42643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Naim, H.; Roth, J.; Sterchi, E.; Lentze, M.; Milla, P.; Schmitz, J.; Hauri, H. Sucrase-isomaltase deficiency in humans. Different mutations disrupt intracellular transport, processing, and function of an intestinal brush border enzyme. J. Clin. Investig. 1988, 82, 667–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlqvist, A. Assay of intestinal disaccharidases. Anal. Biochem. 1968, 22, 99–107. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Coleman, O.I.; Haller, D. ER stress and the UPR in shaping intestinal tissue homeostasis and immunity. Front. Immunol. 2019, 10, 2825. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 2002, 3, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Yoshida, H.; Kokame, K.; Kaufman, R.J.; Mori, K. Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J. Biochem. 2004, 136, 343–350. [Google Scholar] [CrossRef]
- Alfalah, M.; Jacob, R.; Naim, H.Y. Intestinal Dipeptidyl Peptidase IV Is Efficiently Sorted to the Apical Membrane through the Concerted Action of N-andO-Glycans as Well as Association with Lipid Microdomains. J. Biol. Chem. 2002, 277, 10683–10690. [Google Scholar] [CrossRef] [Green Version]
- Langhorst, M.F.; Reuter, A.; Stuermer, C. Scaffolding microdomains and beyond: The function of reggie/flotillin proteins. Cell. Mol. Life Sci. 2005, 62, 2228–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetzel, G.; Heine, M.; Rohwedder, A.; Naim, H.Y. Impact of glycosylation and detergent-resistant membranes on the function of intestinal sucrase-isomaltase. Biol. Chem. 2009, 390, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Suganya, N.; Bhakkiyalakshmi, E.; Suriyanarayanan, S.; Paulmurugan, R.; Ramkumar, K. Quercetin ameliorates tunicamycin-induced endoplasmic reticulum stress in endothelial cells. Cell Prolif. 2014, 47, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wen, D.; Wang, F.; Wang, C.; Yang, L. Curcumin protects against palmitic acid-induced apoptosis via the inhibition of endoplasmic reticulum stress in testicular Leydig cells. Reprod. Biol. Endocrinol. 2019, 17, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Meng, X.-B.; Yu, Y.-L.; Sun, G.-B.; Xu, X.-D.; Zhang, X.-P.; Dong, X.; Ye, J.-X.; Xu, H.-B.; Sun, Y.-F. Elatoside C protects against hypoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes through the reduction of endoplasmic reticulum stress partially depending on STAT3 activation. Apoptosis 2014, 19, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.D.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007, 9, 2277–2294. [Google Scholar] [CrossRef] [Green Version]
- Carrara, M.; Prischi, F.; Nowak, P.R.; Kopp, M.C.; Ali, M.M. Noncanonical binding of BiP ATPase domain to Ire1 and Perk is dissociated by unfolded protein CH1 to initiate ER stress signaling. eLife 2015, 4, e03522. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; He, J.; Xie, H.; Yang, Y.; Li, J.; Zou, Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2014, 93, 54–62. [Google Scholar] [CrossRef]
- Roussou, I.; Lambropoulos, I.; Pagoulatos, G.N.; Fotsis, T.; Roussis, I.G. Decrease of heat shock protein levels and cell populations by wine phenolic extracts. J. Agric. Food Chem. 2004, 52, 1017–1024. [Google Scholar] [CrossRef]
- Yoon, T.; Kang, G.-Y.; Han, A.-R.; Seo, E.-K.; Lee, Y.-S. 2, 4-Bis (4-hydroxybenzyl) phenol inhibits heat shock transcription factor 1 and sensitizes lung cancer cells to conventional anticancer modalities. J. Nat. Prod. 2014, 77, 1123–1129. [Google Scholar] [CrossRef]
- Leonard, A.; Grose, V.; Paton, A.W.; Paton, J.C.; Yule, D.I.; Rahman, A.; Fazal, F. Selective inactivation of intracellular BiP/GRP78 attenuates endothelial inflammation and permeability in acute lung injury. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braakman, I.; Bulleid, N.J. Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 2011, 80, 71–99. [Google Scholar] [CrossRef] [PubMed]
- Pröpsting, M.J.; Jacob, R.; Naim, H.Y. A glutamine to proline exchange at amino acid residue 1098 in sucrase causes a temperature-sensitive arrest of sucrase-isomaltase in the endoplasmic reticulum and cis-Golgi. J. Biol. Chem. 2003, 278, 16310–16314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choy, K.W.; Murugan, D.; Mustafa, M.R. Natural products targeting ER stress pathway for the treatment of cardiovascular diseases. Pharmacol. Res. 2018, 132, 119–129. [Google Scholar] [CrossRef]
- Dauer, P.; Sharma, N.S.; Gupta, V.K.; Durden, B.; Hadad, R.; Banerjee, S.; Dudeja, V.; Saluja, A.; Banerjee, S. ER stress sensor, glucose regulatory protein 78 (GRP78) regulates redox status in pancreatic cancer thereby maintaining “stemness”. Cell Death Dis. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Naim, H.Y.; Joberty, G.; Alfalah, M.; Jacob, R. Temporal Association of the N-andO-Linked Glycosylation Events and Their Implication in the Polarized Sorting of Intestinal Brush Border Sucrase-Isomaltase, Aminopeptidase N, and Dipeptidyl Peptidase IV. J. Biol. Chem. 1999, 274, 17961–17967. [Google Scholar] [CrossRef] [Green Version]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Zahradnik-Bilska, J.; Brzozowski, B.; Magierowski, M.; Mach, T.; Magierowska, K.; Brzozowski, T. The role of intestinal alkaline phosphatase in inflammatory disorders of gastrointestinal tract. Mediat. Inflamm. 2017, 2017, 9074601. [Google Scholar] [CrossRef]
- Lackeyram, D.; Mine, Y.; Archbold, T.; Fan, M. The small intestinal apical hydrolase activities are decreased in the piglet with bowel inflammation induced by dextran sodium sulfate. J. Anim. Sci. 2012, 90, 287–289. [Google Scholar] [CrossRef]
- Brogden, G.; Shammas, H.; Walters, F.; Maalouf, K.; Das, A.M.; Naim, H.Y.; Rizk, S. Different Trafficking Phenotypes of Niemann-Pick C1 Gene Mutations Correlate with Various Alterations in Lipid Storage, Membrane Composition and Miglustat Amenability. Int. J. Mol. Sci. 2020, 21, 2101. [Google Scholar] [CrossRef] [Green Version]
- Ashokkumar, P.; Sudhandiran, G. Protective role of luteolin on the status of lipid peroxidation and antioxidant defense against azoxymethane-induced experimental colon carcinogenesis. Biomed. Pharmacother. 2008, 62, 590–597. [Google Scholar] [CrossRef]
- Gomes, L.; Sorgine, M.; Passos, C.L.A.; Ferreira, C.; de Andrade, I.R.; Silva, J.L.; Atella, G.C.; Mermelstein, C.S.; Fialho, E. Increase in fatty acids and flotillins upon resveratrol treatment of human breast cancer cells. Sci. Rep. 2019, 9, 13960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, K.L.; Soto-Pantoja, D.R.; Clarke, P.A.; Cruz, M.I.; Zwart, A.; Wärri, A.; Hilakivi-Clarke, L.; Roberts, D.D.; Clarke, R. Endoplasmic reticulum stress protein GRP78 modulates lipid metabolism to control drug sensitivity and antitumor immunity in breast cancer. Cancer Res. 2016, 76, 5657–5670. [Google Scholar] [CrossRef] [Green Version]
- Jacob, R.; Naim, H.Y. Apical membrane proteins are transported in distinct vesicular carriers. Curr. Biol. 2001, 11, 1444–1450. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.-L.; Wang, S.-G.; Chan, W.-L.; Lee, C.-H.; Wu, T.-S.; Lin, M.-L.; Chen, S.-S. Impairment of Membrane Lipid Homeostasis by Bichalcone Analog TSWU-BR4 Attenuates Function of GRP78 in Regulation of the Oxidative Balance and Invasion of Cancer Cells. Cells 2020, 9, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Primer | Sequence |
|---|---|
| ATF4 fw ATF4 rev | GTTCTCCAGCGACAAGGCTA ATCCTGCTTGCTGTTGTTGG |
| BiP fw BiP rev | TGTTCAACCAATTATCAGCAAACTC TTCTGCTGTATCCTCTTCACCAGT |
| CHOP fw CHOP rev | AGAACCAGGAAACGGAAACAGA TCTCCTTCATGCGCTGCTTT |
| EDEM fw EDEM rev | CAAGTGTGGGTACGCCACG AAAGAAGCTCTCCATCCGGTC |
| Grp94 fw Grp94 rev | GAAACGGATGCCTGGTGG GCCCCTTCTTCCTGGGTC |
| XBP1 fw XBP1 rev | TGGCCGGGTCTGCTGAGTCCG ATCCATGGGGAGATGTTCTGG |
| GAPDH Fw GAPDH rev | CATGGCCTTCCGTGTTCCTA CCTGCTTCACCACCTTCTTGAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanes, D.; Toutounji, M.; Sebai, H.; Rizk, S.; Naim, H.Y. Rosa canina L. Can Restore Endoplasmic Reticulum Alterations, Protein Trafficking and Membrane Integrity in a Dextran Sulfate Sodium-Induced Inflammatory Bowel Disease Phenotype. Nutrients 2021, 13, 441. https://doi.org/10.3390/nu13020441
Wanes D, Toutounji M, Sebai H, Rizk S, Naim HY. Rosa canina L. Can Restore Endoplasmic Reticulum Alterations, Protein Trafficking and Membrane Integrity in a Dextran Sulfate Sodium-Induced Inflammatory Bowel Disease Phenotype. Nutrients. 2021; 13(2):441. https://doi.org/10.3390/nu13020441
Chicago/Turabian StyleWanes, Dalanda, Mohamad Toutounji, Hichem Sebai, Sandra Rizk, and Hassan Y. Naim. 2021. "Rosa canina L. Can Restore Endoplasmic Reticulum Alterations, Protein Trafficking and Membrane Integrity in a Dextran Sulfate Sodium-Induced Inflammatory Bowel Disease Phenotype" Nutrients 13, no. 2: 441. https://doi.org/10.3390/nu13020441
APA StyleWanes, D., Toutounji, M., Sebai, H., Rizk, S., & Naim, H. Y. (2021). Rosa canina L. Can Restore Endoplasmic Reticulum Alterations, Protein Trafficking and Membrane Integrity in a Dextran Sulfate Sodium-Induced Inflammatory Bowel Disease Phenotype. Nutrients, 13(2), 441. https://doi.org/10.3390/nu13020441