Towards an Optimized Fetal DHA Accretion: Differences on Maternal DHA Supplementation Using Phospholipids vs. Triglycerides during Pregnancy in Different Models
Abstract
:1. Introduction
2. DHA Recommendations and Health Outcomes
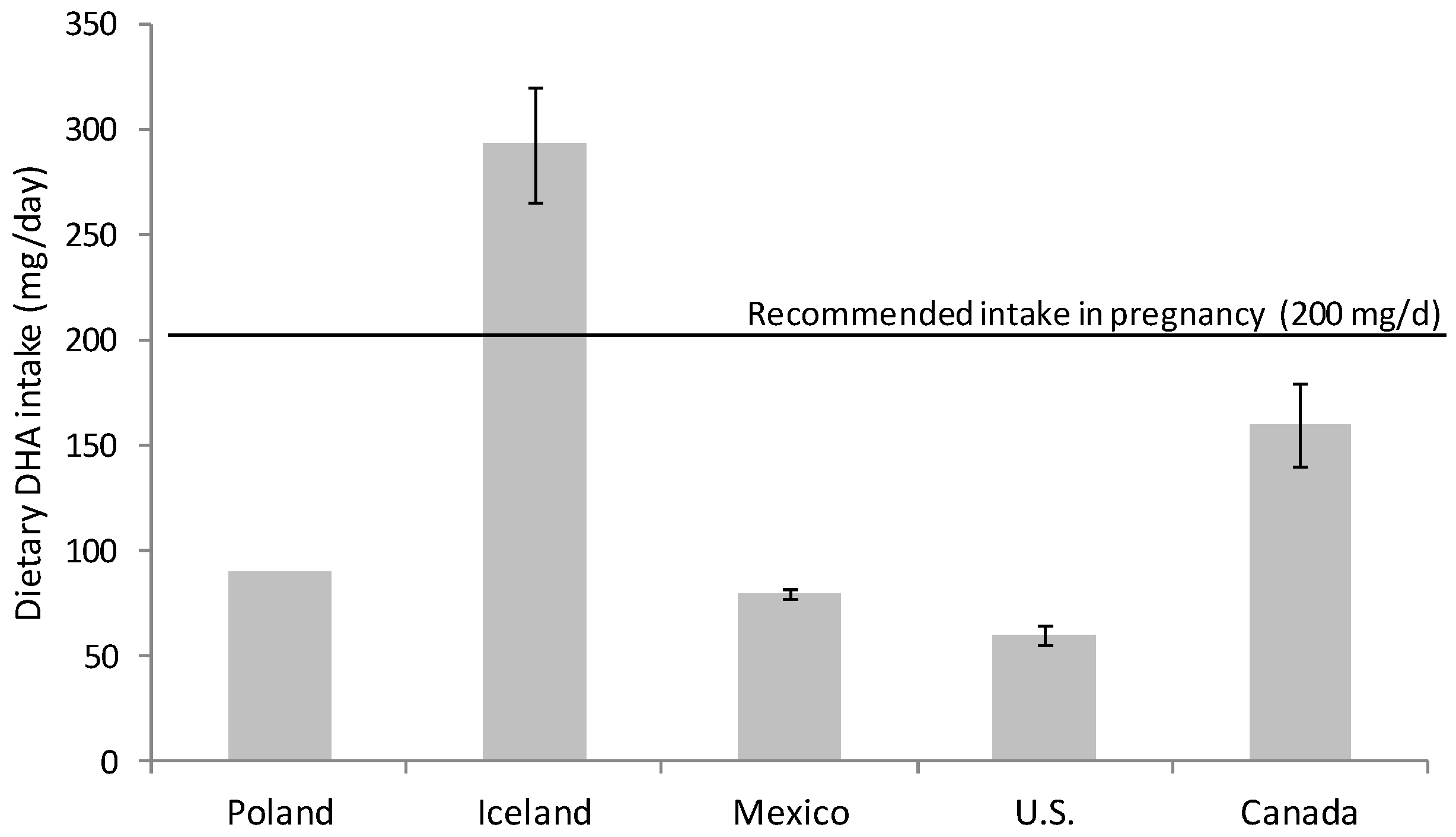
2.1. DHA Intake during the Perinatal Period
2.2. Dietary Recommendation during Pregnancy and Lactation
3. Lipid Sources Utilized in DHA Supplementation
3.1. Fish Oil
3.2. Microalgae Oil
3.3. Enriched Eggs
3.4. Krill Oil
3.5. Lyso-Phospholipids
3.6. Other Sources
3.6.1. Animal Products
3.6.2. Plants
4. Materno-Fetal Bioavailability of Different DHA Sources
4.1. Intestinal Digestion and Absorption
4.2. Circulating DHA and Metabolic Fate
4.3. Placental DHA Uptake and Fetal Accretion
5. DHA Supplementation in Complicated Pregnancies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clandinin, M.T.; Chappell, J.E.; Leong, S.; Heim, T.; Swyer, P.R.; Chance, G.W. Intrauterine fatty acid accretion rates in human brain: Implications for fatty acid requirements. Early Hum. Dev. 1980, 4, 121–129. [Google Scholar] [CrossRef]
- Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef]
- Coletta, J.M.; Bell, S.J.; Roman, A.S. Omega-3 Fatty acids and pregnancy. Rev. Obstet. Gynecol. 2010, 3, 163–171. [Google Scholar] [PubMed]
- Clandinin, M.T.; Chappell, J.E.; Heim, T.; Swyer, P.R.; Chance, G.W. Fatty acid utilization in perinatal de novo synthesis of tissues. Early Hum. Dev. 1981, 5, 355–366. [Google Scholar] [CrossRef]
- Georgieff, M.K.; Innis, S.M. Controversial nutrients that potentially affect preterm neurodevelopment: Essential fatty acids and iron. Pediatr. Res. 2005, 57, 99R–103R. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innis, S.M. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J. Pediatr. 2003, 143, S1–S8. [Google Scholar] [CrossRef] [Green Version]
- Pawlosky, R.J.; Hibbeln, J.R.; Novotny, J.A.; Salem, N., Jr. Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J. Lipid Res. 2001, 42, 1257–1265. [Google Scholar] [CrossRef]
- Goyens, P.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am. J. Clin. Nutr. 2006, 84, 44–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambaz, J.; Ravel, D.; Manier, M.C.; Pepin, D.; Mulliez, N.; Bereziat, G. Essential fatty acids interconversion in the human fetal liver. Biol. Neonate 1985, 47, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Uauy, R.; Hoffman, D.R.; Peirano, P.; Birch, D.G.; Birch, E.E. Essential fatty acids in visual and brain development. Lipids 2001, 36, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Salem, N., Jr.; Wegher, B.; Mena, P.; Uauy, R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc. Natl. Acad. Sci. USA 1996, 93, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef] [PubMed]
- De Groot, R.H.; Hornstra, G.; van Houwelingen, A.C.; Roumen, F. Effect of alpha-linolenic acid supplementation during pregnancy on maternal and neonatal polyunsaturated fatty acid status and pregnancy outcome. Am. J. Clin. Nutr. 2004, 79, 251–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innis, S.M. Polyunsaturated fatty acids in human milk: An essential role in infant development. Adv. Exp. Med. Biol. 2004, 554, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A. Polyunsaturated fatty acids in the food chain in Europe. Am. J. Clin. Nutr. 2000, 71, 176S–178S. [Google Scholar] [CrossRef] [PubMed]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leaf, A.; Weber, P.C. Cardiovascular effects of n-3 fatty acids. N. Engl. J. Med. 1988, 318, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Parra-Cabrera, S.; Stein, A.D.; Wang, M.; Martorell, R.; Rivera, J.; Ramakrishnan, U. Dietary intakes of polyunsaturated fatty acids among pregnant Mexican women. Matern. Child. Nutr. 2011, 7, 140–147. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Lyden, E.; Anderson-Berry, A.; Hanson, C. Omega-3 Fatty Acid Intake of Pregnant Women and Women of Childbearing Age in the United States: Potential for Deficiency? Nutrients 2017, 9, 197. [Google Scholar] [CrossRef] [Green Version]
- Loosemore, E.D.; Judge, M.P.; Lammi-Keefe, C.J. Dietary intake of essential and long-chain polyunsaturated fatty acids in pregnancy. Lipids 2004, 39, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M.; Elias, S.L. Intakes of essential n-6 and n-3 polyunsaturated fatty acids among pregnant Canadian women. Am. J. Clin. Nutr. 2003, 77, 473–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Fulgoni, V.L.; Kris-Etherton, P.M.; Mitmesser, S.H. Dietary Intakes of EPA and DHA Omega-3 Fatty Acids among US Childbearing-Age and Pregnant Women: An Analysis of NHANES 2001–2014. Nutrients 2018, 10, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunnarsdottir, I.; Tryggvadottir, E.A.; Birgisdottir, B.E.; Halldorsson, T.I.; Medek, H.; Geirsson, R.T. Diet and nutrient intake of pregnant women in the capital area in Iceland. Laeknabladid 2016, 102, 378–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wierzejska, R.; Jarosz, M.; Wojda, B.; Siuba-Strzelinska, M. Dietary intake of DHA during pregnancy: A significant gap between the actual intake and current nutritional recommendations. Rocz. Panstw. Zakl. Hig. 2018, 69, 381–386. [Google Scholar] [CrossRef]
- Gil-Sanchez, A.; Larque, E.; Demmelmair, H.; Acien, M.I.; Faber, F.L.; Parrilla, J.J.; Koletzko, B. Maternal-fetal in vivo transfer of [13C]docosahexaenoic and other fatty acids across the human placenta 12 h after maternal oral intake. Am. J. Clin. Nutr. 2010, 92, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larque, E.; Demmelmair, H.; Berger, B.; Hasbargen, U.; Koletzko, B. In vivo investigation of the placental transfer of (13)C-labeled fatty acids in humans. J. Lipid Res. 2003, 44, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, M.A.; Costeloe, K.; Ghebremeskel, K.; Phylactos, A.; Skirvin, L.; Stacey, F. Are deficits of arachidonic and docosahexaenoic acids responsible for the neural and vascular complications of preterm babies? Am. J. Clin. Nutr. 1997, 66, 1032S–1041S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitsanis, D.; Crawford, M.A.; Moodley, T.; Holmsen, H.; Ghebremeskel, K.; Djahanbakhch, O. Arachidonic acid predominates in the membrane phosphoglycerides of the early and term human placenta. J. Nutr. 2005, 135, 2566–2571. [Google Scholar] [CrossRef] [Green Version]
- Crawford, M.A.; Hassam, A.G.; Williams, G. Essential fatty acids and fetal brain growth. Lancet 1976, 1, 452–453. [Google Scholar] [CrossRef]
- Benassayag, C.; Mignot, T.M.; Haourigui, M.; Civel, C.; Hassid, J.; Carbonne, B.; Nunez, E.A.; Ferre, F. High polyunsaturated fatty acid, thromboxane A2, and alpha-fetoprotein concentrations at the human feto-maternal interface. J. Lipid Res. 1997, 38, 276–286. [Google Scholar] [CrossRef]
- Lauritzen, L.; Carlson, S.E. Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status. Matern. Child. Nutr. 2011, 7, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Guesnet, P.; Pugo-Gunsam, P.; Maurage, C.; Pinault, M.; Giraudeau, B.; Alessandri, J.M.; Durand, G.; Antoine, J.M.; Couet, C. Blood lipid concentrations of docosahexaenoic and arachidonic acids at birth determine their relative postnatal changes in term infants fed breast milk or formula. Am. J. Clin. Nutr. 1999, 70, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, S.L.; Innis, S.M. Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length. Am. J. Clin. Nutr. 2001, 73, 807–814. [Google Scholar] [CrossRef]
- Helland, I.B.; Saugstad, O.D.; Smith, L.; Saarem, K.; Solvoll, K.; Ganes, T.; Drevon, C.A. Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics 2001, 108, E82. [Google Scholar] [CrossRef] [Green Version]
- Hibbeln, J.R.; Davis, J.M.; Steer, C.; Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet 2007, 369, 578–585. [Google Scholar] [CrossRef]
- Oken, E.; Wright, R.O.; Kleinman, K.P.; Bellinger, D.; Amarasiriwardena, C.J.; Hu, H.; Rich-Edwards, J.W.; Gillman, M.W. Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort. Environ. Health Perspect. 2005, 113, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Fatty acids and early human development. Early Hum. Dev. 2007, 83, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harslof, L.B.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Emmett, P.M.; Jones, L.R.; Golding, J. Pregnancy diet and associated outcomes in the Avon Longitudinal Study of Parents and Children. Nutr. Rev. 2015, 73, 154–174. [Google Scholar] [CrossRef]
- De Giuseppe, R.; Roggi, C.; Cena, H. n-3 LC-PUFA supplementation: Effects on infant and maternal outcomes. Eur. J. Nutr. 2014, 53, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Smithers, L.G.; Gibson, R.A.; Makrides, M. Maternal supplementation with docosahexaenoic acid during pregnancy does not affect early visual development in the infant: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 1293–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA 2010, 304, 1675–1683. [Google Scholar] [CrossRef] [Green Version]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev. 2018, 11, CD003402. [Google Scholar] [CrossRef]
- Larque, E.; Demmelmair, H.; Gil-Sanchez, A.; Prieto-Sanchez, M.T.; Blanco, J.E.; Pagan, A.; Faber, F.L.; Zamora, S.; Parrilla, J.J.; Koletzko, B. Placental transfer of fatty acids and fetal implications. Am. J. Clin. Nutr. 2011, 94, 1908S–1913S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newberry, S.J.; Chung, M.; Booth, M.; Maglione, M.A.; Tang, A.M.; O’Hanlon, C.E.; Wang, D.D.; Okunogbe, A.; Huang, C.; Motala, A.; et al. Omega-3 Fatty Acids and Maternal and Child Health: An Updated Systematic Review. Evid. Rep. Technol. Assess 2016, 1–826. [Google Scholar] [CrossRef] [Green Version]
- Innis, S.M. The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev. Neurosci. 2000, 22, 474–480. [Google Scholar] [CrossRef]
- Lauritzen, L.; Hansen, H.S.; Jorgensen, M.H.; Michaelsen, K.F. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 2001, 40, 1–94. [Google Scholar] [CrossRef]
- World Health Organization. Fats and fatty acids in human nutrition. In Proceedings of the Joint FAO/WHO Expert Consultation, Geneva, Switzerland, 10–14 November 2008; pp. 5–300. [Google Scholar]
- Koletzko, B.; Cetin, I.; Brenna, J.T. Dietary fat intakes for pregnant and lactating women. Br. J. Nutr. 2007, 98, 873–877. [Google Scholar] [CrossRef] [Green Version]
- Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion of the Panel on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar]
- Lee, J.H.; O’Keefe, J.H.; Lavie, C.J.; Harris, W.S. Omega-3 fatty acids: Cardiovascular benefits, sources and sustainability. Nat. Rev. Cardiol. 2009, 6, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Hill, A.M. N-3 fatty acids: Food or supplements? J. Am. Diet. Assoc. 2008, 108, 1125–1130. [Google Scholar] [CrossRef]
- Valenzuela, A.; Nieto, M.S. Acido docosahexaenoico (DHA) en el desarrollo fetal y en la nutrición materno-infantil. Rev. Méd. Chile 2001, 129, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, B.; Brothersen, C.; McMahon, D.J. Fortification of foods with omega-3 polyunsaturated fatty acids. Crit. Rev. Food Sci. Nutr. 2014, 54, 98–114. [Google Scholar] [CrossRef]
- Martins, D.A.; Custodio, L.; Barreira, L.; Pereira, H.; Ben-Hamadou, R.; Varela, J.; Abu-Salah, K.M. Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar. Drugs 2013, 11, 2259–2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adarme-Vega, T.C.; Lim, D.K.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Fact 2012, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Diez, S. Human health effects of methylmercury exposure. Rev. Environ. Contam. Toxicol. 2009, 198, 111–132. [Google Scholar] [CrossRef]
- Antonelli, M.C.; Pallares, M.E.; Ceccatelli, S.; Spulber, S. Long-term consequences of prenatal stress and neurotoxicants exposure on neurodevelopment. Prog. Neurobiol. 2017, 155, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Adarme-Vega, T.C.; Thomas-Hall, S.R.; Schenk, P.M. Towards sustainable sources for omega-3 fatty acids production. Curr. Opin. Biotechnol. 2014, 26, 14–18. [Google Scholar] [CrossRef]
- Kyle, D.J.; Arterburn, L.M. Single cell oil sources of docosahexaenoic acid: Clinical studies. World Rev. Nutr. Diet. 1998, 83, 116–131. [Google Scholar] [PubMed]
- Hammond, B.G.; Mayhew, D.A.; Naylor, M.W.; Ruecker, F.A.; Mast, R.W.; Sander, W.J. Safety assessment of DHA-rich microalgae from Schizochytrium sp. Regul. Toxicol. Pharmacol. 2001, 33, 192–204. [Google Scholar] [CrossRef]
- Rehault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef] [Green Version]
- Scheideler, S.E.; Froning, G.W. The combined influence of dietary flaxseed variety, level, form, and storage conditions on egg production and composition among vitamin E-supplemented hens. Poult. Sci. 1996, 75, 1221–1226. [Google Scholar] [CrossRef]
- Hargis, P.S.; Van Elswyk, M.E.; Hargis, B.M. Dietary modification of yolk lipid with menhaden oil. Poult. Sci. 1991, 70, 874–883. [Google Scholar] [CrossRef]
- Leskanich, C.O.; Noble, R.C. Manipulation of the n-3 polyunsaturated fatty acid composition of avian eggs and meat. World Poult. Sci. J. 1997, 53, 155–183. [Google Scholar] [CrossRef]
- Schreiner, M.; Moreira, R.G.; Hulan, H.W. Positional distribution of fatty acids in egg yolk lipids. J. Food Lipids 2006, 13, 36–56. [Google Scholar] [CrossRef]
- Makrides, M.; Hawkes, J.S.; Neumann, M.A.; Gibson, R.A. Nutritional effect of including egg yolk in the weaning diet of breast-fed and formula-fed infants: A randomized controlled trial. Am. J. Clin. Nutr. 2002, 75, 1084–1092. [Google Scholar] [CrossRef] [Green Version]
- Farrell, D.J. Enrichment of hen eggs with n-3 long-chain fatty acids and evaluation of enriched eggs in humans. Am. J. Clin. Nutr. 1998, 68, 538–544. [Google Scholar] [CrossRef]
- Jiang, Z.; Sim, J.S. Consumption of n-3 polyunsaturated fatty acid-enriched eggs and changes in plasma lipids of human subjects. Nutrition 1993, 9, 513–518. [Google Scholar] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [Green Version]
- Akoh, C.C. Handbook of Functional Lipids; Taylor & Francis: Boca Raton, FL, USA, 2006; p. 525. [Google Scholar]
- Cohn, J.S.; Kamili, A.; Wat, E.; Chung, R.W.; Tandy, S. Dietary phospholipids and intestinal cholesterol absorption. Nutrients 2010, 2, 116–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blesso, C.N. Egg phospholipids and cardiovascular health. Nutrients 2015, 7, 2731–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kullenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Kwantes, J.M.; Grundmann, O. A brief review of krill oil history, research, and the commercial market. J. Diet Suppl. 2015, 12, 23–35. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E.-D. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef] [Green Version]
- Deutsch, L. Evaluation of the effect of Neptune Krill Oil on chronic inflammation and arthritic symptoms. J. Am. Coll. Nutr. 2007, 26, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, M.; Bernoud, N.; Brossard, N.; Lemaitre-Delaunay, D.; Thies, F.; Croset, M.; Lecerf, J. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 2001, 16, 201–204. [Google Scholar] [CrossRef]
- Thies, F.; Pillon, C.; Moliere, P.; Lagarde, M.; Lecerf, J. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am. J. Physiol. 1994, 267, R1273–R1279. [Google Scholar] [CrossRef] [PubMed]
- Chouinard-Watkins, R.; Lacombe, R.J.S.; Metherel, A.H.; Masoodi, M.; Bazinet, R.P. DHA Esterified to Phosphatidylserine or Phosphatidylcholine is More Efficient at Targeting the Brain than DHA Esterified to Triacylglycerol. Mol. Nutr. Food Res. 2019, 63, e1801224. [Google Scholar] [CrossRef]
- Sugasini, D.; Thomas, R.; Yalagala, P.C.R.; Tai, L.M.; Subbaiah, P.V. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 2017, 7, 11263. [Google Scholar] [CrossRef]
- Sugasini, D.; Yalagala, P.C.R.; Subbaiah, P.V. Efficient Enrichment of Retinal DHA with Dietary Lysophosphatidylcholine-DHA: Potential Application for Retinopathies. Nutrients 2020, 12, 3114. [Google Scholar] [CrossRef]
- Brossard, N.; Croset, M.; Normand, S.; Pousin, J.; Lecerf, J.; Laville, M.; Tayot, J.L.; Lagarde, M. Human plasma albumin transports [13C]docosahexaenoic acid in two lipid forms to blood cells. J. Lipid Res. 1997, 38, 1571–1582. [Google Scholar] [CrossRef]
- Ghebremeskel, K.; Min, Y.; Crawford, M.A.; Nam, J.H.; Kim, A.; Koo, J.N.; Suzuki, H. Blood fatty acid composition of pregnant and nonpregnant Korean women: Red cells may act as a reservoir of arachidonic acid and docosahexaenoic acid for utilization by the developing fetus. Lipids 2000, 35, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, A.; Nieto, S.; Sanhueza, J.; Morgado, N.; Rojas, I.; Zañartu, P. Supplementing female rats with DHA-lysophosphatidylcholine increases docosahexaenoic acid and acetylcholine contents in the brain and improves the memory and learning capabilities of the pups. Grasas Aceites 2010, 61, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Hachem, M.; Geloen, A.; Van, A.L.; Foumaux, B.; Fenart, L.; Gosselet, F.; Da Silva, P.; Breton, G.; Lagarde, M.; Picq, M.; et al. Efficient Docosahexaenoic Acid Uptake by the Brain from a Structured Phospholipid. Mol. Neurobiol. 2016, 53, 3205–3215. [Google Scholar] [CrossRef] [PubMed]
- Chouinard-Watkins, R.; Chen, C.T.; Metherel, A.H.; Lacombe, R.J.S.; Thies, F.; Masoodi, M.; Bazinet, R.P. Phospholipid class-specific brain enrichment in response to lysophosphatidylcholine docosahexaenoic acid infusion. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1092–1098. [Google Scholar] [CrossRef]
- Sugasini, D.; Yalagala, P.C.R.; Goggin, A.; Tai, L.M.; Subbaiah, P.V. Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: Lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J. Nutr. Biochem. 2019, 74, 108231. [Google Scholar] [CrossRef]
- Hosomi, R.; Fukunaga, K.; Nagao, T.; Tanizaki, T.; Miyauchi, K.; Yoshida, M.; Kanda, S.; Nishiyama, T.; Takahashi, K. Effect of Dietary Partial Hydrolysate of Phospholipids, Rich in Docosahexaenoic Acid-Bound Lysophospholipids, on Lipid and Fatty Acid Composition in Rat Serum and Liver. J. Food Sci. 2019, 84, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Hachem, M.; Nacir, H.; Picq, M.; Belkouch, M.; Bernoud-Hubac, N.; Windust, A.; Meiller, L.; Sauvinet, V.; Feugier, N.; Lambert-Porcheron, S.; et al. Docosahexaenoic Acid (DHA) Bioavailability in Humans after Oral Intake of DHA-Containing Triacylglycerol or the Structured Phospholipid AceDoPC((R)). Nutrients 2020, 12, 251. [Google Scholar] [CrossRef] [Green Version]
- Croset, M.; Brossard, N.; Polette, A.; Lagarde, M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem. J. 2000, 345, 61–67. [Google Scholar] [CrossRef]
- Lo Van, A.; Sakayori, N.; Hachem, M.; Belkouch, M.; Picq, M.; Lagarde, M.; Osumi, N.; Bernoud-Hubac, N. Mechanisms of DHA transport to the brain and potential therapy to neurodegenerative diseases. Biochimie 2016, 130, 163–167. [Google Scholar] [CrossRef]
- Subbaiah, P.V.; Dammanahalli, K.J.; Yang, P.; Bi, J.; O’Donnell, J.M. Enhanced incorporation of dietary DHA into lymph phospholipids by altering its molecular carrier. Biochim. Biophys. Acta 2016, 1861, 723–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polette, A.; Deshayes, C.; Chantegrel, B.; Croset, M.; Armstrong, J.M.; Lagarde, M. Synthesis of acetyl,docosahexaenoyl-glycerophosphocholine and its characterization using nuclear magnetic resonance. Lipids 1999, 34, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, M.; Hachem, M.; Bernoud-Hubac, N.; Picq, M.; Vericel, E.; Guichardant, M. Biological properties of a DHA-containing structured phospholipid (AceDoPC) to target the brain. Prostaglandins Leukot Essent Fatty Acids 2015, 92, 63–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Givens, D.I.; Kliem, K.E.; Gibbs, R.A. The role of meat as a source of n-3 polyunsaturated fatty acids in the human diet. Meat Sci. 2006, 74, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Rymer, C.; Givens, D.I. n-3 fatty acid enrichment of edible tissue of poultry: A review. Lipids 2005, 40, 121–130. [Google Scholar] [CrossRef]
- Lee, S.A.; Whenham, N.; Bedford, M.R. Review on docosahexaenoic acid in poultry and swine nutrition: Consequence of enriched animal products on performance and health characteristics. Anim. Nutr. 2019, 5, 11–21. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Malau-Aduli, B.S.; Cavalieri, J.; Malau-Aduli, A.E.O.; Nichols, P.D. Enhancing Omega-3 Long-Chain Polyunsaturated Fatty Acid Content of Dairy-Derived Foods for Human Consumption. Nutrients 2019, 11, 743. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Lopez, N.; Usher, S.; Sayanova, O.V.; Napier, J.A.; Haslam, R.P. Modifying the lipid content and composition of plant seeds: Engineering the production of LC-PUFA. Appl. Microbiol. Biotechnol. 2015, 99, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, 1183–1194. [Google Scholar] [CrossRef] [Green Version]
- Gil-Sanchez, A.; Demmelmair, H.; Parrilla, J.J.; Koletzko, B.; Larque, E. Mechanisms involved in the selective transfer of long chain polyunsaturated Fatty acids to the fetus. Front. Genet. 2011, 2, 57. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, I.; Sasaki, E.; Yasunami, H.; Nomiyama, S.; Nakayama, M.; Sugano, M.; Imaizumi, K.; Yazawa, K. Digestion and lymphatic transport of eicosapentaenoic and docosahexaenoic acids given in the form of triacylglycerol, free acid and ethyl ester in rats. Biochim. Biophys. Acta 1995, 1259, 297–304. [Google Scholar] [CrossRef]
- Tou, J.C.; Altman, S.N.; Gigliotti, J.C.; Benedito, V.A.; Cordonier, E.L. Different sources of omega-3 polyunsaturated fatty acids affects apparent digestibility, tissue deposition, and tissue oxidative stability in growing female rats. Lipids Health Dis. 2011, 10, 179. [Google Scholar] [CrossRef] [Green Version]
- Amate, L.; Gil, A.; Ramirez, M. Dietary long-chain polyunsaturated fatty acids from different sources affect fat and fatty acid excretions in rats. J. Nutr. 2001, 131, 3216–3221. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Fujimoto, K.; Miyazawa, T. Polyunsaturated (n-3) fatty acids susceptible to peroxidation are increased in plasma and tissue lipids of rats fed docosahexaenoic acid-containing oils. J. Nutr. 2000, 130, 3028–3033. [Google Scholar] [CrossRef] [Green Version]
- Gazquez, A.; Hernandez-Albaladejo, I.; Larque, E. Docosahexaenoic acid supplementation during pregnancy as phospholipids did not improve the incorporation of this fatty acid into rat fetal brain compared with the triglyceride form. Nutr. Res. 2017, 37, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, A.; Nieto, S.; Sanhueza, J.; Nunez, M.J.; Ferrer, C. Tissue accretion and milk content of docosahexaenoic acid in female rats after supplementation with different docosahexaenoic acid sources. Ann. Nutr. Metab. 2005, 49, 325–332. [Google Scholar] [CrossRef]
- Amate, L.; Gil, A.; Ramirez, M. Feeding infant piglets formula with long-chain polyunsaturated fatty acids as triacylglycerols or phospholipids influences the distribution of these fatty acids in plasma lipoprotein fractions. J. Nutr. 2001, 131, 1250–1255. [Google Scholar] [CrossRef] [Green Version]
- Mathews, S.A.; Oliver, W.T.; Phillips, O.T.; Odle, J.; Diersen-Schade, D.A.; Harrell, R.J. Comparison of triglycerides and phospholipids as supplemental sources of dietary long-chain polyunsaturated fatty acids in piglets. J. Nutr. 2002, 132, 3081–3089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, J.; Boza, J.; Suárez, M.D.; Gil, A. The effect of a formula supplemented with n-3 and n-6 long-chain polyunsaturated fatty acids on plasma phospholipid, liver microsomal, retinal, and brain fatty acid composition in neonatal piglets. J. Nutr. Biochem. 1997, 8, 217–223. [Google Scholar] [CrossRef]
- Alessandri, J.M.; Goustard, B.; Guesnet, P.; Durand, G. Docosahexaenoic acid concentrations in retinal phospholipids of piglets fed an infant formula enriched with long-chain polyunsaturated fatty acids: Effects of egg phospholipids and fish oils with different ratios of eicosapentaenoic acid to docosahexaenoic acid. Am. J. Clin. Nutr. 1998, 67, 377–385. [Google Scholar]
- Gazquez, A.; Ruiz-Palacios, M.; Larque, E. DHA supplementation during pregnancy as phospholipids or TAG produces different placental uptake but similar fetal brain accretion in neonatal piglets. Br. J. Nutr. 2017, 118, 981–988. [Google Scholar] [CrossRef] [Green Version]
- Carnielli, V.P.; Verlato, G.; Pederzini, F.; Luijendijk, I.; Boerlage, A.; Pedrotti, D.; Sauer, P.J. Intestinal absorption of long-chain polyunsaturated fatty acids in preterm infants fed breast milk or formula. Am. J. Clin. Nutr. 1998, 67, 97–103. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Castellote, A.I.; Campoy, C.; Rivero, M.; Rodriguez-Palmero, M.; Lopez-Sabater, M.C. The source of long-chain PUFA in formula supplements does not affect the fatty acid composition of plasma lipids in full-term infants. J. Nutr. 2004, 134, 868–873. [Google Scholar] [CrossRef] [Green Version]
- Vaisman, N.; Kaysar, N.; Zaruk-Adasha, Y.; Pelled, D.; Brichon, G.; Zwingelstein, G.; Bodennec, J. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: Effect of dietary n-3 fatty acids containing phospholipids. Am. J. Clin. Nutr. 2008, 87, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Le Kim, D.; Betzing, H. Intestinal absorption of polyunsaturated phosphatidylcholine in the rat. Hoppe Seylers Z. Physiol. Chem. 1976, 357, 1321–1331. [Google Scholar] [CrossRef]
- Subbaiah, P.V.; Ganguly, J. Studies on the phospholipases of rat intestinal mucosa. Biochem. J. 1970, 118, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Yang, Y.; Braunstein, E.; Georgeson, K.E.; Harmon, C.M. Gut expression and regulation of FAT/CD36: Possible role in fatty acid transport in rat enterocytes. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E916–E923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niot, I.; Poirier, H.; Tran, T.T.; Besnard, P. Intestinal absorption of long-chain fatty acids: Evidence and uncertainties. Prog. Lipid Res. 2009, 48, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.T.; Tso, P. Intestinal lipid absorption and transport. Front. Biosci. 2001, 6, D299–D319. [Google Scholar] [CrossRef] [Green Version]
- Tso, P.; Drake, D.S.; Black, D.D.; Sabesin, S.M. Evidence for separate pathways of chylomicron and very low-density lipoprotein assembly and transport by rat small intestine. Am. J. Physiol. 1984, 247, G599–G610. [Google Scholar] [CrossRef] [PubMed]
- Amate, L.; Gil, A.; Ramirez, M. Dietary long-chain PUFA in the form of TAG or phospholipids influence lymph lipoprotein size and composition in piglets. Lipids 2002, 37, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Zierenberg, O.; Grundy, S.M. Intestinal absorption of polyenephosphatidylcholine in man. J. Lipid Res. 1982, 23, 1136–1142. [Google Scholar] [CrossRef]
- Krimbou, L.; Hajj Hassan, H.; Blain, S.; Rashid, S.; Denis, M.; Marcil, M.; Genest, J. Biogenesis and speciation of nascent apoA-I-containing particles in various cell lines. J. Lipid Res. 2005, 46, 1668–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, C.; Davies, L.; Corcoran, F.; Stammers, J.; Colley, J.; Spencer, S.A.; Hull, D. Fatty acid balance studies in term infants fed formula milk containing long-chain polyunsaturated fatty acids. Acta Paediatr. 1998, 87, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Salem, N., Jr.; Kuratko, C.N. A reexamination of krill oil bioavailability studies. Lipids Health Dis. 2014, 13, 137. [Google Scholar] [CrossRef] [Green Version]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids 2013, 89, 1–8. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Schneider, I.; Meyer, H.; Neubronner, J.; von Schacky, C.; Hahn, A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations—A comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis. 2011, 10, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, P.; Zhu, H.; Breivik, J.F.; Hjelle, J.I.; Zeng, Y. Determination and structural elucidation of triacylglycerols in krill oil by chromatographic techniques. Lipids 2014, 49, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Srigley, C.T.; Orr-Tokle, I.C. Presence of Fatty-Acid Ethyl Esters in Krill Oil Dietary Supplements. Lipids 2018, 53, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Moughan, P.J.; Birtles, M.J.; Cranwell, P.D.; Smith, W.C.; Pedraza, M. The piglet as a model animal for studying aspects of digestion and absorption in milk-fed human infants. World Rev. Nutr. Diet 1992, 67, 40–113. [Google Scholar] [CrossRef]
- Small, D.M. The effects of glyceride structure on absorption and metabolism. Annu. Rev. Nutr. 1991, 11, 413–434. [Google Scholar] [CrossRef]
- Pufal, D.A.; Quinlan, P.T.; Salter, A.M. Effect of dietary triacylglycerol structure on lipoprotein metabolism: A comparison of the effects of dioleoylpalmitoylglycerol in which palmitate is esterified to the 2- or 1(3)-position of the glycerol. Biochim. Biophys. Acta 1995, 1258, 41–48. [Google Scholar] [CrossRef]
- Amate, L.; Ramirez, M.; Gil, A. Positional analysis of triglycerides and phospholipids rich in long-chain polyunsaturated fatty acids. Lipids 1999, 34, 865–871. [Google Scholar] [CrossRef]
- Myher, J.J.; Kuksis, A.; Geher, K.; Park, P.W.; Diersen-Schade, D.A. Stereospecific analysis of triacylglycerols rich in long-chain polyunsaturated fatty acids. Lipids 1996, 31, 207–215. [Google Scholar] [CrossRef]
- Christensen, M.S.; Hoy, C.E.; Becker, C.C.; Redgrave, T.G. Intestinal absorption and lymphatic transport of eicosapentaenoic (EPA), docosahexaenoic (DHA), and decanoic acids: Dependence on intramolecular triacylglycerol structure. Am. J. Clin. Nutr. 1995, 61, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Carnielli, V.P.; Luijendijk, I.H.; van Goudoever, J.B.; Sulkers, E.J.; Boerlage, A.A.; Degenhart, H.J.; Sauer, P.J. Feeding premature newborn infants palmitic acid in amounts and stereoisomeric position similar to that of human milk: Effects on fat and mineral balance. Am. J. Clin. Nutr. 1995, 61, 1037–1042. [Google Scholar] [CrossRef]
- Goustard-Langelier, B.; Guesnet, P.; Durand, G.; Antoine, J.M.; Alessandri, J.M. n-3 and n-6 fatty acid enrichment by dietary fish oil and phospholipid sources in brain cortical areas and nonneural tissues of formula-fed piglets. Lipids 1999, 34, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sanchez, A.; Koletzko, B.; Larque, E. Current understanding of placental fatty acid transport. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Lindegaard, M.L.; Olivecrona, G.; Christoffersen, C.; Kratky, D.; Hannibal, J.; Petersen, B.L.; Zechner, R.; Damm, P.; Nielsen, L.B. Endothelial and lipoprotein lipases in human and mouse placenta. J. Lipid Res. 2005, 46, 2339–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haggarty, P. Placental regulation of fatty acid delivery and its effect on fetal growth—A review. Placenta 2002, 23, S28–S38. [Google Scholar] [CrossRef] [PubMed]
- Dutta-Roy, A.K. Transport mechanisms for long-chain polyunsaturated fatty acids in the human placenta. Am. J. Clin. Nutr. 2000, 71, 315S–322S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauster, M.; Rechberger, G.; Sovic, A.; Horl, G.; Steyrer, E.; Sattler, W.; Frank, S. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J. Lipid Res. 2005, 46, 1517–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCoy, M.G.; Sun, G.S.; Marchadier, D.; Maugeais, C.; Glick, J.M.; Rader, D.J. Characterization of the lipolytic activity of endothelial lipase. J. Lipid Res. 2002, 43, 921–929. [Google Scholar] [CrossRef]
- Chen, S.; Subbaiah, P.V. Phospholipid and fatty acid specificity of endothelial lipase: Potential role of the enzyme in the delivery of docosahexaenoic acid (DHA) to tissues. Biochim. Biophys. Acta 2007, 1771, 1319–1328. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- Hanebutt, F.L.; Demmelmair, H.; Schiessl, B.; Larque, E.; Koletzko, B. Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin. Nutr. 2008, 27, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Larque, E.; Ruiz-Palacios, M.; Koletzko, B. Placental regulation of fetal nutrient supply. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 292–297. [Google Scholar] [CrossRef]
- Ferchaud-Roucher, V.; Kramer, A.; Silva, E.; Pantham, P.; Weintraub, S.T.; Jansson, T.; Powell, T.L. A potential role for lysophosphatidylcholine in the delivery of long chain polyunsaturated fatty acids to the fetal circulation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 394–402. [Google Scholar] [CrossRef]
- Perazzolo, S.; Hirschmugl, B.; Wadsack, C.; Desoye, G.; Lewis, R.M.; Sengers, B.G. The influence of placental metabolism on fatty acid transfer to the fetus. J. Lipid Res. 2017, 58, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazquez, A.; Prieto-Sanchez, M.T.; Blanco-Carnero, J.E.; van Harskamp, D.; Perazzolo, S.; Oosterink, J.E.; Demmelmair, H.; Schierbeek, H.; Sengers, B.G.; Lewis, R.M.; et al. In vivo kinetic study of materno-fetal fatty acid transfer in obese and normal weight pregnant women. J. Physiol. 2019. [Google Scholar] [CrossRef]
- Klingler, M.; Demmelmair, H.; Larque, E.; Koletzko, B. Analysis of FA contents in individual lipid fractions from human placental tissue. Lipids 2003, 38, 561–566. [Google Scholar] [CrossRef]
- Johnsen, G.M.; Weedon-Fekjaer, M.S.; Tobin, K.A.; Staff, A.C.; Duttaroy, A.K. Long-chain polyunsaturated fatty acids stimulate cellular fatty acid uptake in human placental choriocarcinoma (BeWo) cells. Placenta 2009, 30, 1037–1044. [Google Scholar] [CrossRef]
- Tomedi, L.E.; Chang, C.C.; Newby, P.K.; Evans, R.W.; Luther, J.F.; Wisner, K.L.; Bodnar, L.M. Pre-pregnancy obesity and maternal nutritional biomarker status during pregnancy: A factor analysis. Public Health Nutr. 2013, 16, 1414–1418. [Google Scholar] [CrossRef] [Green Version]
- Vidakovic, A.J.; Gishti, O.; Voortman, T.; Felix, J.F.; Williams, M.A.; Hofman, A.; Demmelmair, H.; Koletzko, B.; Tiemeier, H.; Jaddoe, V.W.; et al. Maternal plasma PUFA concentrations during pregnancy and childhood adiposity: The Generation R Study. Am. J. Clin. Nutr. 2016, 103, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Ghebremeskel, K.; Thomas, B.; Lowy, C.; Min, Y.; Crawford, M.A. Type 1 diabetes compromises plasma arachidonic and docosahexaenoic acids in newborn babies. Lipids 2004, 39, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Lowy, C.; Ghebremeskel, K.; Thomas, B.; Offley-Shore, B.; Crawford, M. Unfavorable effect of type 1 and type 2 diabetes on maternal and fetal essential fatty acid status: A potential marker of fetal insulin resistance. Am. J. Clin. Nutr. 2005, 82, 1162–1168. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B.A.; Ghebremeskel, K.; Lowy, C.; Offley-Shore, B.; Crawford, M.A. Plasma fatty acids of neonates born to mothers with and without gestational diabetes. Prostaglandins Leukot Essent Fatty Acids 2005, 72, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Pagan, A.; Prieto-Sanchez, M.T.; Blanco-Carnero, J.E.; Gil-Sanchez, A.; Parrilla, J.J.; Demmelmair, H.; Koletzko, B.; Larque, E. Materno-fetal transfer of docosahexaenoic acid is impaired by gestational diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E826–E833. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Sanchez, M.T.; Ruiz-Palacios, M.; Blanco-Carnero, J.E.; Pagan, A.; Hellmuth, C.; Uhl, O.; Peissner, W.; Ruiz-Alcaraz, A.J.; Parrilla, J.J.; Koletzko, B.; et al. Placental MFSD2a transporter is related to decreased DHA in cord blood of women with treated gestational diabetes. Clin. Nutr. 2017, 36, 513–521. [Google Scholar] [CrossRef]
- Soygur, B.; Sati, L.; Demir, R. Altered expression of human endogenous retroviruses syncytin-1, syncytin-2 and their receptors in human normal and gestational diabetic placenta. Histol. Histopathol. 2016, 31, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Palacios, M.; Prieto-Sanchez, M.T.; Ruiz-Alcaraz, A.J.; Blanco-Carnero, J.E.; Sanchez-Campillo, M.; Parrilla, J.J.; Larque, E. Insulin Treatment May Alter Fatty Acid Carriers in Placentas from Gestational Diabetes Subjects. Int. J. Mol. Sci. 2017, 18, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dube, E.; Ethier-Chiasson, M.; Lafond, J. Modulation of cholesterol transport by insulin-treated gestational diabetes mellitus in human full-term placenta. Biol. Reprod. 2013, 88, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dube, E.; Gravel, A.; Martin, C.; Desparois, G.; Moussa, I.; Ethier-Chiasson, M.; Forest, J.C.; Giguere, Y.; Masse, A.; Lafond, J. Modulation of fatty acid transport and metabolism by maternal obesity in the human full-term placenta. Biol. Reprod. 2012, 87, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, G.; Fabrizi, M.; Rava, L.; Ciofi Degli Atti, M.; Vernocchi, P.; Vallone, C.; Pietrantoni, E.; Lanciotti, R.; Signore, F.; Manco, M. Influence of Maternal Obesity and Gestational Weight Gain on Maternal and Foetal Lipid Profile. Nutrients 2016, 8, 368. [Google Scholar] [CrossRef] [Green Version]
- Gazquez, A.; Uhl, O.; Ruiz-Palacios, M.; Gill, C.; Patel, N.; Koletzko, B.; Poston, L.; Larque, E. Placental lipid droplet composition: Effect of a lifestyle intervention (UPBEAT) in obese pregnant women. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 998–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, Y.; Djahanbakhch, O.; Hutchinson, J.; Eram, S.; Bhullar, A.S.; Namugere, I.; Ghebremeskel, K. Efficacy of docosahexaenoic acid-enriched formula to enhance maternal and fetal blood docosahexaenoic acid levels: Randomized double-blinded placebo-controlled trial of pregnant women with gestational diabetes mellitus. Clin. Nutr. 2016, 35, 608–614. [Google Scholar] [CrossRef] [Green Version]
- Monthe-Dreze, C.; Penfield-Cyr, A.; Smid, M.C.; Sen, S. Maternal Pre-Pregnancy Obesity Attenuates Response to Omega-3 Fatty Acids Supplementation During Pregnancy. Nutrients 2018, 10, 1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Ref. | Age | Pregnant | DHA Sources | DHA Dose | Mode of Administration | Outcomes Measured | Major Findings |
|---|---|---|---|---|---|---|---|
| Rat/mouse models | |||||||
| [79] | Young male rats | No | 3H-DHA as Lyso-phosphatidylcholine (Lyso-PC) and NEFA | 12 nmol | Tracer infusion | 3H-DHA enrichment in brain, liver, kidney and heart | ↑ Incorporation of 3H-DHA as Lyso-PC in the brain. Similar or ↓ incorporation in other tissues compared to NEFA |
| [81] | Adult male mice | No | Lyso-PC and NEFA | 40 mg/kg/d | Oral intake (30 days) | Plasma, liver, adipose and different brain regions fatty acids (FA). Brain function and memory tests | Lyso-PC but not NEFA increase brain DHA content. No differences in other tissues. ↑ Improvement of brain function and memory with Lyso-PC |
| [85] | Adult female rats | Yes | Monoacylglycerol and Lyso-PC | 8 mg/kg/d | Maternal supplementation (9 weeks) | Blood, liver and adipose tissue FA in mothers. Brain regions FA in the offspring. Learning and memory skills | ↑ Incorporation of DHA in cerebellum and hippocampus of pups with Lyso-PC DHA while no differences in frontal and occipital cortex. Better learning and memory scores in Lyso-PC offspring |
| [86] | Young male rats | No | 14C-DHA as Lyso-PC and NEFA | 100 nmol | Tracer infusion | 14C-DHA enrichment in plasma, brain, heart, eyes and liver FA | ↑ 14C-DHA incorporation in brain after Lyso-PC administration. No differences in other tissues |
| [87] | Old male rats | No | 14C-DHA as Lyso-PC and NEFA | 10 μCi | Tracer infusion | 14C-DHA enrichment in plasma and different brain PL pools | ↓ Net rate of DHA entry into the brain with Lyso-PC ↑ 14C-DHA incorporation in brain PC but ↓ in ethanolamine PL with Lyso-PC |
| [88] | Adult male rats | No | Lyso-PC, PL and TG | 40 mg/kg/d | Oral intake (30 days) | Plasma, liver, heart, adipose tissue and different brain regions FA | Incorporation of DHA in plasma and liver: Lyso-PC > PL > TG. ↑ Incorporation of DHA from TG in heart and adipose tissue. Incorporation of DHA in brain regions: Lyso-PC > PL while no effect of DHA TG |
| [89] | Adult male rats | No | Lyso-PL and TG | 23.5 mmol/kg diet | Oral intake (28 days) | Serum and liver FA | No differences of DHA incorporation in serum. ↑ Incorporation of DHA from TG in liver |
| Human studies | |||||||
| [90] | Adult men | No | 13C-DHA as Lyso-PC and in the form of TG | 50 mg | Single oral intake | 13C-DHA enrichment in plasma and red blood cells PL FA | ↑ 13C-DHA incorporation in plasma PL with Lyso-PC. No differences in red blood cells PL |
| Ref. | Age | Pregnant | DHA Sources | DHA Dose | Time of Administration | Outcomes Measured | Major Findings |
|---|---|---|---|---|---|---|---|
| Rat models | |||||||
| [104] | Adult female | No | Fish oil (TG) and krill oil (PL) | 1.9–4.6% | 8 weeks | FA apparent digestibility and brain fatty acids (FA) | ↓ Intestinal absorption and brain DHA deposition after administration as PL |
| [105] | Adult female | No | Tuna/fungal oil (TG) and pig brain concentrate (PL) | 0.9% | 3 weeks | FA excretions and fat apparent absorption | ↓ Apparent absorption of DHA from pig brain PL |
| [105] | Adult female | No | Egg TG and egg PL | 0.9% | 3 weeks | FA excretions and fat apparent absorption | ↑ Apparent absorption of DHA from egg PL |
| [106] | Adult male | No | TG and PL oils (not specified) | ~1% | 3 weeks | Plasma, liver and kidney FA | ↓ DHA in plasma and liver after PL oil administration |
| [107] | Adult female | Yes | Microalgae oil (TG) and egg yolk (PL) | 2.5% | 3 weeks | Maternal plasma and liver FA, total fetus and fetal brain FA, placenta FA | No DHA differences in maternal plasma, fetus or placenta. ↑ DHA in maternal liver fractions with TG source |
| [108] | Adult female | Yes | Microalgae oil (TG), egg yolk (PL) | 8 mg/kg/d | 9 weeks | Maternal plasma, red blood cells, liver, adipose tissue and milk FA | No differences in maternal plasma. ↑ DHA in red blood cells and milk FA with PL source |
| Pig models | |||||||
| [109] | Piglets | No | Tuna/fungal oil (TG) and egg yolk (PL) | 0.3% | 4 weeks | Plasma and plasma lipoprotein lipid fractions FA | ↑ DHA incorporation in HDL-PL fraction with egg yolk source (PL) |
| [110] | Piglets | No | Tuna/fungal oil (TG) and egg yolk (PL) | 0.3% | 16 days | Plasma FA and dry matter digestibility | ↓ Intestinal absorption and plasma concentration of DHA after administration as PL |
| [111] | Piglets | No | Sow milk (TG) and pig brain concentrate (PL) | 0.3–0.4% | 17 days | Plasma PL and liver microsomes FA | ↑ DHA incorporation in plasma PL and liver with DHA-PL source |
| [112] | Piglets | No | Fish oil (TG) and egg yolk (PL) | 0.2–0.4% | 2 weeks | Plasma and red blood cells FA | ↑ DHA incorporation in plasma PL and with DHA-PL source |
| [113] | Adult female | Yes | Microalgae oil (TG) and egg yolk (PL) | 0.8% | 6 weeks | Maternal plasma, lipoproteins and liver FA, fetal plasma and brain FA, placenta FA | ↑ DHA content in placenta with PL source but no differences in fetal tissues |
| Human studies | |||||||
| [114] | Preterm infants | No | Breast milk/algae oil (TG) and egg yolk (PL) | 0.24–0.64% | ≥5 weeks | Fecal output and FA balance | ↑ Intestinal absorption of DHA administered as PL |
| [115] | Full term infants | No | Microalgae oil (TG) and egg yolk (PL) | 0.1% | 3 months | Plasma lipid fractions FA | No differences in plasma DHA |
| [116] | Children 8–13 y | No | Fish oil (TG) and enriched PL (not specified) | 100 mg/d | 3 months | Plasma and red blood cells PL fraction FA | No differences in plasma or red blood cells DHA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gázquez, A.; Larqué, E. Towards an Optimized Fetal DHA Accretion: Differences on Maternal DHA Supplementation Using Phospholipids vs. Triglycerides during Pregnancy in Different Models. Nutrients 2021, 13, 511. https://doi.org/10.3390/nu13020511
Gázquez A, Larqué E. Towards an Optimized Fetal DHA Accretion: Differences on Maternal DHA Supplementation Using Phospholipids vs. Triglycerides during Pregnancy in Different Models. Nutrients. 2021; 13(2):511. https://doi.org/10.3390/nu13020511
Chicago/Turabian StyleGázquez, Antonio, and Elvira Larqué. 2021. "Towards an Optimized Fetal DHA Accretion: Differences on Maternal DHA Supplementation Using Phospholipids vs. Triglycerides during Pregnancy in Different Models" Nutrients 13, no. 2: 511. https://doi.org/10.3390/nu13020511






