Impact of Maternal Food Restriction on Heart Proteome in Appropriately Grown and Growth-Restricted Wistar—Rat Offspring
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Proteomic Analysis
2.2.1. Quantitative Proteomics Sample Processing
2.2.2. Database Searching
2.2.3. Bioinformatics Analysis
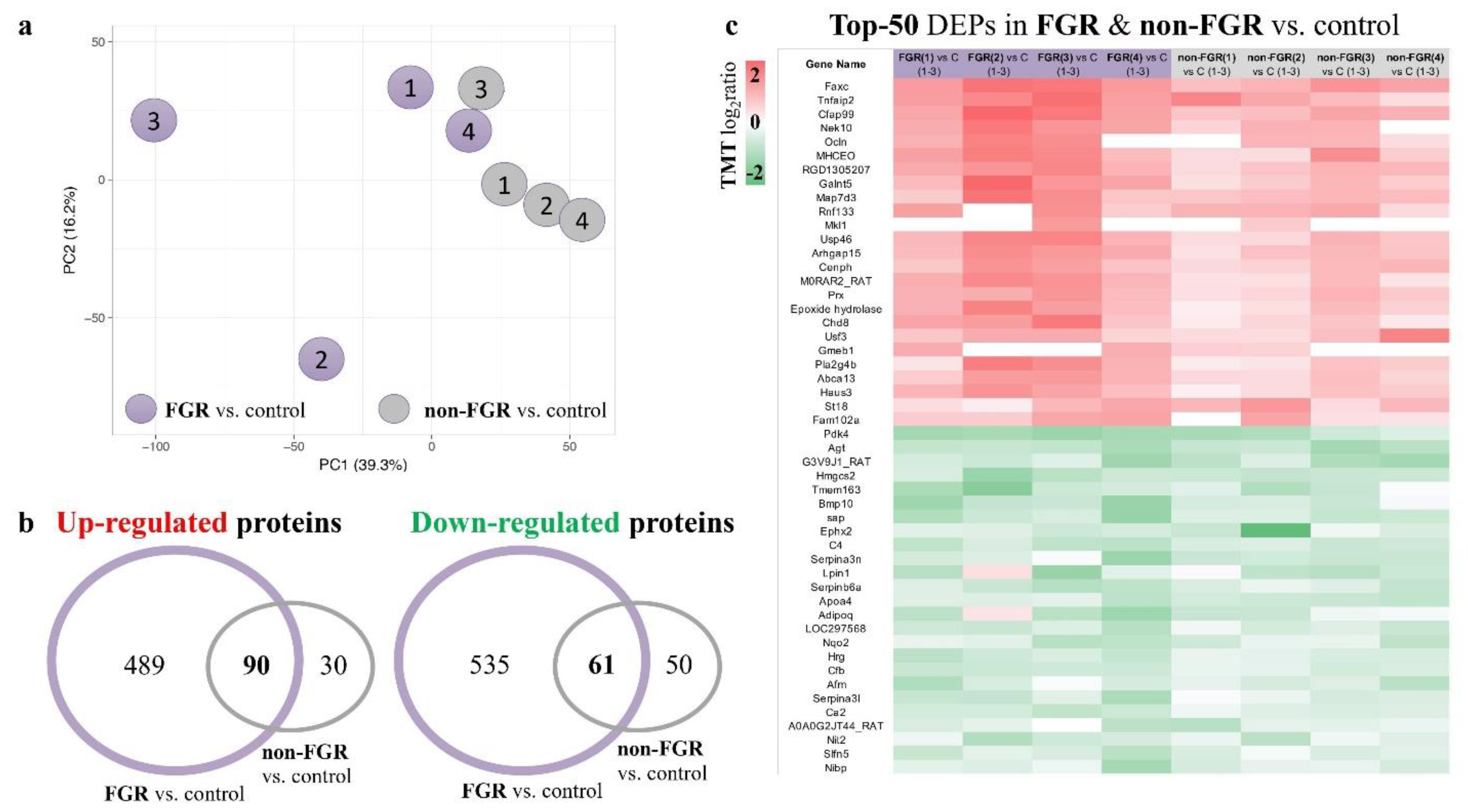
3. Results
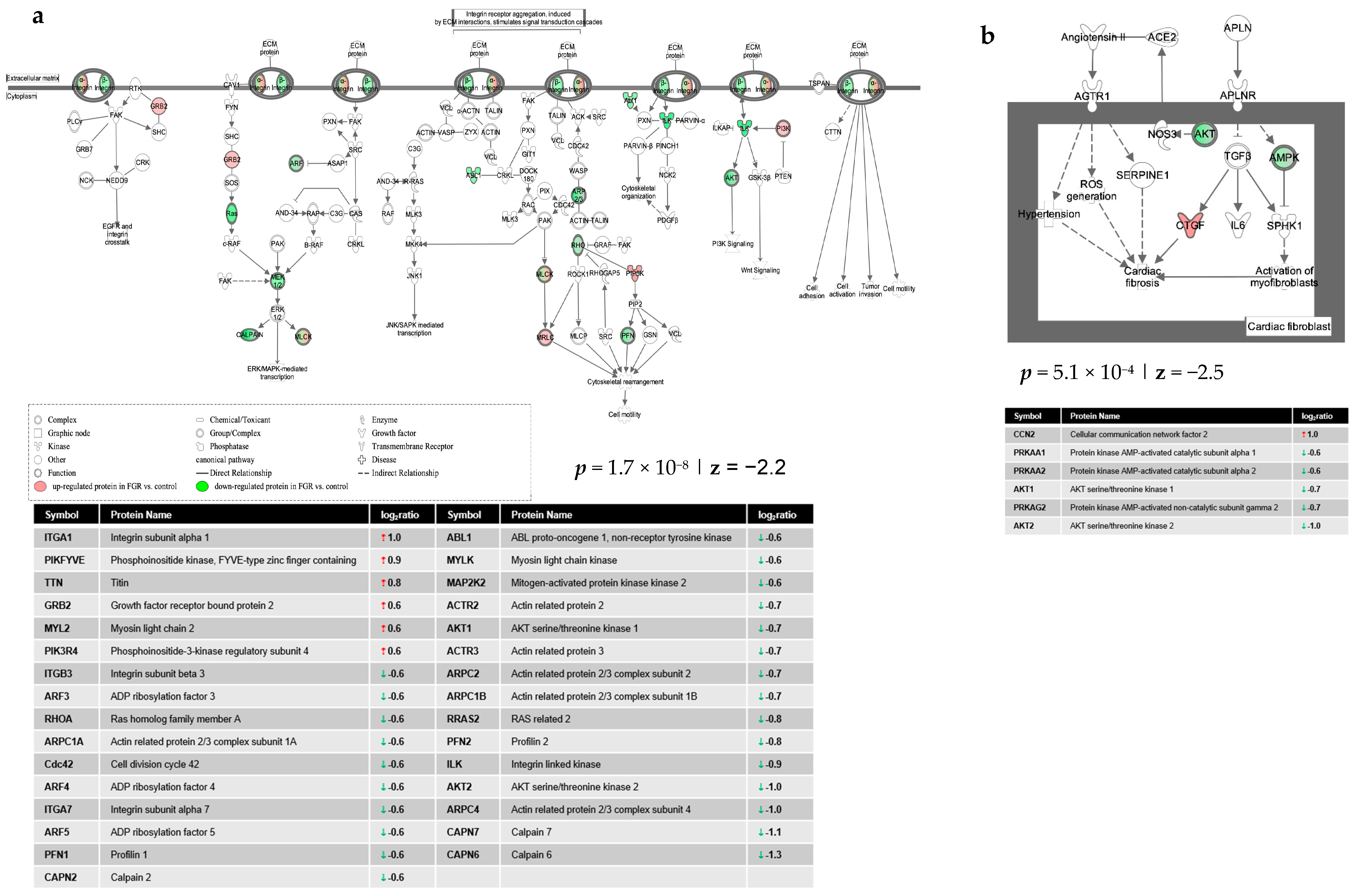
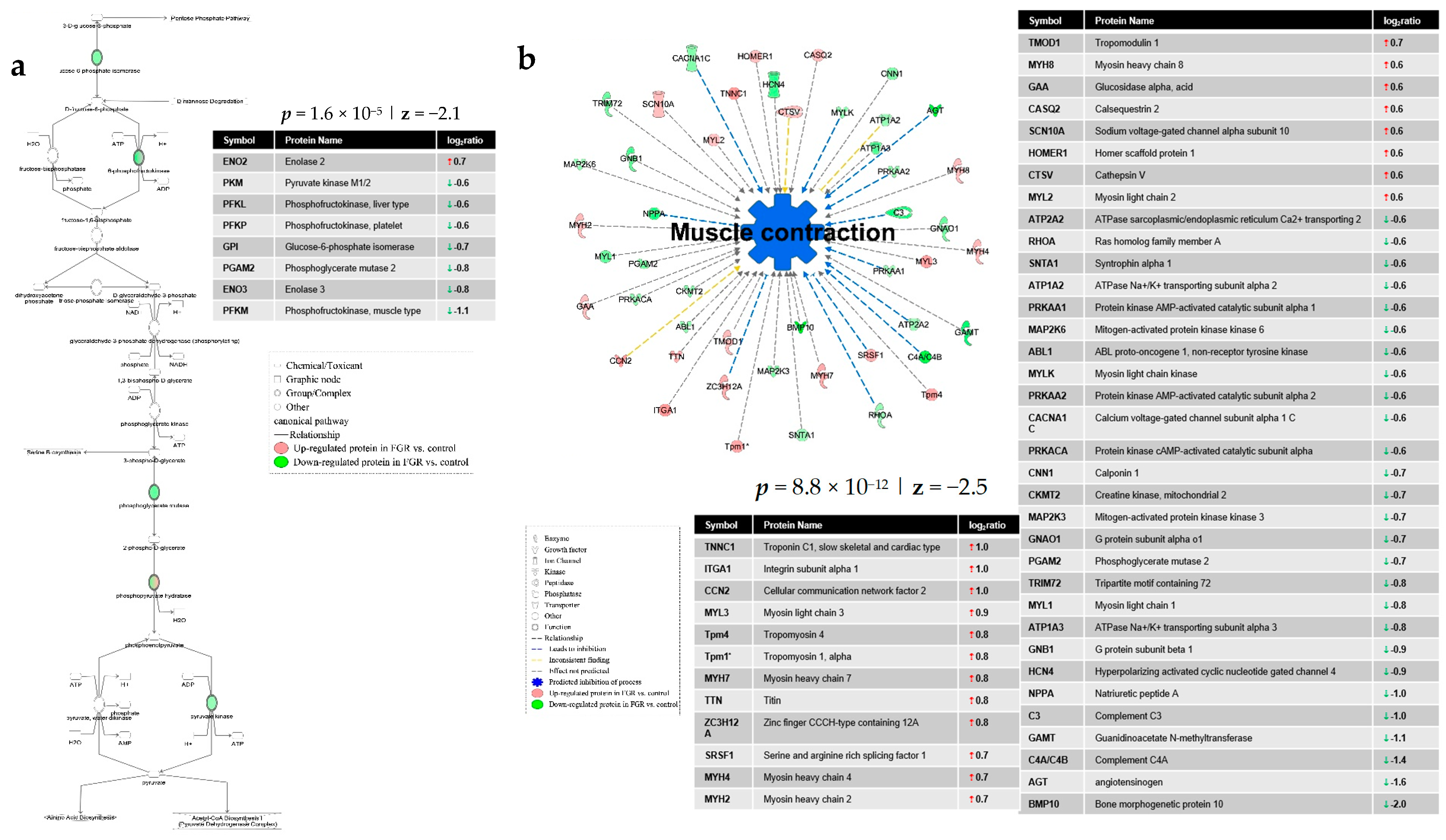
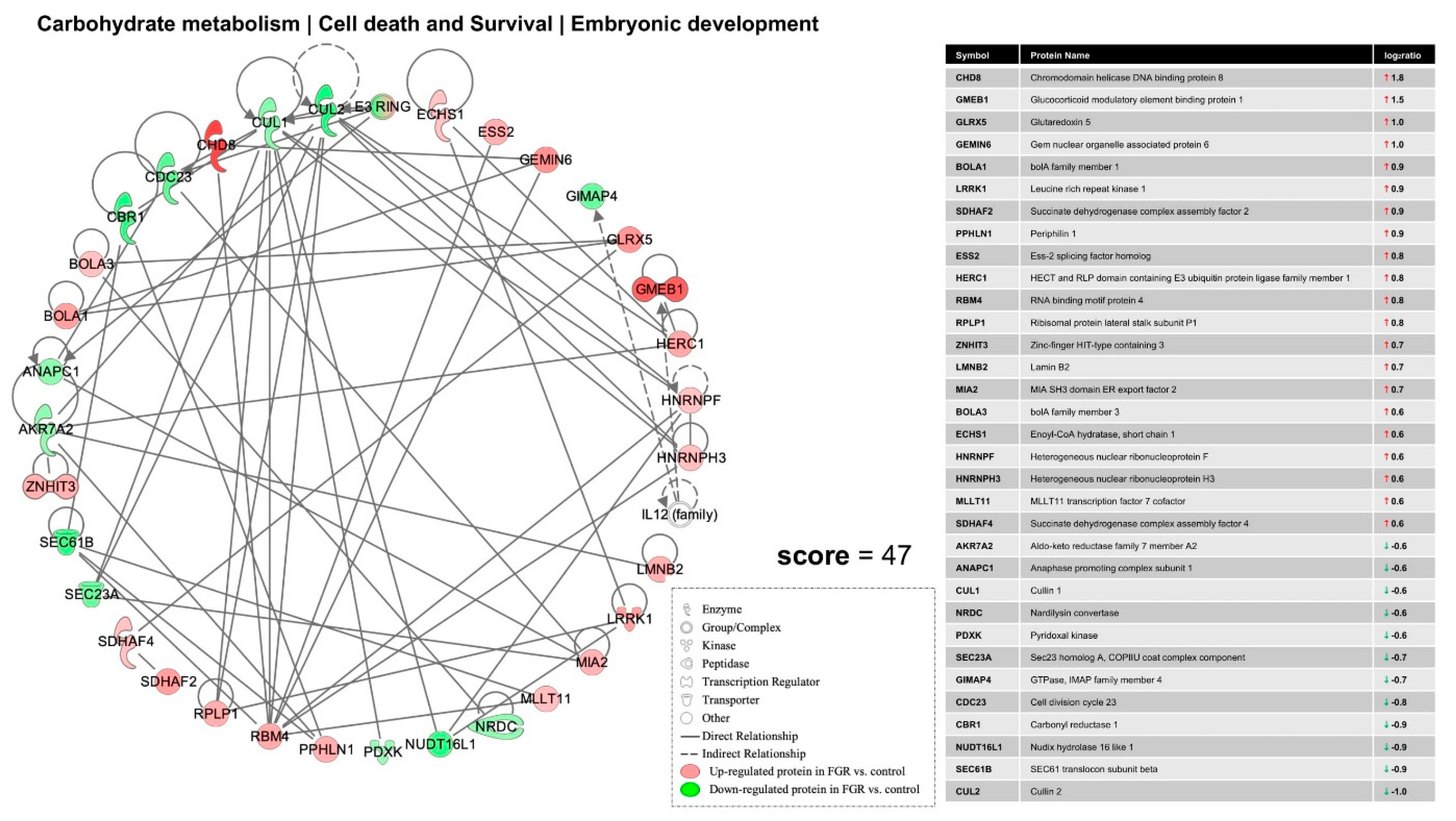
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, D. Infant mortality, childhood nutrition, and ischaemic heart disease in england and wales. Lancet 1986, 327, 1077–1081. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C.; Golding, J.; Kuh, D.; Wadsworth, M.E. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 1989, 298, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Barker, D. The fetal and infant origins of adult disease the womb may be more important than the home. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.J.; Kim, Y.J. What is fetal programming? A lifetime health is under the control of in utero health. Obstet. Gynecol. Sci. 2017, 60, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Belkacemi, L.; Nelson, D.M.; Desai, M.; Ross, M.G. Maternal Undernutrition Influences Placental-Fetal Development. Biol. Reprod. 2010, 83, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Vuguin, P.M. Animal models for small for gestational age and fetal programming of adult disease. Horm. Res. 2007, 68, 113–123. [Google Scholar] [CrossRef]
- Stein, Z.; Susser, M. The Dutch Famine, 1944-1945, and the Reproductive Process. II. Interrelations of Caloric Rations and Six Indices at Birth. Pediatr. Res. 1975, 9, 76–83. [Google Scholar] [CrossRef]
- Pasternak, Y.; Weintraub, A.Y.; Shoham-Vardi, I.; Sergienko, R.; Guez, J.; Wiznitzer, A.; Shalev, H.; Sheiner, E. Obstetric and Perinatal Outcomes in Women with Eating Disorders. J. Womens Health 2012, 21, 61–65. [Google Scholar] [CrossRef]
- Royal College of Obstetricians and Gynaecologists. Green-Top Guideline 31: The Investigation and Manangement of the Small-for-Gestational-Age Fetus. Available online: https://www.rcog.org.uk/globalassets/documents/guidelines/gtg_31.pdf (accessed on 5 October 2020).
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Lees, C.C.; Lees, C.; Baschat, A.A.; Costa, F.D.S.; Ferrazzi, E.M.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Stampfer, M.J.; Manson, E.J.; Rosner, B.; Hankinson, E.S.; Colditz, A.G.; Hennekens, C.H.; Willet, W.C. Birth weight and risk of cardiovascular disease in a cohort of women followed up since. BMJ 1997, 315, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Leon, D.A.; Lithell, O.H.; Vågerö, D.; Koupilová, I.; Mohsen, R.; Berglund, L.; Lithell, U.-B.; McKeigue, P.M. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: Cohort study of 15 000 Swedish men and women born 1915–1929. BMJ 1998, 317, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Ronalds, G.; Clark, H.; Smith, G.D.; Leon, D.A. Birth Weight Is Inversely Associated With Incident Coronary Heart Disease and Stroke among Individuals Born in the 1950s. Circulation 2005, 112, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Lawani, O.S.; Demerath, E.W.; Norby, F.L.; Soliman, E.Z.; Huxley, R.R.; Rose, K.M.; Alonso, A. Birth weight and the risk of atrial fibrillation in whites and African Americans: The Atherosclerosis Risk In Communities (ARIC) study. BMC Cardiovasc. Disord. 2014, 14, 69. [Google Scholar] [CrossRef]
- Larsson, S.C.; Drca, N.; Jensen-Urstad, M.; Wolk, A. Incidence of atrial fibrillation in relation to birth weight and preterm birth. Int. J. Cardiol. 2015, 178, 149–152. [Google Scholar] [CrossRef]
- Tekola-Ayele, F.; Workalemahu, T.; Gorfu, G.; Shrestha, D.; Tycko, B.; Wapner, R.; Zhang, C.; Louis, G.M.B. Sex differences in the associations of placental epigenetic aging with fetal growth. Aging 2019, 11, 5412–5432. [Google Scholar] [CrossRef]
- Davis, G.K.; Newsome, A.D.; Ojeda, N.B.; Alexander, B.T. Effects of Intrauterine Growth Restriction and Female Sex on Future Blood Pressure and Cardiovascular Disease. Curr. Hypertens. Rep. 2017, 19, 13. [Google Scholar] [CrossRef]
- Crispi, F.; Miranda, J.; Gratacós, E. Long-term cardiovascular consequences of fetal growth restriction: Biology, clinical implications, and opportunities for prevention of adult disease. Am. J. Obstet. Gynecol. 2018, 218, S869–S879. [Google Scholar] [CrossRef]
- Cohen, E.; Wong, F.; Horne, R.S.; Yiallourou, S.R. Intrauterine growth restriction: Impact on cardiovascular development and function throughout infancy. Pediatr. Res. 2016, 79, 821–830. [Google Scholar] [CrossRef]
- Swanson, A.; David, A.L. Animal models of fetal growth restriction: Considerations for translational medicine. Placenta 2015, 36, 623–630. [Google Scholar] [CrossRef]
- Lane-Petter, W. Cannibalism in rats and mice. Proc. R. Soc. Med. 1968, 61, 1295–1296. [Google Scholar] [PubMed]
- Huss, M.K.; Chum, H.H.; Chang, A.G.; Jampachairsi, K.; Pacharinsak, C. The Physiologic Effects of Isoflurane, Sevoflurane, and Hypothermia Used for Anesthesia in Neonatal Rats (Rattus norvegicus). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 83–88. [Google Scholar] [PubMed]
- Eleftheriades, M.; Pervanidou, P.; Vafaei, H.; Vaggos, G.; Dontas, A.I.; Skenderi, K.; Sebire, N.J.; Nicolaides, K. Metabolic profiles of adult Wistar rats in relation to prenatal and postnatal nutritional manipulation: The role of birthweight. Hormones 2014, 13, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriades, M.; Vafaei, H.; Dontas, A.I.; Vaggos, G.; Marinou, K.; Pervanidou, P.; Sebire, N.J.; Chrousos, G.P.; Nicolaides, K.H. Assessment of body composition in Wistar rat offspring by DXA in relation to prenatal and postnatal nutritional manipulation. Pediatr. Res. 2016, 80, 319–325. [Google Scholar] [CrossRef]
- Aravidou, E.; Eleftheriades, M.; Malamitsi-Puchner, A.; Anagnostopoulos, A.K.; Aravantinos, L.; Dontas, A.I.; Aravidis, C.; Creatsas, G.; Tsangaris, G.; Chrousos, G.P. Protein expression in the brain of rat offspring in relation to prenatal caloric restriction. J. Matern. Neonatal Med. 2015, 29, 1–8. [Google Scholar] [CrossRef]
- Billiard, F.; Karaliota, S.; Wang, B.; Stellas, D.; Serafimidis, I.; Manousopoulou, A.; Koutmani, Y.; Ninou, E.; Golubov, J.; DaNave, A.; et al. Delta-like Ligand-4-Notch Signaling Inhibition Regulates Pancreatic Islet Function and Insulin Secretion. Cell Rep. 2018, 22, 895–904. [Google Scholar] [CrossRef]
- Morianos, I.; Trochoutsou, A.I.; Papadopoulou, G.; Semitekolou, M.; Banos, A.; Konstantopoulos, D.; Manousopoulou, A.; Kapasa, M.; Wei, P.; Lomenick, B.; et al. Activin-A limits Th17 pathogenicity and autoimmune neuroinflammation via CD39 and CD73 ectonucleotidases and Hif1-α–dependent pathways. Proc. Natl. Acad. Sci. USA 2020, 117, 12269–12280. [Google Scholar] [CrossRef]
- Manousopoulou, A.; Hayden, A.; Mellone, M.; Garay-Baquero, D.J.; White, C.H.; Noble, F.; Lopez, M.; Thomas, G.J.; Underwood, T.J.; Garbis, S.D. Quantitative proteomic profiling of primary cancer-associated fibroblasts in oesophageal adenocarcinoma. Br. J. Cancer 2018, 118, 1200–1207. [Google Scholar] [CrossRef]
- Manousopoulou, A.; Koutmani, Y.; Karaliota, S.; Woelk, C.H.; Manolakos, E.S.; Karalis, K.; Garbis, S.D. Hypothalamus proteomics from mouse models with obesity and anorexia reveals therapeutic targets of appetite regulation. Nutr. Diabetes 2016, 6, e204. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Ross, R.S.; Borg, T.K. Integrins and the Myocardium. Circ. Res. 2001, 88, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Shai, S.-Y.; Harpf, A.E.; Babbitt, C.J.; Jordan, M.C.; Fishbein, M.C.; Chen, J.; Omura, M.; Leil, T.A.; Becker, K.D.; Jiang, M.; et al. Cardiac Myocyte-Specific Excision of the β1 Integrin Gene Results in Myocardial Fibrosis and Cardiac Failure. Circ. Res. 2002, 90, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Masoumy, E.P.; Sawyer, A.A.; Sharma, S.; Patel, J.A.; Gordon, P.M.K.; Regnault, T.R.H.; Matushewski, B.; Weintraub, N.L.; Richardson, B.; Thompson, J.A.; et al. The lifelong impact of fetal growth restriction on cardiac development. Pediatr. Res. 2018, 84, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.-C.; Zhang, Z.-Z.; Wang, W.; McKinnie, S.M.K.; Vederas, J.C.; Oudit, G.Y. Targeting the apelin pathway as a novel therapeutic approach for cardiovascular diseases. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Zimanyi, M.; Black, M.J. Effect of Maternal Protein Restriction in Rats on Cardiac Fibrosis and Capillarization in Adulthood. Pediatr. Res. 2006, 60, 83–87. [Google Scholar] [CrossRef]
- Folino, A.; Montarolo, P.G.; Samaja, M.; Raffaella, R. Effects of apelin on the cardiovascular system. Heart Fail. Rev. 2015, 20, 505–518. [Google Scholar] [CrossRef]
- Tsirka, A.; Gruetzmacher, E.; Kelley, D.; Ritov, V.; Devaskar, S.; Lane, R.H. Myocardial gene expression of glucose transporter 1 and glucose transporter 4 in response to uteroplacental insufficiency in the rat. J. Endocrinol. 2001, 169, 373–380. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Jaswal, J.S. Energy Metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J. Cardiovasc. Pharmacol. 2010, 56, 130–140. [Google Scholar] [CrossRef]
- Donthi, R.V.; Ye, G.; Wu, C.; McClain, D.A.; Lange, A.J.; Epstein, P.N. Cardiac expression of kinase-deficient 6-Phosphofructo-2-kinase/Fructose-2,6-bisphosphatase inhibits glycolysis, promotes hypertrophy, impairs myocyte function, and reduces insulin sensitivity. J. Biol. Chem. 2004, 279, 48085–48090. [Google Scholar] [CrossRef]
- Kourliouros, A.; Yin, X.; Didangelos, A.; Hosseini, M.T.; Valencia, O.; Mayr, M.; Jahangiri, M. Substrate modifications precede the development of atrial fibrillation after cardiac surgery: A proteomic study. Ann. Thorac. Surg. 2011, 92, 104–110. [Google Scholar] [CrossRef]
- Corstius, H.B.; Zimanyi, M.; Maka, N.; Herath, T.; Thomas, W.G.; Van Der Laarse, A.; Wreford, N.G.; Black, M.J. Effect of intrauterine growth restriction on the number of cardiomyocytes in rat hearts. Pediatr. Res. 2005, 57, 796–800. [Google Scholar] [CrossRef] [PubMed]
| Group | Mean | SD | p-Value | |
|---|---|---|---|---|
| Length of gestation (days) | Starved | 21.22 | 0.47 | 0.081 |
| Control | 20.73 | 0.06 | ||
| Litter size (pups) | Starved | 10.83 | 1.72 | 0.530 |
| Control | 11.50 | 1.29 | ||
| Post- delivery maternal weight (g) | Starved | 268.83 | 26.75 | 0.304 |
| Control | 264.50 | 10.47 | ||
| Birth weight (g) | Starved | 5.423 | 0.610 | <0.001 |
| Control | 6.419 | 0.436 | ||
| Heart weight (g) | Starved | 0.027 | 0.006 | <0.001 |
| Control | 0.035 | 0.008 | ||
| FGR | 0.025 | 0.005 | 0.003 | |
| Non-FGR | 0.030 | 0.006 | ||
| Heart to body weight ratio | Starved | 0.00507 | 0.00103 | 0.043 |
| Control | 0.00552 | 0.00113 | ||
| FGR | 0.00515 | 0.00112 | 0.359 | |
| Non-FGR | 0.00498 | 0.00094 | ||
| Brain weight (g) | Starved | 0.150 | 0.045 | 0.001 |
| Control | 0.180 | 0.044 | ||
| FGR | 0.151 | 0.058 | 0.783 | |
| Non-FGR | 0.148 | 0.043 | ||
| Brain to body weight ratio | Starved | 0.02826 | 0.00968 | 0.905 |
| Control | 0.02806 | 0.00598 | ||
| FGR | 0.03157 | 0.01093 | 0.009 | |
| Non-FGR | 0.02496 | 0.00698 | ||
| Liver weight (g) | Starved | 0.245 | 0.070 | 0.117 |
| Control | 0.266 | 0.057 | ||
| FGR | 0.211 | 0.047 | <0.001 | |
| Non-FGR | 0.280 | 0.073 | ||
| Liver to body weight ratio | Starved | 0.04498 | 0.01026 | 0.112 |
| Control | 0.41753 | 0.00946 | ||
| FGR | 0.04274 | 0.00743 | 0.103 | |
| Non-FGR | 0.04721 | 0.01220 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouridis, A.; Manousopoulou, A.; Potiris, A.; Sarli, P.-M.; Aravantinos, L.; Pervanidou, P.; Deligeoroglou, E.; Garbis, S.D.; Eleftheriades, M. Impact of Maternal Food Restriction on Heart Proteome in Appropriately Grown and Growth-Restricted Wistar—Rat Offspring. Nutrients 2021, 13, 466. https://doi.org/10.3390/nu13020466
Zouridis A, Manousopoulou A, Potiris A, Sarli P-M, Aravantinos L, Pervanidou P, Deligeoroglou E, Garbis SD, Eleftheriades M. Impact of Maternal Food Restriction on Heart Proteome in Appropriately Grown and Growth-Restricted Wistar—Rat Offspring. Nutrients. 2021; 13(2):466. https://doi.org/10.3390/nu13020466
Chicago/Turabian StyleZouridis, Andreas, Antigoni Manousopoulou, Anastasios Potiris, Polyxeni-Maria Sarli, Leon Aravantinos, Panagiota Pervanidou, Efthymios Deligeoroglou, Spiros D. Garbis, and Makarios Eleftheriades. 2021. "Impact of Maternal Food Restriction on Heart Proteome in Appropriately Grown and Growth-Restricted Wistar—Rat Offspring" Nutrients 13, no. 2: 466. https://doi.org/10.3390/nu13020466
APA StyleZouridis, A., Manousopoulou, A., Potiris, A., Sarli, P.-M., Aravantinos, L., Pervanidou, P., Deligeoroglou, E., Garbis, S. D., & Eleftheriades, M. (2021). Impact of Maternal Food Restriction on Heart Proteome in Appropriately Grown and Growth-Restricted Wistar—Rat Offspring. Nutrients, 13(2), 466. https://doi.org/10.3390/nu13020466











