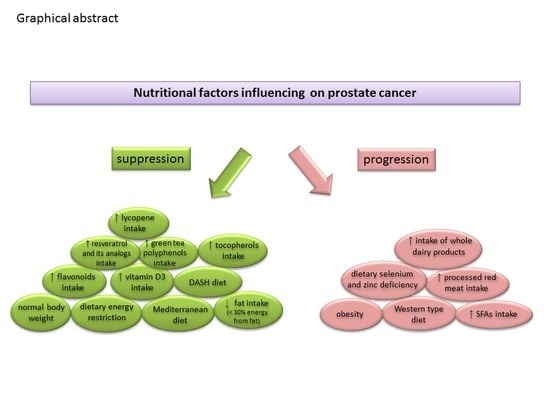
Dietary Factors and Prostate Cancer Development, Progression, and Reduction
Abstract
1. Introduction
2. Prostate Cancer—Etiology and Pathogenesis
3. The Role of Nutritional Factors and Foodstuffs on Prostate Cancer Risk
3.1. Nutrients and Dietary Patterns
3.1.1. Excessive Consumption of Selected Nutrients
3.1.2. Dietary Fat
3.1.3. Meat Consumption
3.1.4. Selenium and Zinc
3.1.5. Nutrients in Dairy Products
3.2. Phytonutrients
3.2.1. Vitamin E and Tocopherols
3.2.2. Selected Flavonoids (Isoflavones, Catechins, and Resveratrol and Its Analogs)
Isoflavones
Green Tea Polyphenols (Catechins)
Resveratrol and Its Analogs
3.2.3. Lycopene
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Cancer Today (Powered by GLOBOCAN 2018). Available online: https://gco.iarc.fr/today (accessed on 23 October 2020).
- Center, M.M.; Jemal, A.; Lortet-Tieulent, J.; Ward, E.; Ferlay, J.; Brawley, O.; Bray, F. International variation in prostate cancer incidence and mortality rates. Eur. Urol. 2012, 61, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Culp, M.B.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.L.; Fritschi, L.; de Klerk, N.H.; Mackerras, D.; Leavy, J. Dietary Patterns Identified Using Factor Analysis and Prostate Cancer Risk: A Case Control Study in Western Australia. Ann. Epidemiol. 2008, 18, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Jalilpiran, Y.; Dianatinasab, M.; Zeighami, S.; Bahmanpour, S.; Ghiasvand, R.; Mohajeri, S.A.R.; Faghih, S. Western Dietary Pattern, But not Mediterranean Dietary Pattern, Increases the Risk of Prostate Cancer. Nutr. Cancer 2018, 70, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Niclis, C.; Román, M.D.; Osella, A.R.; Eynard, A.R.; Diáz, M.D.P. Traditional Dietary Pattern Increases Risk of Prostate Cancer in Argentina: Results of a Multilevel Modeling and Bias Analysis from a Case-Control Study. J. Cancer Epidemiol. 2015, 2015, 179562. [Google Scholar] [CrossRef]
- Walker, M.; Aronson, K.J.; King, W.; Wilson, J.W.L.; Fan, W.; Heaton, J.P.W.; MacNeily, A.; Nickel, J.C.; Morales, A. Dietary patterns and risk of prostate cancer in Ontario, Canada. Int. J. Cancer 2005, 116, 592–598. [Google Scholar] [CrossRef]
- Shin, S.; Saito, E.; Sawada, N.; Ishihara, J.; Takachi, R.; Nanri, A.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Sasazuki, S.; et al. Dietary patterns and prostate cancer risk in Japanese: The Japan Public Health Center-based Prospective Study (JPHC Study). Cancer Causes Control. 2018, 29, 589–600. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Yamthe, L.R.T.; Tali, B.T.; Adetunji, C.O.; Rahavian, A.; Mudau, F.N.; Martorell, M.; Setzer, W.N.; Rodrigues, C.F.; et al. Phytochemicals in Prostate Cancer: From Bioactive Molecules to Upcoming Therapeutic Agents. Nutrients 2019, 11, 1483. [Google Scholar] [CrossRef]
- Thomas-Charles, C.; Fennell, H. Anti-Prostate Cancer Activity of Plant-Derived Bioactive Compounds: A Review. Curr. Mol. Biol. Rep. 2019, 5, 140–151. [Google Scholar] [CrossRef]
- Nelson, W.G.; De Marzo, A.M.; Isaacs, W.B. Prostate cancer. N. Engl. J. Med. 2003, 349, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cornago, A.; Key, T.J.; Allen, N.E.; Fensom, G.K.; Bradbury, K.E.; Martin, R.M.; Travis, R.C. Prospective investigation of risk factors for prostate cancer in the UK Biobank cohort study. Br. J. Cancer 2017, 117, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Egawa, S. Epidemiology of prostate cancer in Asian countries. Int. J. Urol. 2018, 25, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Peisch, S.F.; Van Blarigan, E.L.; Chan, J.M.; Stampfer, M.J.; Kenfield, S.A. Prostate cancer progression and mortality: A review of diet and lifestyle factors. World J. Urol. 2017, 35, 867–874. [Google Scholar] [CrossRef]
- Kgatle, M.M.; Kalla, A.A.; Islam, M.M.; Sathekge, M.; Moorad, R. Prostate Cancer: Epigenetic Alterations, Risk Factors, and Therapy. Prostate Cancer 2016, 2016, 5653862. [Google Scholar] [CrossRef]
- Brawley, O.W. Prostate cancer epidemiology in the United States. World J. Urol. 2012, 30, 195–200. [Google Scholar] [CrossRef]
- Malik, S.S.; Batool, R.; Masood, N.; Yasmin, A. Risk factors for prostate cancer: A multifactorial case-control study. Curr. Probl. Cancer 2018, 42, 337–343. [Google Scholar] [CrossRef]
- Dess, R.T.; Hartman, H.E.; Mahal, B.A.; Soni, P.D.; Jackson, W.C.; Cooperberg, M.R.; Amling, C.L.; Aronson, W.J.; Kane, C.J.; Terris, M.K.; et al. Association of Black Race with Prostate Cancer-Specific and Other-Cause Mortality. JAMA Oncol. 2019, 5, 975–983. [Google Scholar] [CrossRef]
- Nettey, O.S.; Walker, A.J.; Keeter, M.K.; Singal, A.; Nugooru, A.; Martin, I.K.; Ruden, M.; Gogana, P.; Dixon, M.A.; Osuma, T.; et al. Self-reported Black race predicts significant prostate cancer independent of clinical setting and clinical and socioeconomic risk factors. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 501.e1–501.e8. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; David, R.A.; Aderemi, A.V.; Iseolorunkanmi, A.; Oyedokun, A.; Iweala, E.E.J.; Omoregbe, N.; Ayo, C.K. An estimate of the incidence of prostate cancer in Africa: A systematic review and meta-analysis. PLoS ONE 2016, 11, 0153496. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Lortet-Tieulent, J.; Ferlay, J.; Forman, D.; Auvinen, A. Prostate cancer incidence and mortality trends in 37 European countries: An overview. Eur. J. Cancer 2010, 46, 3040–3052. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.P.; Anderson, W.F.; Rosenberg, P.S.; Cook, M.B. Past, Current, and Future Incidence Rates and Burden of Metastatic Prostate Cancer in the United States. Eur. Urol. Focus 2018, 4, 121–127. [Google Scholar] [CrossRef]
- Humphrey, P.A. Histological variants of prostatic carcinoma and their significance. Histopathology 2012, 60, 59–74. [Google Scholar] [CrossRef]
- Andreoiu, M.; Cheng, L. Multifocal prostate cancer: Biologic, prognostic, and therapeutic implications. Hum. Pathol. 2010, 41, 781–793. [Google Scholar] [CrossRef]
- Lee, C.H.; Akin-Olugbade, O.; Kirschenbaum, A. Overview of Prostate Anatomy, Histology, and Pathology. Endocrinol. Metab. Clin. N. Am. 2011, 40, 565–575. [Google Scholar] [CrossRef]
- Gonzalez-Moreno, O.; Boque, N.; Redrado, M.; Milagro, F.; Campion, J.; Endermann, T.; Takahashi, K.; Saito, Y.; Catena, R.; Schomburg, L.; et al. Selenoprotein-P is down-regulated in prostate cancer, which results in lack of protection against oxidative damage. Prostate 2011, 71, 824–834. [Google Scholar] [CrossRef]
- Coleman, W.B. Molecular pathogenesis of prostate cancer. In Molecular Pathology: The Molecular Basis of Human Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 555–568. ISBN 9780128027615. [Google Scholar]
- Aaron, L.T.; Franco, O.E.; Hayward, S.W. Review of Prostate Anatomy and Embryology and the Etiology of Benign Prostatic Hyperplasia. Urol. Clin. N. Am. 2016, 43, 279–288. [Google Scholar] [CrossRef]
- Dobbs, R.W.; Malhotra, N.R.; Greenwald, D.T.; Wang, A.Y.; Prins, G.S.; Abern, M.R. Estrogens and prostate cancer. Prostate Cancer Prostatic Dis. 2019, 22, 185–194. [Google Scholar] [CrossRef]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen signaling in prostate cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030452. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, J.W.; Kim, J.K.; Lee, S.M.; Song, C.; Jeong, I.G.; Hong, J.H.; Kim, C.S.; Ahn, H. Association between serum levels of insulin-like growth factor-1, bioavailable testosterone, and pathologic Gleason score. Cancer Med. 2018, 7, 4170–4180. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Niu, Y.; Huang, H. Posttranslational regulation of androgen dependent and independent androgen receptor activities in prostate cancer. Asian J. Urol. 2020, 7, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Srinidhi, S.; Vishwanatha, J. Oncogenic activation in prostate cancer progression and metastasis: Molecular insights and future challenges. J. Carcinog. 2012, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Nordström, T.; Akre, O.; Aly, M.; Grönberg, H.; Eklund, M. Prostate-specific antigen (PSA) density in the diagnostic algorithm of prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 57–63. [Google Scholar] [CrossRef]
- Kopp, W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef]
- Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Available online: https://www.wcrf.org/dietandcancer (accessed on 11 October 2020).
- Lope, V.; Martín, M.; Castelló, A.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Antolín, S.; Ramos-Vázquez, M.; García-Sáenz, J.Á.; Muñoz, M.; et al. Overeating, caloric restriction and breast cancer risk by pathologic subtype: The EPIGEICAM study. Sci. Rep. 2019, 9, 3904. [Google Scholar] [CrossRef]
- Mazidi, M.; Mikhailidis, D.P.; Sattar, N.; Toth, P.P.; Judd, S.; Blaha, M.J.; Hernandez, A.V.; Penson, P.E.; Banach, M. Association of types of dietary fats and all-cause and cause-specific mortality: A prospective cohort study and meta-analysis of prospective studies with 1,148,117 participants. Clin. Nutr. 2020, 39, 3677–3686. [Google Scholar] [CrossRef]
- Arthur, A.E.; Goss, A.M.; Demark-Wahnefried, W.; Mondul, A.M.; Fontaine, K.R.; Chen, Y.T.; Carroll, W.R.; Spencer, S.A.; Rogers, L.Q.; Rozek, L.S.; et al. Higher carbohydrate intake is associated with increased risk of all-cause and disease-specific mortality in head and neck cancer patients: Results from a prospective cohort study. Int. J. Cancer 2018, 143, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Maino Vieytes, C.A.; Taha, H.M.; Burton-Obanla, A.A.; Douglas, K.G.; Arthur, A.E. Carbohydrate Nutrition and the Risk of Cancer. Curr. Nutr. Rep. 2019, 8, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Turesky, R.J. Mechanistic evidence for red meat and processed meat intake and cancer risk: A follow-up on the international agency for research on cancer evaluation of 2015. Chimia (Aarau) 2018, 72, 718–724. [Google Scholar] [CrossRef]
- Li, Y.; Schoufour, J.; Wang, D.D.; Dhana, K.; Pan, A.; Liu, X.; Song, M.; Liu, G.; Shin, H.J.; Sun, Q.; et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: Prospective cohort study. BMJ 2020, 368. [Google Scholar] [CrossRef]
- Matsushita, M.; Fujita, K.; Nonomura, N. Influence of Diet and Nutrition on Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 1447. [Google Scholar] [CrossRef]
- Vidal, A.C.; Howard, L.E.; Moreira, D.M.; Castro-Santamaria, R.; Andriole, G.L.; Freedland, S.J. Obesity increases the risk for high-grade prostate cancer: Results from the REDUCE study. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2936–2942. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, G.; Sun, B.; Zhao, G.; Liu, D.; Sun, J.; Liu, C.; Guo, H. Impact of obesity upon prostate cancer-associated mortality: A meta-analysis of 17 cohort studies. Oncol. Lett. 2015, 9, 1307–1312. [Google Scholar] [CrossRef]
- Harrison, S.; Tilling, K.; Turner, E.L.; Martin, R.M.; Lennon, R.; Lane, J.A.; Donovan, J.L.; Hamdy, F.C.; Neal, D.E.; Bosch, J.L.H.R.; et al. Systematic review and meta-analysis of the associations between body mass index, prostate cancer, advanced prostate cancer, and prostate-specific antigen. Cancer Causes Control. 2020, 31, 431–449. [Google Scholar] [CrossRef]
- Langlais, C.S.; Cowan, J.E.; Neuhaus, J.; Kenfield, S.A.; van Blarigan, E.L.; Broering, J.M.; Cooperberg, M.R.; Carroll, P.; Chan, J.M. Obesity at diagnosis and prostate cancer prognosis and recurrence risk following primary treatment by radical prostatectomy. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Blando, J.; Moore, T.; Hursting, S.; Jiang, G.; Saha, A.; Beltran, L.; Shen, J.; Repass, J.; Strom, S.; DiGiovanni, J. Dietary Energy Balance Modulates Prostate Cancer Progression in Hi-Myc Mice. Cancer Prev. Res. 2011, 4, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.K.; Giovannucci, E. Diet, Nutrition, and Prostate cancer. Annu. Rev. Nutr. 1998, 18, 413–440. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Huang, P.H.; Chu, P.C.; Chen, M.C.; Chou, C.C.; Wang, D.; Kulp, S.K.; Teng, C.M.; Wang, Q.; Chen, C.S. Energy restriction-mimetic agents induce apoptosis in prostate cancer cells in part through epigenetic activation of KLF6 tumor suppressor gene expression. J. Biol. Chem. 2011, 286, 9968–9976. [Google Scholar] [CrossRef] [PubMed]
- Safdie, F.M.; Dorff, T.; Quinn, D.; Fontana, L.; Wei, M.; Lee, C.; Cohen, P.; Longo, V.D. Fasting and cancer treatment in humans: A case series report. Aging 2009, 1, 988–1007. [Google Scholar] [CrossRef]
- Kopeina, G.S.; Senichkin, V.V.; Zhivotovsky, B. Caloric restriction—A promising anti-cancer approach: From molecular mechanisms to clinical trials. Biochim. Biophys. Acta Rev. Cancer 2017, 1867, 29–41. [Google Scholar] [CrossRef]
- Renehan, A.G.; Frystyk, J.; Flyvbjerg, A. Obesity and cancer risk: The role of the insulin-IGF axis. Trends Endocrinol. Metab. 2006, 17, 328–336. [Google Scholar] [CrossRef]
- Di Sebastiano, K.M.; Bell, K.E.; Mitchell, A.S.; Quadrilatero, J.; Dubin, J.A.; Mourtzakis, M. Glucose metabolism during the acute prostate cancer treatment trajectory: The influence of age and obesity. Clin. Nutr. 2018, 37, 195–203. [Google Scholar] [CrossRef]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, W.; McGinley, J.; Wolfe, P.; Thompson, H.J. Effects of dietary energy repletion and IGF-1 infusion on the inhibition of mammary carcinogenesis by dietary energy restriction. Mol. Carcinog. 2005, 42, 170–176. [Google Scholar] [CrossRef]
- Platz, E.A. Energy imbalance and prostate cancer. J. Nutr. 2002, 132, 3471S–3481S. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Kenfield, S.A.; Van Blarigan, E.L.; Batista, J.L.; Sesso, H.D.; Ma, J.; Stampfer, M.J.; Chavarro, J.E. Dietary Patterns after Prostate Cancer Diagnosis in Relation to Disease-Specific and Total Mortality. Cancer Prev. Res. 2015, 8, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.C.; Severi, G.; Baglietto, L.; Krishnan, K.; English, D.R.; Hopper, J.L.; Giles, G.G. Dietary patterns and prostate cancer risk. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3126–3129. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Tulloch-Reid, M.; Walker, S.; McFarlane-Anderson, N.; Bennett, F.; Francis, D.; Coard, K. Dietary patterns as predictors of prostate cancer in Jamaican men. Nutr. Cancer 2013, 65, 367–374. [Google Scholar] [CrossRef]
- Castelló, A.; Boldo, E.; Amiano, P.; Castaño-Vinyals, G.; Aragonés, N.; Gómez-Acebo, I.; Peiró, R.; Jimenez-Moleón, J.J.; Alguacil, J.; Tardón, A.; et al. Mediterranean Dietary Pattern is Associated with Low Risk of Aggressive Prostate Cancer: MCC-Spain Study. J. Urol. 2018, 199, 430–437. [Google Scholar] [CrossRef]
- Schneider, L.; Su, L.J.; Arab, L.; Bensen, J.T.; Farnan, L.; Fontham, E.T.H.; Song, L.; Hussey, J.; Merchant, A.T.; Mohler, J.L.; et al. Dietary patterns based on the Mediterranean diet and DASH diet are inversely associated with high aggressive prostate cancer in PCaP. Ann. Epidemiol. 2019, 29, 16–22.e1. [Google Scholar] [CrossRef]
- Escrich, E.; Moral, R.; Grau, L.; Costa, I.; Solanas, M. Molecular mechanisms of the effects of olive oil and other dietary lipids on cancer. Mol. Nutr. Food Res. 2007, 51, 1279–1292. [Google Scholar] [CrossRef]
- Cheng, S.; Zheng, Q.; Ding, G.; Li, G. Mediterranean dietary pattern and the risk of prostate cancer a meta-analysis. Medicine 2019, 98, e16341. [Google Scholar] [CrossRef]
- Pelser, C.; Mondul, A.M.; Hollenbeck, A.R.; Park, Y. Dietary Fat, Fatty Acids, and Risk of Prostate Cancer in the NIH-AARP Diet and Health Study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 697–707. [Google Scholar] [CrossRef]
- Liss, M.A.; Al-Bayati, O.; Gelfond, J.; Goros, M.; Ullevig, S.; DiGiovanni, J.; Hamilton-Reeves, J.; O’Keefe, D.; Bacich, D.; Weaver, B.; et al. Higher baseline dietary fat and fatty acid intake is associated with increased risk of incident prostate cancer in the SABOR study. Prostate Cancer Prostatic Dis. 2019, 22, 244–251. [Google Scholar] [CrossRef]
- Ohwaki, K.; Endo, F.; Kachi, Y.; Hattori, K.; Muraishi, O.; Nishikitani, M.; Yano, E. Relationship between Dietary Factors and Prostate-Specific Antigen in Healthy Men. Urol. Int. 2012, 89, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Ugge, H.; Downer, M.K.; Carlsson, J.; Bowden, M.; Davidsson, S.; Mucci, L.A.; Fall, K.; Andersson, S.O.; Andrén, O. Circulating inflammation markers and prostate cancer. Prostate 2019, 79, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Gucalp, A.; Iyengar, N.M.; Zhou, X.K.; Giri, D.D.; Falcone, D.J.; Wang, H.; Williams, S.; Krasne, M.D.; Yaghnam, I.; Kunzel, B.; et al. Periprostatic adipose inflammation is associated with high-grade prostate cancer. Prostate Cancer Prostatic Dis. 2017, 20, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.; Livas, L.; Kyprianou, N. Inflammation in prostate cancer progression and therapeutic targeting. Transl. Androl. Urol. 2015, 4, 455–463. [Google Scholar] [PubMed]
- Gurel, B.; Lucia, M.S.; Thompson, I.M.; Goodman, P.J.; Tangen, C.M.; Kristal, A.R.; Parnes, H.L.; Hoque, A.; Lippman, S.M.; Sutcliffe, S.; et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol. Biomark. Prev. 2014, 23, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Richman, E.L.; Kenfield, S.A.; Chavarro, J.E.; Stampfer, M.J.; Giovannucci, E.L.; Willett, W.C.; Chan, J.M. Fat Intake After Diagnosis and Risk of Lethal Prostate Cancer and All-Cause Mortality. JAMA Intern. Med. 2013, 173, 1318. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Arab, L.; Su, L.J.; Farnan, L.; Fontham, E.T.H.; Mohler, J.L.; Bensen, J.T.; Steck, S.E. Saturated fat intake and prostate cancer aggressiveness: Results from the population-based North Carolina-Louisiana Prostate Cancer Project. Prostate Cancer Prostatic Dis. 2017, 20, 48–54. [Google Scholar] [CrossRef]
- Kristal, A.R.; Cohen, J.H.; Qu, P.; Stanford, J.L. Associations of energy, fat, calcium, and vitamin D with prostate cancer risk. Cancer Epidemiol. Biomark. Prev. 2002, 11, 719–725. [Google Scholar]
- Sonn, G.A.; Aronson, W.; Litwin, M.S. Impact of diet on prostate cancer: A review. Prostate Cancer Prostatic Dis. 2005, 8, 304–310. [Google Scholar] [CrossRef]
- Kim, S.; Yang, X.; Li, Q.; Wu, M.; Costyn, L.; Beharry, Z.; Bartlett, M.G.; Cai, H. Myristoylation of Src kinase mediates Src-induced and high-fat diet–accelerated prostate tumor progression in mice. J. Biol. Chem. 2017, 292, 18422–18433. [Google Scholar] [CrossRef]
- Barnard, R.J.; Ngo, T.H.; Leung, P.S.; Aronson, W.J.; Golding, L.A. A low-fat diet and/or strenuous exercise alters the IGF axis in vivo and reduces prostate tumor cell growth in vitro. Prostate 2003, 56, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, J.; Sampieri, K.; Clohessy, J.G.; Mendez, L.; Gonzalez-Billalabeitia, E.; Liu, X.S.; Lee, Y.R.; Fung, J.; Katon, J.M.; et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat. Genet. 2018, 50, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Fujita, K.; Nojima, S.; Hayashi, Y.; Nakano, K.; Ishizuya, Y.; Wang, C.; Yamamoto, Y.; Kinouchi, T.; Matsuzaki, K.; et al. High-fat diet-induced inflammation accelerates prostate cancer growth via IL6 signaling. Clin. Cancer Res. 2018, 24, 4309–4318. [Google Scholar] [CrossRef]
- Vykhovanets, E.V.; Shankar, E.; Vykhovanets, O.V.; Shukla, S.; Gupta, S. High-fat diet increases NF-κB signaling in the prostate of reporter mice. Prostate 2011, 71, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M. Chemoprevention of Prostate Cancer: Agents and Study Designs. J. Urol. 2007, 178, S9–S13. [Google Scholar] [CrossRef]
- Akinsete, J.A.; Ion, G.; Witte, T.R.; Hardman, W.E. Consumption of high ω-3 fatty acid diet suppressed prostate tumorigenesis in C3(1) Tag mice. Carcinogenesis 2012, 33, 140–148. [Google Scholar] [CrossRef]
- Epstein, M.M.; Kasperzyk, J.L.; Mucci, L.A.; Giovannucci, E.; Price, A.; Wolk, A.; Håkansson, N.; Fall, K.; Andersson, S.O.; Andrén, O. Dietary fatty acid intake and prostate cancer survival in Örebro county, Sweden. Am. J. Epidemiol. 2012, 176, 240–252. [Google Scholar] [CrossRef]
- Park, S.Y.; Murphy, S.P.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Fat and meat intake and prostate cancer risk: The Multiethnic Cohort Study. Int. J. Cancer 2007, 121, 1339–1345. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Stampfer, M.J.; Hall, M.N.; Sesso, H.D.; Ma, J. A 22-y prospective study of fish intake in relation to prostate cancer incidence and mortality. Am. J. Clin. Nutr. 2008, 88, 1297–1303. [Google Scholar] [CrossRef]
- Szymanski, K.M.; Wheeler, D.C.; Mucci, L.A. Fish consumption and prostate cancer risk: A review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1223–1233. [Google Scholar] [CrossRef]
- Bidoli, E.; Talamini, R.; Bosetti, C.; Negri, E.; Maruzzi, D.; Montella, M.; Franceschi, S.; La Vecchia, C. Macronutrients, fatty acids, cholesterol and prostate cancer risk. Ann. Oncol. 2005, 16, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Brasky, T.M.; Darke, A.K.; Song, X.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L.; et al. Plasma phospholipid fatty acids and prostate cancer risk in the select trial. J. Natl. Cancer Inst. 2013, 105, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Kristal, A.R.; Arnold, K.B.; Neuhouser, M.L.; Goodman, P.; Platz, E.A.; Albanes, D.; Thompson, I.M. Diet, supplement use, and prostate cancer risk: Results from the prostate cancer prevention trial. Am. J. Epidemiol. 2010, 172, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Garzotto, M.; Beer, T.M.; Thuillier, P.; Lieberman, S.; Mori, M.; Stoller, W.A.; Farris, P.E.; Shannon, J. Effects of ω-3 Fatty Acids and Catechins on Fatty Acid Synthase in the Prostate: A Randomized Controlled Trial. Nutr. Cancer 2016, 68, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Weinberg, V.; Magbanua, M.J.; Sosa, E.; Simko, J.; Shinohara, K.; Federman, S.; Mattie, M.; Hughes-Fulford, M.; Haqq, C.; et al. Nutritional supplements, COX-2 and IGF-1 expression in men on active surveillance for prostate cancer. Cancer Causes Control. 2011, 22, 141–150. [Google Scholar] [CrossRef]
- Dhaka, V.; Gulia, N.; Ahlawat, K.S.; Khatkar, B.S. Trans fats-sources, health risks and alternative approach—A review. J. Food Sci. Technol. 2011, 48, 534–541. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Song, J.; Wang, A.; Zou, Y.; Ding, L.; Wen, Y. Plasma trans-fatty acids levels and mortality: A cohort study based on 1999–2000 National Health and Nutrition Examination Survey (NHANES). Lipids Health Dis. 2017, 16, 176. [Google Scholar] [CrossRef]
- Islam, M.A.; Amin, M.N.; Siddiqui, S.A.; Hossain, M.P.; Sultana, F.; Kabir, M.R. Trans fatty acids and lipid profile: A serious risk factor to cardiovascular disease, cancer and diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1643–1647. [Google Scholar] [CrossRef]
- Hu, J.; La Vecchia, C.; De Groh, M.; Negri, E.; Morrison, H.; Mery, L. Dietary transfatty acids and cancer risk. Eur. J. Cancer Prev. 2011, 20, 530–538. [Google Scholar] [CrossRef]
- Liu, X.; Schumacher, F.R.; Plummer, S.J.; Jorgenson, E.; Casey, G.; Witte, J.S. trans-Fatty acid intake and increased risk of advanced prostate cancer: Modification by RNASEL R462Q variant. Carcinogenesis 2007, 28, 1232–1236. [Google Scholar] [CrossRef]
- Fleshner, N.; Bagnell, P.S.; Klotz, L.; Venkateswaran, V.; Kristal, A.R.; Thompson, I.M.; Klein, E.; Denis, L.J.; Ford, L.G. Dietary fat and prostate cancer. J. Urol. 2004, 171, S19–S24. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Park, Y.; Graubard, B.I.; Leitzmann, M.F.; Hollenbeck, A.; Schatzkin, A.; Cross, A.J. Meat and meat-related compounds and risk of prostate cancer in a large prospective cohort study in the United States. Am. J. Epidemiol. 2009, 170, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Bylsma, L.C.; Alexander, D.D. A review and meta-analysis of prospective studies of red and processed meat, meat cooking methods, heme iron, heterocyclic amines and prostate cancer. Nutr. J. 2015, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.D.; Mink, P.J.; Cushing, C.A.; Sceurman, B. A review and meta-analysis of prospective studies of red and processed meat intake and prostate cancer. Nutr. J. 2010, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- John, E.M.; Stern, M.C.; Sinha, R.; Koo, J. Meat consumption, Cooking Practices, Meat Mutagens and Risk of Prostate Cancer. Nutr. Cancer 2011, 63, 525. [Google Scholar] [CrossRef]
- Koutros, S.; Cross, A.J.; Sandler, D.P.; Hoppin, J.A.; Ma, X.; Zheng, T.; Alavanja, M.C.R.; Sinha, R. Meat and meat mutagens and risk of prostate cancer in the agricultural health study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 80–87. [Google Scholar] [CrossRef]
- Michaud, D.S.; Augustsson, K.; Rimm, E.B.; Stampfer, M.J.; Willet, W.C.; Giovannucci, E. A prospective study on intake of animal products and risk of prostate cancer. Cancer Causes Control. 2001, 12, 557–567. [Google Scholar] [CrossRef]
- Rohrmann, S.; Platz, E.A.; Kavanaugh, C.J.; Thuita, L.; Hoffman, S.C.; Helzlsouer, K.J. Meat and dairy consumption and subsequent risk of prostate cancer in a US cohort study. Cancer Causes Control. 2007, 18, 41–50. [Google Scholar] [CrossRef]
- Amin, M.; Jeyaganth, S.; Fahmy, N.; Bégin, L.R.; Aronson, S.; Jacobson, S.; Tanguay, S.; Kassouf, W.; Aprikian, A. Dietary habits and prostate cancer detection: A case-control study. Can. Urol. Assoc. J. 2008, 2, 510–515. [Google Scholar] [CrossRef]
- You, W.; Henneberg, M. Prostate cancer incidence is correlated to total meat intake- A cross-national ecologic analysis of 172 countries. Asian Pacific J. Cancer Prev. 2018, 19, 2229–2239. [Google Scholar] [CrossRef]
- Kolonel, L.N. Fat, meat, and prostate cancer. Epidemiol. Rev. 2001, 23, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; Stewart, B.W.; et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Shirai, T.; Sano, M.; Tamano, S.; Takahashi, S.; Hirose, M.; Futakuchi, M.; Hasegawa, R.; Imaida, K.; Matsumoto, K.I.; Wakabayashi, K.; et al. The prostate: A target for carcinogenicity of 2-amino-1-methyl-6- phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997, 57, 195–198. [Google Scholar] [PubMed]
- Hrubá, E.; Vondráček, J.; Líbalová, H.; Topinka, J.; Bryja, V.; Souček, K.; Machala, M. Gene expression changes in human prostate carcinoma cells exposed to genotoxic and nongenotoxic aryl hydrocarbon receptor ligands. Toxicol. Lett. 2011, 206, 178–188. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc: Role in immunity, oxidative stress and chronic inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 646–652. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Banji, D.; Banji, O.J.F.; Reddy, M.; Annamalai, A.R. Impact of zinc, selenium and lycopene on capsaicin induced mutagenicity and oxidative damage in mice. J. Trace Elem. Med. Biol. 2013, 27, 230–235. [Google Scholar] [CrossRef]
- Wolonciej, M.; Milewska, E.; Roszkowska-Jakimiec, W. Trace elements as an activator of antioxidant enzymes. Postepy Hig. Med. Dosw. 2016, 70, 1483–1498. [Google Scholar] [CrossRef]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef]
- Diamond, A.M. Selenoproteins of the Human Prostate: Unusual Properties and Role in Cancer Etiology. Biol. Trace Elem. Res. 2019, 192, 51–59. [Google Scholar] [CrossRef]
- Sayehmiri, K.; Azami, M.; Mohammadi, Y.; Soleymani, A.; Tardeh, Z. The association between selenium and prostate cancer: A systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2018, 19, 1431–1437. [Google Scholar] [PubMed]
- Cui, Z.; Liu, D.; Liu, C.; Liu, G. Serum selenium levels and prostate cancer risk. Medicine 2017, 96, e5944. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.; Hooper, L.; Norat, T.; Lau, R.; Aune, D.; Greenwood, D.C.; Vieira, R.; Collings, R.; Harvey, L.J.; Sterne, J.A.; et al. Selenium and prostate cancer: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 96, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Travis, R.C.; Appleby, P.N.; Albanes, D.; Barnett, M.J.; Black, A.; Bueno-De-Mesquita, H.B.; Deschasaux, M.; Galan, P.; Goodman, G.E.; et al. Selenium and prostate cancer: Analysis of individual participant data from fifteen prospective studies. J. Natl. Cancer Inst. 2016, 108, djw153. [Google Scholar] [CrossRef]
- Platz, E.A.; Helzlsouer, K.J. Selenium, zinc, and prostate cancer. Epidemiol. Rev. 2001, 23, 93–101. [Google Scholar] [CrossRef]
- Robberecht, H.; De Bruyne, T.; Davioud-Charvet, E.; Mackrill, J.; Hermans, N. Selenium Status in Elderly People: Longevity and Age-Related Diseases. Curr. Pharm. Des. 2019, 25, 1694–1706. [Google Scholar] [CrossRef]
- Outzen, M.; Tjonneland, A.; Larsen, E.H.; Friis, S.; Larsen, S.B.; Christensen, J.; Overvad, K.; Olsen, A. Selenium status and risk of prostate cancer in a Danish population. Br. J. Nutr. 2016, 115, 1669–1677. [Google Scholar] [CrossRef]
- Waters, D.J.; Shen, S.; Glickman, L.T.; Cooley, D.M.; Bostwick, D.G.; Qian, J.; Combs, G.F.; Morris, J.S. Prostate cancer risk and DNA damage: Translational significance of selenium supplementation in a canine model. Carcinogenesis 2005, 26, 1256–1262. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The selenium and vitamin E cancer prevention trial (SELECT). JAMA J. Am. Med. Assoc. 2009, 301, 39–51. [Google Scholar] [CrossRef]
- Kenfield, S.A.; Van Blarigan, E.L.; DuPre, N.; Stampfer, M.J.; Giovannucci, E.L.; Chan, J.M. Selenium supplementation and prostate cancer mortality. J. Natl. Cancer Inst. 2015, 107, dju360. [Google Scholar] [CrossRef]
- Kristal, A.R.; Darke, A.K.; Morris, J.S.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L.; et al. Baseline selenium status and effects of selenium and vitamin E supplementation on prostate cancer risk. J. Natl. Cancer Inst. 2014, 106, djt456. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.D.; Jiang, C.; Malewicz, B.; Dong, Y.; Young, C.Y.F.; Kang, K.S.; Lee, Y.S.; Ip, C.; Lü, J. Methyl selenium metabolites decrease prostate-specific antigen expression by inducing protein degradation and suppressing androgen-stimulated transcription. Mol. Cancer Ther. 2004, 3, 605–611. [Google Scholar] [PubMed]
- Dong, Y.; Lee, S.O.; Zhang, H.; Marshall, J.; Gao, A.C.; Ip, C. Prostate Specific Antigen Expression Is Down-Regulated by Selenium through Disruption of Androgen Receptor Signaling. Cancer Res. 2004, 64, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Santi, R.; Tamanini, I.; Galli, I.C.; Perletti, G.; Bjerklund Johansen, T.E.; Nesi, G. Current knowledge of the potential links between inflammation and prostate cancer. Int. J. Mol. Sci. 2019, 20, 3833. [Google Scholar] [CrossRef]
- Kim, H.W.; Ha, U.S.; Woo, J.C.; Kim, S.J.; Yoon, B.I.; Lee, S.J.; Cho, Y.H. Preventive effect of selenium on chronic bacterial prostatitis. J. Infect. Chemother. 2012, 18, 30–34. [Google Scholar] [CrossRef]
- Omabe, M.; Ezeani, M. Infection, inflammation and prostate carcinogenesis. Infect. Genet. Evol. 2011, 11, 1195–1198. [Google Scholar] [CrossRef]
- Willis, M.S.; Wians, F.H. The role of nutrition in preventing prostate cancer: A review of the proposed mechanism of action of various dietary substances. Clin. Chim. Acta 2003, 330, 57–83. [Google Scholar] [CrossRef]
- Sanmartin, C.; Plano, D.; Palop, J. Selenium Compounds and Apoptotic Modulation: A New Perspective in Cancer Therapy. Mini-Rev. Med. Chem. 2008, 8, 1020–1031. [Google Scholar] [CrossRef]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.; Björnstedt, M. Redox-Active Selenium Compounds—From Toxicity and Cell Death to Cancer Treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef]
- Xu, J.; Gong, Y.; Sun, Y.; Cai, J.; Liu, Q.; Bao, J.; Yang, J.; Zhang, Z. Impact of Selenium Deficiency on Inflammation, Oxidative Stress, and Phagocytosis in Mouse Macrophages. Biol. Trace Elem. Res. 2020, 194, 237–243. [Google Scholar] [CrossRef]
- Avery, J.; Hoffmann, P. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Shahvazi, S.; Soltani, S.; Ahmadi, S.; de Souza, R.; Salehi-Abargouei, A. The Effect of Vitamin D Supplementation on Prostate Cancer: A Systematic Review and Meta-Analysis of Clinical Trials. Horm. Metab. Res. 2019, 51, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, Á. Reported dietary intake and food sources of zinc, selenium, and vitamins a, e and c in the spanish population: Findings from the anibes study. Nutrients 2017, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Yamasaki, S.; Nishida, K.; Murakami, M.; Hirano, T. Zinc homeostasis and signaling in health and diseases. J. Biol. Inorg. Chem. 2011, 16, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; Teixeira, F.J.; Schoenfeld, B.J. Dietary vs. pharmacological doses of zinc: A clinical review. Clin. Nutr. 2020, 39, 1345–1353. [Google Scholar] [CrossRef]
- Epstein, M.M.; Kasperzyk, J.L.; Andrén, O.; Giovannucci, E.L.; Wolk, A.; Håkansson, N.; Andersson, S.O.; Johansson, J.E.; Fall, K.; Mucci, L.A. Dietary zinc and prostate cancer survival in a Swedish cohort. Am. J. Clin. Nutr. 2011, 93, 586–593. [Google Scholar] [CrossRef]
- Gutiérrez-González, E.; Castelló, A.; Fernández-Navarro, P.; Castaño-Vinyals, G.; Llorca, J.; Salas, D.; Salcedo-Bellido, I.; Aragonés, N.; Fernández-Tardón, G.; Alguacil, J.; et al. Dietary zinc and risk of prostate cancer in Spain: MCC-Spain study. Nutrients 2019, 11, 18. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Al-Alem, U.; Dabbous, F.; Ali, M.M.; Batai, K.; Shah, E.; Kittles, R.A. Zinc intake and risk of prostate cancer: Case-control study and meta-analysis. PLoS ONE 2016, 11, 0165956. [Google Scholar] [CrossRef]
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc supplement use and risk of prostate cancer. J. Natl. Cancer Inst. 2003, 95, 1004–1007. [Google Scholar] [CrossRef]
- Xue, Y.N.; Yu, B.B.; Liu, Y.N.; Guo, R.; Li, J.L.; Zhang, L.C.; Su, J.; Sun, L.K.; Li, Y. Zinc promotes prostate cancer cell chemosensitivity to paclitaxel by inhibiting epithelial-mesenchymal transition and inducing apoptosis. Prostate 2019, 79, 647–656. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Tang, X.; Guo, R.; Li, J.; Chen, Y.; Guo, H.; Su, J.; Sun, L.; Liu, Y. Zinc enhances chemosensitivity to paclitaxel in PC-3 prostate cancer cells. Oncol. Rep. 2018, 40, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.Y.; Jing, R.; Smalley, K.J.; Wang, Z.X.; Taccioli, C.; Fan, S.; Chen, H.; Alder, H.; Huebner, K.; Farber, J.L.; et al. Human-like hyperplastic prostate with low ZIP1 induced solely by Zn deficiency in rats. Proc. Natl. Acad. Sci. USA 2018, 115, E11091–E11100. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, A.; Kaya, Y.; Tanriverdi, O. Effect of the Interaction Between Selenium and Zinc on DNA Repair in Association With Cancer Prevention. J. Cancer Prev. 2019, 24, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Daragó, A.; Klimczak, M.; Stragierowicz, J.; Stasikowska-Kanicka, O.; Kilanowicz, A. The Effect of Zinc, Selenium, and Their Combined Supplementation on Androgen Receptor Protein Expression in the Prostate Lobes and Serum Steroid Hormone Concentrations of Wistar Rats. Nutrients 2020, 12, 153. [Google Scholar] [CrossRef]
- Capiod, T.; Delongchamps, N.B.; Pigat, N.; Souberbielle, J.C.; Goffin, V. Do dietary calcium and Vitamin D matter in men with prostate cancer? Nat. Rev. Urol. 2018, 15, 453–461. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Bobrowska-Korczak, B.; Tokarz, A. Disorders of Mechanisms of Calcium Metabolism Control as Potential Risk Factors of Prostate Cancer. Curr. Med. Chem. 2017, 24, 4229–4244. [Google Scholar] [CrossRef]
- López-Plaza, B.; Bermejo, L.M.; Santurino, C.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Gómez-Candela, C. Milk and Dairy Product Consumption and Prostate Cancer Risk and Mortality: An Overview of Systematic Reviews and Meta-analyses. Adv. Nutr. 2019, 10, S212. [Google Scholar] [CrossRef]
- Downer, M.K.; Batista, J.L.; Mucci, L.A.; Stampfer, M.J.; Epstein, M.M.; Håkansson, N.; Wolk, A.; Johansson, J.E.; Andrén, O.; Fall, K.; et al. Dairy intake in relation to prostate cancer survival. Int. J. Cancer 2017, 140, 2060–2069. [Google Scholar] [CrossRef]
- Ahn, J.; Albanes, D.; Peters, U.; Schatzkin, A.; Lim, U.; Freedman, M.; Chatterjee, N.; Andriole, G.L.; Leitzmann, M.F.; Hayes, R.B.; et al. Dairy Products, Calcium Intake, and Risk of Prostate Cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2623–2630. [Google Scholar] [CrossRef]
- Travis, R.C.; Perez-Cornago, A.; Appleby, P.N.; Albanes, D.; Joshu, C.E.; Lutsey, P.L.; Mondul, A.M.; Platz, E.A.; Weinstein, S.J.; Layne, T.M.; et al. A collaborative analysis of individual participant data from 19 prospective studies assesses circulating Vitamin D and prostate cancer risk. Cancer Res. 2019, 79, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Trump, D.; Aragon-Ching, J. Vitamin D in prostate cancer. Asian J. Androl. 2018, 20, 244–252. [Google Scholar] [CrossRef]
- Gao, J.; Wei, W.; Wang, G.; Zhou, H.; Fu, Y.; Liu, N. Circulating vitamin D concentration and risk of prostate cancer: A dose–response meta-analysis of prospective studies. Ther. Clin. Risk Manag. 2018, 14, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Yao, Q.; Zhuo, Z.; Ma, Z.; Chen, G. Circulating vitamin d level and mortality in prostate cancer patients: A dose–response meta-analysis. Endocr. Connect. 2018, 7, R294–R303. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Giovannucci, E.L. Diet: Dairy Products, Calcium, and Vitamin D and Risk of Prostate Cancer. Epidemiol. Rev. 2001, 23, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Tat, D.; Kenfield, S.A.; Cowan, J.E.; Broering, J.M.; Carroll, P.R.; Van Blarigan, E.L.; Chan, J.M. Milk and other dairy foods in relation to prostate cancer recurrence: Data from the cancer of the prostate strategic urologic research endeavor (CaPSURETM). Prostate 2018, 78, 32–39. [Google Scholar] [CrossRef]
- Torfadottir, J.E.; Steingrimsdottir, L.; Mucci, L.; Aspelund, T.; Kasperzyk, J.L.; Olafsson, O.; Fall, K.; Tryggvadottir, L.; Harris, T.B.; Launer, L.; et al. Milk Intake in Early Life and Risk of Advanced Prostate Cancer. Am. J. Epidemiol. 2012, 175, 144. [Google Scholar] [CrossRef]
- Watters, J.L.; Gail, M.H.; Weinstein, S.J.; Virtamo, J.; Albanes, D. Associations between α-tocopherol, β-carotene, and retinol and prostate cancer survival. Cancer Res. 2009, 69, 3833–3841. [Google Scholar] [CrossRef]
- Hada, M.; Mondul, A.M.; Weinstein, S.J.; Albanes, D. Serum Retinol and Risk of Overall and Site-Specific Cancer in the ATBC Study. Am. J. Epidemiol. 2020, 189, 532–542. [Google Scholar] [CrossRef]
- Cui, R.; Liu, Z.-Q.; Xu, Q. Blood α-Tocopherol, γ-Tocopherol Levels and Risk of Prostate Cancer: A Meta-Analysis of Prospective Studies. PLoS ONE 2014, 9, e93044. [Google Scholar] [CrossRef]
- Antwi, S.O.; Steck, S.E.; Zhang, H.; Stumm, L.; Zhang, J.; Hurley, T.G.; Hebert, J.R. Plasma carotenoids and tocopherols in relation to prostate-specific antigen (PSA) levels among men with biochemical recurrence of prostate cancer. Cancer Epidemiol. 2015, 39, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.J.; Wright, M.E.; Lawson, K.A.; Snyder, K.; Männistö, S.; Taylor, P.R.; Virtamo, J.; Albanes, D. Serum and dietary vitamin E in relation to prostate cancer risk. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Albanes, D.; Till, C.; Klein, E.A.; Goodman, P.J.; Mondul, A.M.; Weinstein, S.J.; Taylor, P.R.; Parnes, H.L.; Gaziano, J.M.; Song, X.; et al. Plasma Tocopherols and Risk of Prostate Cancer in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Cancer Prev. Res. 2014, 7, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Wada, S. Chemoprevention of Tocotrienols: The Mechanism of Antiproliferative Effects. In Food Factors for Health Promotion; KARGER: Basel, Switherlands, 2009; pp. 204–216. [Google Scholar]
- Barve, A.; Khor, T.O.; Nair, S.; Reuhl, K.; Suh, N.; Reddy, B.; Newmark, H.; Kong, A.-N. γ-Tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int. J. Cancer 2009, 124, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, K.A.; De Pascual-Tereasa, S.; Needs, P.W.; Bao, Y.P.; O’Brien, N.M.; Williamson, G. Effect of flavonoids and Vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 551, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Martins, N.; Barros, L. Phenolic Compounds and Its Bioavailability: In Vitro Bioactive Compounds or Health Promoters? In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2017; Volume 82, pp. 1–44. [Google Scholar]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.R.; Liu, D. Flavonoids Influence Epigenetic-Modifying Enzyme Activity: Structure-Function Relationships and the Therapeutic Potential for Cancer. Curr. Med. Chem. 2010, 17, 1756–1768. [Google Scholar] [CrossRef]
- Sonoda, T.; Nagata, Y.; Mori, M.; Miyanaga, N.; Takashima, N.; Okumura, K.; Goto, K.; Naito, S.; Fujimoto, K.; Hirao, Y.; et al. A case-control study of diet and prostate cancer in Japan: Possible protective effect of traditional Japanese diet. Cancer Sci. 2004, 95, 238–242. [Google Scholar] [CrossRef]
- Kurahashi, N.; Iwasaki, M.; Sasazuki, S.; Otani, T.; Inoue, M.; Tsugane, S. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol. Biomark. Prev. 2007, 16, 538–545. [Google Scholar] [CrossRef]
- Yan, L.; Spitznagel, E.L. Soy consumption and prostate cancer risk in men: A revisit of a meta-analysis. Am. J. Clin. Nutr. 2009, 89, 1155–1163. [Google Scholar] [CrossRef]
- Van Die, M.D.; Bone, K.M.; Williams, S.G.; Pirotta, M.V. Soy and soy isoflavones in prostate cancer: A systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014, 113, E119–E130. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.W.; Kim, S.Y.; Jee, S.H.; Kim, Y.N.; Nam, C.M. Soy food consumption and risk of prostate cancer: A meta-analysis of observational studies. Nutr. Cancer 2009, 61, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Applegate, C.C.; Rowles, J.L.; Ranard, K.M.; Jeon, S.; Erdman, J.W. Soy consumption and the risk of prostate cancer: An updated systematic review and meta-analysis. Nutrients 2018, 10, 40. [Google Scholar] [CrossRef]
- Kumar, N.B.; Cantor, A.; Allen, K.; Riccardi, D.; Besterman-Dahan, K.; Seigne, J.; Helal, M.; Salup, R.; Pow-Sang, J. The Specific Role of Isoflavones in Reducing Prostate Cancer Risk. Prostate 2004, 59, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Sivoňová, M.K.; Kaplán, P.; Tatarková, Z.; Lichardusová, L.; Dušenka, R.; Jurečeková, J. Androgen receptor and soy isoflavones in prostate cancer (Review). Mol. Clin. Oncol. 2019, 10, 191–204. [Google Scholar] [CrossRef]
- Gonzalez-Menendez, P.; Hevia, D.; Rodriguez-Garcia, A.; Mayo, J.C.; Sainz, R.M. Regulation of GLUT Transporters by Flavonoids in Androgen-Sensitive and -Insensitive Prostate Cancer Cells. Endocrinology 2014, 155, 3238–3250. [Google Scholar] [CrossRef]
- Miyata, Y.; Shida, Y.; Hakariya, T.; Sakai, H. Anti-Cancer Effects of Green Tea Polyphenols Against Prostate Cancer. Molecules 2019, 24, 193. [Google Scholar] [CrossRef]
- Filippini, T.; Malavolti, M.; Borrelli, F.; Izzo, A.A.; Fairweather-Tait, S.J.; Horneber, M.; Vinceti, M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2020, 2020. [Google Scholar] [CrossRef]
- Guo, Y.; Zhi, F.; Chen, P.; Zhao, K.; Xiang, H.; Mao, Q.; Wang, X.; Zhang, X. Green tea and the risk of prostate cancer: A systematic review and meta-analysis. Medicine 2017, 96, e6426. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Zhao, C.-N.; Cao, S.-Y.; Tang, G.-Y.; Gan, R.-Y.; Li, H.-B. Effects and mechanisms of tea for the prevention and management of cancers: An updated review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1693–1705. [Google Scholar] [CrossRef]
- Wang, P.; Henning, S.M.; Magyar, C.E.; Elshimali, Y.; Heber, D.; Vadgama, J.V. Green tea and quercetin sensitize PC-3 xenograft prostate tumors to docetaxel chemotherapy. J. Exp. Clin. Cancer Res. 2016, 35, 73. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.J.; Chen, B.H. Preparation of catechin extracts and nanoemulsions from green tea leaf waste and their inhibition effect on prostate cancer cell PC-3. Int. J. Nanomed. 2016, 11, 1907–1926. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Cancer and metastasis: Prevention and treatment by green tea. Cancer Metastasis Rev. 2010, 29, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Bavaresco, L.; Fregoni, C. Physiological Role and Molecular Aspects of Grapevine Stilbenic Compounds. In Molecular Biology & Biotechnology of the Grapevine; Springer: Dordrecht, The Netherlands, 2001; pp. 153–182. [Google Scholar]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Kopp, P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the “French paradox”? Eur. J. Endocrinol. 1998, 138, 619–620. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. npj Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Shukla, Y.; Singh, R. Resveratrol and cellular mechanisms of cancer prevention. Ann. N. Y. Acad. Sci. 2011, 1215, 1–8. [Google Scholar] [CrossRef]
- Jasiński, M.; Jasińska, L.; Ogrodowczyk, M. Resveratrol in prostate diseases—A short review. Cent. Eur. J. Urol. 2013, 66, 144–149. [Google Scholar]
- Jang, Y.G.; Go, R.E.; Hwang, K.A.; Choi, K.C. Resveratrol inhibits DHT-induced progression of prostate cancer cell line through interfering with the AR and CXCR4 pathway. J. Steroid Biochem. Mol. Biol. 2019, 192, 105406. [Google Scholar] [CrossRef]
- Hsieh, T.; Wu, J.M. Resveratrol Suppresses Prostate Cancer Epithelial Cell Scatter/Invasion by Targeting Inhibition of Hepatocyte Growth Factor (HGF) Secretion by Prostate Stromal Cells and Upregulation of E-cadherin by Prostate Cancer Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 1760. [Google Scholar] [CrossRef]
- Ye, M.; Tian, H.; Lin, S.; Mo, J.; Li, Z.; Chen, X.; Liu, J. Resveratrol inhibits proliferation and promotes apoptosis via the androgen receptor splicing variant 7 and PI3K/AKT signaling pathway in LNCaP prostate cancer cells. Oncol. Lett. 2020, 20. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Z.; Zhang, X. Resveratrol induces apoptosis in murine prostate cancer cells via hypoxia-inducible factor 1-alpha (HIF-1α)/reactive oxygen species (ROS)/P53 signaling. Med. Sci. Monit. 2018, 24, 8970–8976. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wu, X.; Li, Y.; Gao, J.; Wang, F.; Jin, Y.; Chong, T.; Malhotra, A. Evaluation of biophysical as well as biochemical potential of curcumin and resveratrol during prostate cancer. J. Drug Target. 2020, 28, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Siddiqui, I.A.; Panackal, J.E.; Mintie, C.A.; Ahmad, N. Quercetin–resveratrol combination for prostate cancer management in TRAMP mice. Cancers 2020, 12, 2141. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed]
- Levenson, A.S. Metastasis-associated protein 1-mediated antitumor and anticancer activity of dietary stilbenes for prostate cancer chemoprevention and therapy. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, J.; Tringali, C.; Oskarsson, A. Resveratrol, piceatannol and analogs inhibit activation of both wild-type and T877A mutant androgen receptor. J. Steroid Biochem. Mol. Biol. 2017, 174, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dholakia, K.; Sikorska, G.; Martinez, L.A.; Levenson, A.S. Mta1-dependent anticancer activity of gnetin c in prostate cancer. Nutrients 2019, 11, 2096. [Google Scholar] [CrossRef]
- Kato, E.; Tokunaga, Y.; Sakan, F. Stilbenoids isolated from the seeds of melinjo (Gnetum gnemon L.) and their biological activity. J. Agric. Food Chem. 2009, 57, 2544–2549. [Google Scholar] [CrossRef]
- Li, K.; Dias, S.J.; Rimando, A.M.; Dhar, S.; Mizuno, C.S.; Penman, A.D.; Lewin, J.R.; Levenson, A.S. Pterostilbene Acts through Metastasis-Associated Protein 1 to Inhibit Tumor Growth, Progression and Metastasis in Prostate Cancer. PLoS ONE 2013, 8, 0057542. [Google Scholar] [CrossRef]
- Kido, L.A.; Hahm, E.-R.; Kim, S.-H.; Baseggio, A.M.; Cagnon, V.H.A.; Singh, S.; Maróstica, M.R. Prevention of Prostate Cancer in Transgenic Adenocarcinoma of the Mouse Prostate Mice by Yellow Passion Fruit Extract and Antiproliferative Effects of Its Bioactive Compound Piceatannol. J. Cancer Prev. 2020, 25, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Solís, C.; Pedraza-Chaverrí, J.; Torres-Ramos, M.; Jiménez-Farfán, D.; Cruz Salgado, A.; Serrano-García, N.; Osorio-Rico, L.; Sotelo, J. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid. Based Complement. Altern. Med. 2013, 2013, 705121. [Google Scholar] [CrossRef] [PubMed]
- Rowles, J.L.; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Zu, K.; Mucci, L.; Rosner, B.A.; Clinton, S.K.; Loda, M.; Stampfer, M.J.; Giovannucci, E. Dietary lycopene, angiogenesis, and prostate cancer: A prospective study in the prostate-specific antigen era. J. Natl. Cancer Inst. 2014, 106, djt430. [Google Scholar] [CrossRef] [PubMed]
- Elgass, S.; Cooper, A.; Chopra, M. Lycopene treatment of prostate cancer cell lines inhibits adhesion and migration properties of the cells. Int. J. Med. Sci. 2014, 11, 948–954. [Google Scholar] [CrossRef][Green Version]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020, 129, 110459. [Google Scholar] [CrossRef]
- Biernacka, K.M.; Holly, J.M.P.; Martin, R.M.; Frankow, A.; Bull, C.J.; Hamdy, F.C.; Donovan, J.L.; Neal, D.E.; Metcalfe, C.; Lane, A. Effect of green tea and lycopene on the insulin-like growth factor system: The ProDiet randomized controlled trial. Eur. J. Cancer Prev. 2019, 28, 569–575. [Google Scholar] [CrossRef]
- Lee, L.K.; Foo, K.Y. An appraisal of the therapeutic value of lycopene for the chemoprevention of prostate cancer: A nutrigenomic approach. Food Res. Int. 2013, 54, 1217–1228. [Google Scholar] [CrossRef]
- Ansari, M.S.; Gupta, N.P.; Hemal, A.K. Chemoprevention of carcinoma prostate: A review. Int. Urol. Nephrol. 2002, 34, 207–214. [Google Scholar] [CrossRef]
- Wang, Y.; Jacobs, E.J.; Newton, C.C.; McCullough, M.L. Lycopene, tomato products and prostate cancer-specific mortality among men diagnosed with nonmetastatic prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Int. J. Cancer 2016, 138, 2846–2855. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oczkowski, M.; Dziendzikowska, K.; Pasternak-Winiarska, A.; Włodarek, D.; Gromadzka-Ostrowska, J. Dietary Factors and Prostate Cancer Development, Progression, and Reduction. Nutrients 2021, 13, 496. https://doi.org/10.3390/nu13020496
Oczkowski M, Dziendzikowska K, Pasternak-Winiarska A, Włodarek D, Gromadzka-Ostrowska J. Dietary Factors and Prostate Cancer Development, Progression, and Reduction. Nutrients. 2021; 13(2):496. https://doi.org/10.3390/nu13020496
Chicago/Turabian StyleOczkowski, Michał, Katarzyna Dziendzikowska, Anna Pasternak-Winiarska, Dariusz Włodarek, and Joanna Gromadzka-Ostrowska. 2021. "Dietary Factors and Prostate Cancer Development, Progression, and Reduction" Nutrients 13, no. 2: 496. https://doi.org/10.3390/nu13020496
APA StyleOczkowski, M., Dziendzikowska, K., Pasternak-Winiarska, A., Włodarek, D., & Gromadzka-Ostrowska, J. (2021). Dietary Factors and Prostate Cancer Development, Progression, and Reduction. Nutrients, 13(2), 496. https://doi.org/10.3390/nu13020496