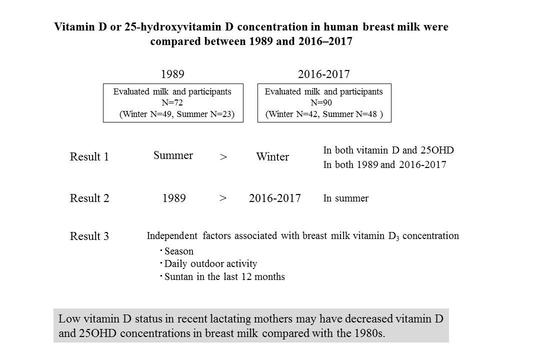
Comparison of Vitamin D and 25-Hydroxyvitamin D Concentrations in Human Breast Milk between 1989 and 2016–2017
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Breast Milk Samples
2.3. Sample Preparation
2.4. LC-MS/MS
2.5. Lifestyle Parameters of the Lactating Women in 2016–2017
2.6. Calculation of Antirachitic Activity
2.7. Statistical Analyses
3. Results
3.1. Comparison of Breast Milk Vitamin D and 25OHD Concentrations between 1989 and 2016–2017
3.2. Effects of Lifestyle Parameters on Breast Milk Vitamin D and 25OHD Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M.; et al. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid. Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Balk, S.J. Council on Environmental Health, Section on Dermatology, Ultraviolet radiation: A hazard to children and adolescents. Pediatrics 2011, 127, 588–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramanian, S.; Ganesh, R. Vitamin D deficiency in exclusively breast-fed infants. Indian J. Med. Res. 2008, 127, 250–255. [Google Scholar] [PubMed]
- Dawodu, A.; Tsang, R.C. Maternal vitamin D status: Effect on milk vitamin D content and vitamin D status of breastfeeding infants. Adv. Nutr. 2012, 3, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Thorne-Lyman, A.; Fawzi, W.W. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and metaanalysis. Paediatr. Perinat. Epidemiol. 2012, 26, 75–90. [Google Scholar] [CrossRef] [Green Version]
- Nakao, H. Nutritional significance of human milk vitamin D in neonatal period. Kobe J. Med. Sci. 1988, 34, 121–128. [Google Scholar] [PubMed]
- Specker, B.L.; Valanis, B.; Hertzberg, V.; Edwards, N.; Tsang, R.C. Sunshine exposure and serum 25-hydroxyvitamin D concentrations in exclusively breast-fed infants. J. Pediatr. 1985, 107, 372–376. [Google Scholar] [CrossRef]
- Kamao, M.; Tsugawa, N.; Suhara, Y.; Wada, A.; Mori, T.; Murata, K.; Nishino, R.; Ukita, T.; Uenishi, K.; Tanaka, K.; et al. Quantification of fat-soluble vitamins in human breast milk by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 859, 192–200. [Google Scholar] [CrossRef]
- Hollis, B.W.; Roos, B.A.; Draper, H.H.; Lambert, P.W. Vitamin D and its metabolites in human and bovine milk. J. Nutr. 1981, 111, 1240–1248. [Google Scholar] [CrossRef]
- Sakurai, T.; Furukawa, M.; Asoh, M.; Kanno, T.; Kojima, T.; Yonekubo, A. Fat-soluble and water-soluble vitamin contents of breast milk from Japanese women. J. Nutr. Sci. Vitaminol. 2005, 51, 239–247. [Google Scholar] [CrossRef]
- Markestad, T. Plasma concentrations of vitamin D metabolites in unsupplemented breast-fed infants. Eur. J. Pediatr. 1983, 141, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboezem, T.; Degenhart, H.J.; de Muinck Keizer-Schrama, S.M.; Bouillon, R.; Grose, W.F.; Hackeng, W.H.; Visser, H.K. Vitamin D metabolism in breast-fed infants and their mothers. Pediatr. Res. 1989, 25, 623–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollis, B.W.; Pittard, W.B., III; Reinhardt, T.A. Relationships among vitamin D, 25-hydroxyvitamin D, and vitamin D-binding protein concentrations in the plasma and milk of human subjects. J. Clin. Endocrinol. Metab. 1986, 62, 41–44. [Google Scholar] [CrossRef]
- Oberhelman, S.S.; Meekins, M.E.; Fischer, P.R.; Lee, B.R.; Singh, R.J.; Cha, S.S.; Gardner, B.M.; Pettifor, J.M.; Croghan, I.T.; Thacher, T.D. Maternal vitamin D supplementation to improve the vitamin D status of breast-fed infants: A randomized controlled trial. Mayo Clin. Proc. 2013, 88, 1378–1387. [Google Scholar] [CrossRef] [Green Version]
- við Streym, S.; Højskov, C.S.; Møller, U.K.; Heickendorff, L.; Vestergaard, P.; Mosekilde, L.; Rejnmark, L. Vitamin D content in human breast milk: A 9-mo follow-up study. Am. J. Clin. Nutr. 2016, 103, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikata, H.; Tsugawa, N.; Watanabe, Y.; Tsuburai, T.; Chaki, O.; Hirahara, F.; Miyagi, E.; Sakakibara, H.; Uenishi, K.; Okano, T. 25-Hydroxyvitamin D profiles and maternal bone mass during pregnancy and lactation in Japanese women. J. Bone Miner. Metab. 2020, 38, 99–108. [Google Scholar] [CrossRef]
- Cesur, Y.; Doğan, M.; Ariyuca, S.; Basaranoglu, M.; Bektas, M.S.; Peker, E.; Akbayram, S.; Caksen, H. Evaluation of children with nutritional rickets. J. Pediatr. Endocrinol. Metab. 2011, 24, 35–43. [Google Scholar] [CrossRef]
- Singleton, R.; Lescher, R.; Gessner, B.D.; Benson, M.; Bulkow, L.; Rosenfeld, J.; Thomas, T.; Holman, R.C.; Haberling, D.; Bruce, M.; et al. Rickets and vitamin D deficiency in Alaska native children. J. Pediatr. Endocrinol. Metab. 2015, 28, 815–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, B.J.; Dickson, N.P.; Houghton, L.A.; Ward, L.M.; Taylor, B.J. Incidence and characteristics of vitamin D deficiency rickets in New Zealand children: A New Zealand Paediatric Surveillance Unit study. Aust. N. Z. J. Public Health 2015, 39, 380–383. [Google Scholar] [CrossRef]
- Thacher, T.D.; Fischer, P.R.; Tebben, P.J.; Singh, R.J.; Cha, S.S.; Maxson, J.A.; Yawn, B.P. Increasing incidence of nutritional rickets: A population-based study in Olmsted County, Minnesota. Mayo Clin. Proc. 2013, 88, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Akazawa, Y.; Shiohara, M.; Amano, Y.; Uchida, N.; Nakamura, S.; Minami, I.; Yasui, K.; Kurata, K.; Koike, K. The clinical characteristics of vitamin D deficiency in childhood: A systematic literature review of Japanese patients. J. Pediatr. Endocrinol. Metab. 2010, 23, 675–684. [Google Scholar] [CrossRef]
- Matsuo, K.; Mukai, T.; Suzuki, S.; Fujieda, K. Prevalence and risk factors of vitamin D deficiency rickets in Hokkaido, Japan. Pediatr. Int. 2009, 51, 559–562. [Google Scholar] [CrossRef]
- Yorifuji, J.; Yorifuji, T.; Tachibana, K.; Nagai, S.; Kawai, M.; Momoi, T.; Nagasaka, H.; Hatayama, H.; Nakahata, T. Craniotabes in normal newborns: The earliest sign of subclinical vitamin D deficiency. J. Clin. Endocrinol. Metab. 2008, 93, 1784–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, T.; Kotani, T.; Miyoshi, Y.; Santo, Y.; Hirai, H.; Namba, N.; Shima, M.; Shimizu, K.; Nakajima, S.; Ozono, K. A spectrum of clinical presentations in seven Japanese patients with vitamin D deficiency. Clin. Pediatr. Endocrinol. 2006, 15, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, T.; Nakayama, H.; Kitaoka, T.; Nakamura, Y.; Fukumoto, S.; Fujiwara, I.; Hasegawa, Y.; Ihara, K.; Kitanaka, S.; Koyama, S.; et al. Incidence rate and characteristics of symptomatic vitamin D deficiency in children: A nationwide survey in Japan. Endocr. J. 2018, 65, 593–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuoka, Y.; Idota, T. The concentration of epidermal growth factor in Japanese mother’s milk. J. Nutr. Sci. Vitaminol. 1995, 41, 241–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nojiri, K.; Kobayashi, S.; Higurashi, S.; Takahashi, T.; Tsujimori, Y.; Ueno, H.M.; Watanabe-Matsuhashi, S.; Toba, Y.; Yamamura, J.; Nakano, T.; et al. Maternal health and nutrition status, human milk composition, and growth and development of infants and children: A prospective Japanese Human Milk Study protocol. Int. J. Environ. Res. Public Health 2020, 17, 1869. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Tsugawa, N.; Suhara, Y.; Kamao, M.; Okano, T. Determination of 25-hydroxyvitamin D in human plasma using high-performance liquid chromatography-tandem mass spectrometry. Anal. Chem. 2005, 77, 3001–3007. [Google Scholar] [CrossRef]
- Higashi, T.; Awada, D.; Shimada, K. Simultaneous determination of 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 in human plasma by liquid chromatography-tandem mass spectrometry employing derivatization with a Cookson-type reagent. Biol. Pharm. Bull. 2001, 24, 738–743. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Nabak, A.C.; Johnson, R.E.; Keuler, N.S.; Hansen, K.E. Can a questionnaire predict vitamin D status in postmenopausal women? Public Health Nutr. 2014, 17, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Cashman, K.D.; Seamans, K.M.; Lucey, A.J.; Stöcklin, E.; Weber, P.; Kiely, M.; Hill, T.R. Relative effectiveness of oral 25-hydroxyvitamin D3 and vitamin D3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am. J. Clin. Nutr. 2012, 95, 1350–1356. [Google Scholar] [CrossRef] [Green Version]
- Renken, S.A.; Warthesen, J.J. Vitamin D Stability in Milk. J. Food Sci. 1993, 58, 552–555. [Google Scholar] [CrossRef]
- Upreti, P.; Mistry, V.V.; Warthesen, J.J. Estimation and fortification of vitamin D3 in pasteurized process cheese. J. Dairy Sci. 2002, 85, 3173–3181. [Google Scholar] [CrossRef] [Green Version]
- Borai, A.; Bahijri, S.; Livingstone, C.; Nawajha, M.; Bawazeer, A.; Baarmah, Z.; Shanaa, A.; Kadam, I.; Abdelaal, M. Assessment of Becton Dickinson plain and serum separator tubes in measurement of 25-hydroxyvitamin D3 (25OHD3) by HPLC and immunoassay methods. J. Clin. Lab. Anal. 2016, 30, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Colak, A.; Toprak, B.; Dogan, N.; Ustuner, F. Effect of sample type, centrifugation and storage conditions on vitamin D concentration. Biochem. Med. 2013, 23, 321–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiraishi, M.; Haruna, M.; Matsuzaki, M.; Murayama, R. Demographic and lifestyle factors associated with vitamin D status in pregnant Japanese women. J. Nutr. Sci. Vitaminol. 2014, 60, 420–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ala-Houhala, M.; Koskinen, T.; Parviainen, M.T.; Visakorpi, J.K. 25-Hydroxyvitamin D and vitamin D in human milk: Effects of supplementation and season. Am. J. Clin. Nutr. 1988, 48, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Non-Ionizing Radiation Protection. Global Solar UV Index; World Health Organization: Genève, Switzerland, 1995; p. 21. [Google Scholar]
- Wagner, C.L.; Taylor, S.N.; Johnson, D.D.; Hollis, B.W. The role of vitamin D in pregnancy and lactation: Emerging concepts. Womens Health 2012, 8, 323–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, A.; Okano, T.; Tsugawa, N.; Tasaka, Y.; Kobayashi, T.; Kodama, S.; Matsuo, T. Effects of ergocalciferol supplementation on the concentration of vitamin D and its metabolites in human milk. J. Nutr. 1989, 119, 1639–1646. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hulsey, T.C.; Fanning, D.; Ebeling, M.; Hollis, B.W. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: A 6-month follow-up pilot study. Breastfeed. Med. 2006, 1, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Basile, L.A.; Taylor, S.N.; Wagner, C.L.; Horst, R.L.; Hollis, B.W. The effect of high-dose vitamin D supplementation on serum vitamin D levels and milk calcium concentration in lactating women and their infants. Breastfeed. Med. 2006, 1, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawodu, A.; Salameh, K.M.; Al-Janahi, N.S.; Bener, A.; Elkum, N. The Effect of High-Dose Postpartum Maternal Vitamin D supplementation alone compared with maternal plus infant vitamin D supplementation in breastfeeding infants in a high-risk population. A randomized controlled trial. Nutrients 2019, 11, 1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollis, B.W.; Wagner, C.L. Vitamin D requirements during lactation: High-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am. J. Clin. Nutr. 2004, 80, 1752S–1758S. [Google Scholar] [CrossRef] [PubMed]



| Participants | Total | Regions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kanto (35–37° N) | Kyushu (31–34° N) | Hokkaido (41–46° N) | ||||||||||
| N | Age | N | Age | N | Age | N | Age | |||||
| <1989> | ||||||||||||
| All | 72 | 27.7 | ±3.8 | 28 | 26.8 | ±3.7 | 24 | 28.0 | ±3.5 | 20 | 28.8 | ±4.2 |
| Summer | 23 | 27.3 | ±4.3 | 10 | 26.2 | ±4.8 | 9 | 28.1 | ±3.7 | 4 | 28.5 | ±4.4 |
| Winter | 49 | 27.9 | ±3.6 | 18 | 27.2 | ±3.0 | 15 | 27.9 | ±3.6 | 16 | 28.8 | ±4.3 |
| <2016~2017> | ||||||||||||
| All | 90 | 30.3 | ±3.7 | 34 | 30.5 | ±3.6 | 28 | 30.3 | ±3.5 | 28 | 30.3 | ±4.2 |
| Summer | 48 | 30.2 | ±3.6 | 20 | 31.1 | ±3.6 | 13 | 29.6 | ±3.0 | 15 | 29.6 | ±4.1 |
| Winter | 42 | 30.5 | ±3.9 | 14 | 29.6 | ±3.7 | 15 | 30.8 | ±4.0 | 13 | 31.1 | ±4.3 |
| Survey Year | Vitamin D3 | Vitamin D2 | Total Vitamin D | 25OHD3 | 25OHD2 | Total 25OHD |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| <1989> | 0.252 ± 0.477 | 0.073 ± 0.120 | 0.325 ± 0.492 | 0.322 ± 0.312 | 0.006 ± 0.014 | 0.328 ± 0.319 |
| ND: 5% | ND: 22% | ND: 0% | ND: 82% | |||
| <2016–2017> | 0.161 ± 0.174 | 0.045 ± 0.138 | 0.206 ± 0.238 | 0.261 ± 0.130 | 0.018 ± 0.035 | 0.279 ± 0.133 |
| ND: 0% | ND: 3.2% | ND: 4.4% | ND: 16% | |||
| p * | 0.094 | ― | 0.044 | 0.094 | ― | 0.191 |
| Parameters | Mean (SD) or N (%) | |
|---|---|---|
| Maternal height (cm) | 158.4 | (5.3) |
| Maternal body weight (kg) | 54.1 | (6.9) |
| Maternal body mass index (BMI: kg/m2) | 21.6 | (2.7) |
| Parity (times) | 1.5 | (0.5) |
| Vitamin D intake (μg/day) | 11.9 | (7.1) |
| Daily outdoor activity (min/day) | 101.2 | (70.8) |
| Suntan in the last 12 months [Yes] | 21 | (23%) |
| Behavior to avoid sunlight exposure [Yes] (e.g., using parasol or walking in the shade) | 45 | (50%) |
| Frequency of sunscreen use per week | ||
| 1: 7 days | 18 | (20%) |
| 2: 5–6 days | 16 | (18%) |
| 3: 3–4 days | 9 | (10%) |
| 4: 1–2 days | 20 | (22%) |
| 5: 0 day | 27 | (30%) |
| Sunlight exposure time per day in the last one week | ||
| 1: >30 min | 39 | (43%) |
| 2: 15–30 min | 27 | (30%) |
| 3: 5–15 min | 17 | (19%) |
| 4: <5 min | 7 | (8%) |
| Parameters | Vitamin D3 Concentration | 25OHD3 Concentration | ||||
|---|---|---|---|---|---|---|
| β | R2 | P | β | R2 | P | |
| Age (years) a | −2.6 × 10−3 | 0.003 | 0.600 | 1.7 × 10−3 | 0.002 | 0.649 |
| BMI (kg/m2) a | 3.8 × 10−4 | <0.001 | 0.956 | 2.7 × 10−3 | 0.003 | 0.605 |
| Vitamin D intake (µg/day) a | 2.1 × 10−3 | 0.007 | 0.429 | −5.3 × 10−4 | 0.001 | 0.784 |
| Daily outdoor activity (min) a | 6.0 × 10−4 | 0.059 | 0.021 | 1.4 × 10−4 | 0.006 | 0.470 |
| Season [Winter-Summer] b | −0.113 | 0.107 | 0.002 | −0.115 | 0.196 | <0.001 |
| Suntan in last 12 months [Yes-No] b | 0.107 | 0.069 | 0.012 | −0.007 | 0.001 | 0.824 |
| Regions [Kanto, Kyusyu, Hokkaido] b | — | 0.008 | 0.697 | — | 0.045 | 0.135 |
| Frequency of sunscreen use per week [1: 7 days, 2: 5–6 days, 3: 3–4 days, 4: 1–2 days, 5: 0 days] b | — | 0.043 | 0.433 | — | 0.055 | 0.298 |
| Parameters | Vitamin D3 Concentration | 25OHD3 Concentration | ||||
|---|---|---|---|---|---|---|
| β | R2 | P | β | R2 | P | |
| Season [Winter-Summer] | −0.050 | 0.107 | 0.002 | −0.060 | 0.196 | <0.001 |
| Daily outdoor activity time (min) | 6.0 × 10−4 | 0.059 | 0.015 | 1.5 × 104 | 0.006 | 0.410 |
| Suntan in last 12 months [Yes-No] | −0.042 | 0.040 | 0.040 | −0.018 | 0.013 | 0.232 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsugawa, N.; Nishino, M.; Kuwabara, A.; Ogasawara, H.; Kamao, M.; Kobayashi, S.; Yamamura, J.; Higurashi, S. Comparison of Vitamin D and 25-Hydroxyvitamin D Concentrations in Human Breast Milk between 1989 and 2016–2017. Nutrients 2021, 13, 573. https://doi.org/10.3390/nu13020573
Tsugawa N, Nishino M, Kuwabara A, Ogasawara H, Kamao M, Kobayashi S, Yamamura J, Higurashi S. Comparison of Vitamin D and 25-Hydroxyvitamin D Concentrations in Human Breast Milk between 1989 and 2016–2017. Nutrients. 2021; 13(2):573. https://doi.org/10.3390/nu13020573
Chicago/Turabian StyleTsugawa, Naoko, Mayu Nishino, Akiko Kuwabara, Honami Ogasawara, Maya Kamao, Shunjiro Kobayashi, Junichi Yamamura, and Satoshi Higurashi. 2021. "Comparison of Vitamin D and 25-Hydroxyvitamin D Concentrations in Human Breast Milk between 1989 and 2016–2017" Nutrients 13, no. 2: 573. https://doi.org/10.3390/nu13020573
APA StyleTsugawa, N., Nishino, M., Kuwabara, A., Ogasawara, H., Kamao, M., Kobayashi, S., Yamamura, J., & Higurashi, S. (2021). Comparison of Vitamin D and 25-Hydroxyvitamin D Concentrations in Human Breast Milk between 1989 and 2016–2017. Nutrients, 13(2), 573. https://doi.org/10.3390/nu13020573