Omega-3 Polyunsaturated Fatty Acids Prevent Nonalcoholic Steatohepatitis (NASH) and Stimulate Adipogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Fish Oil Supplementation
2.2. Experimental Procedure
2.3. Biochemical Plasma and Lipid Analysis in the Liver
2.4. Frozen Liver Tissue H/E and Oil Red O Staining
2.5. Adipocytes and AdSC Isolation
2.6. AdSC Culture and Differentiation
2.7. In Vitro Treatment with Fatty Acids
2.8. Differentiation of Pre-Adipocytes from 3T3-L1 Cell Lineage and Browning Induction
2.9. Cell Proliferation Assay
2.10. Oil Red O Staining and Lipid Content Determination
2.11. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.12. Statistical Analysis
3. Results
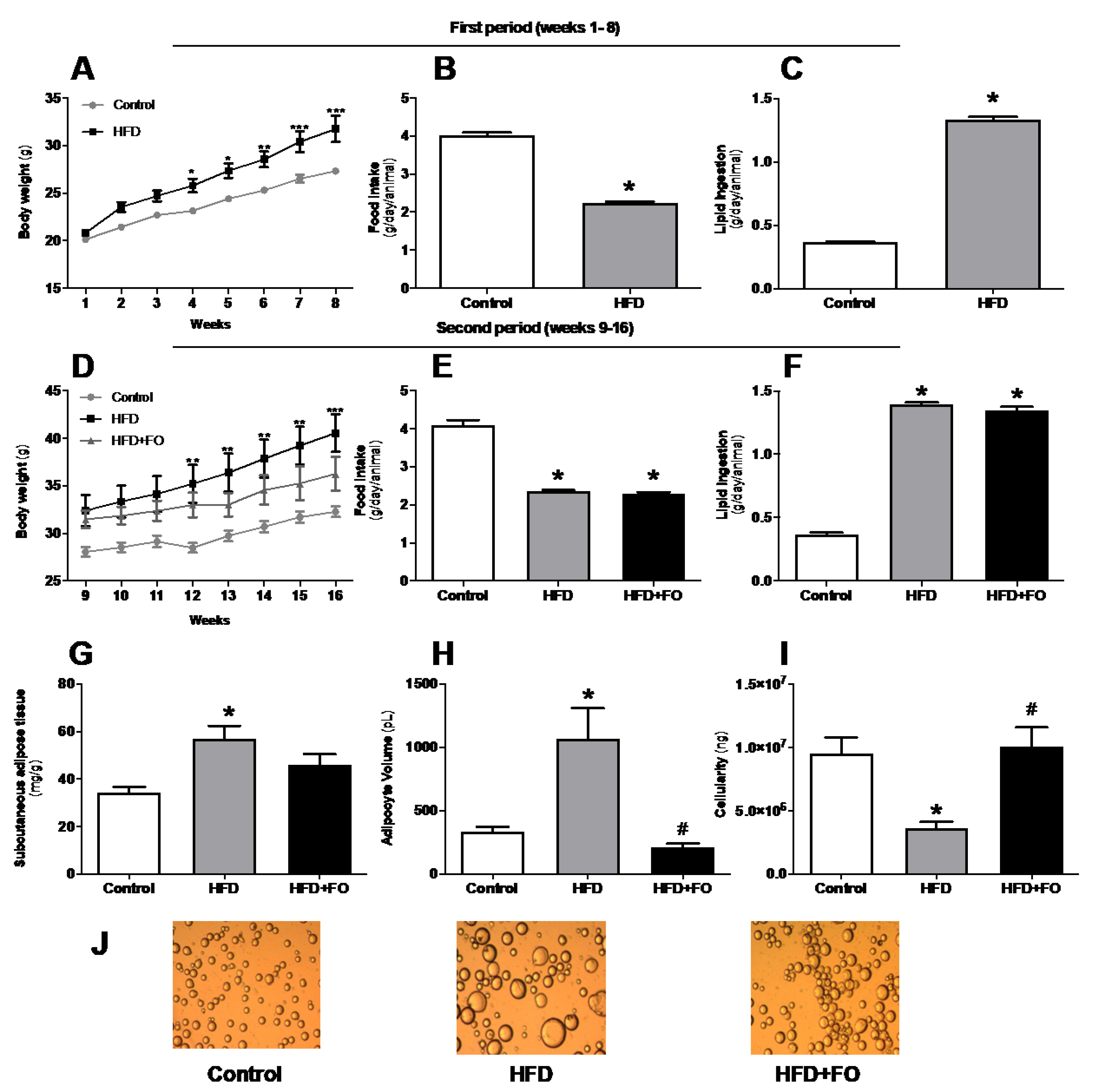
3.1. Effects of FO Treatment on Body Weight, Adiposity, and Hypertrophy of ING Adipocytes from Mice with HFD-Induced Obesity
3.2. Plasma Lipid Profile and Blood Glucose
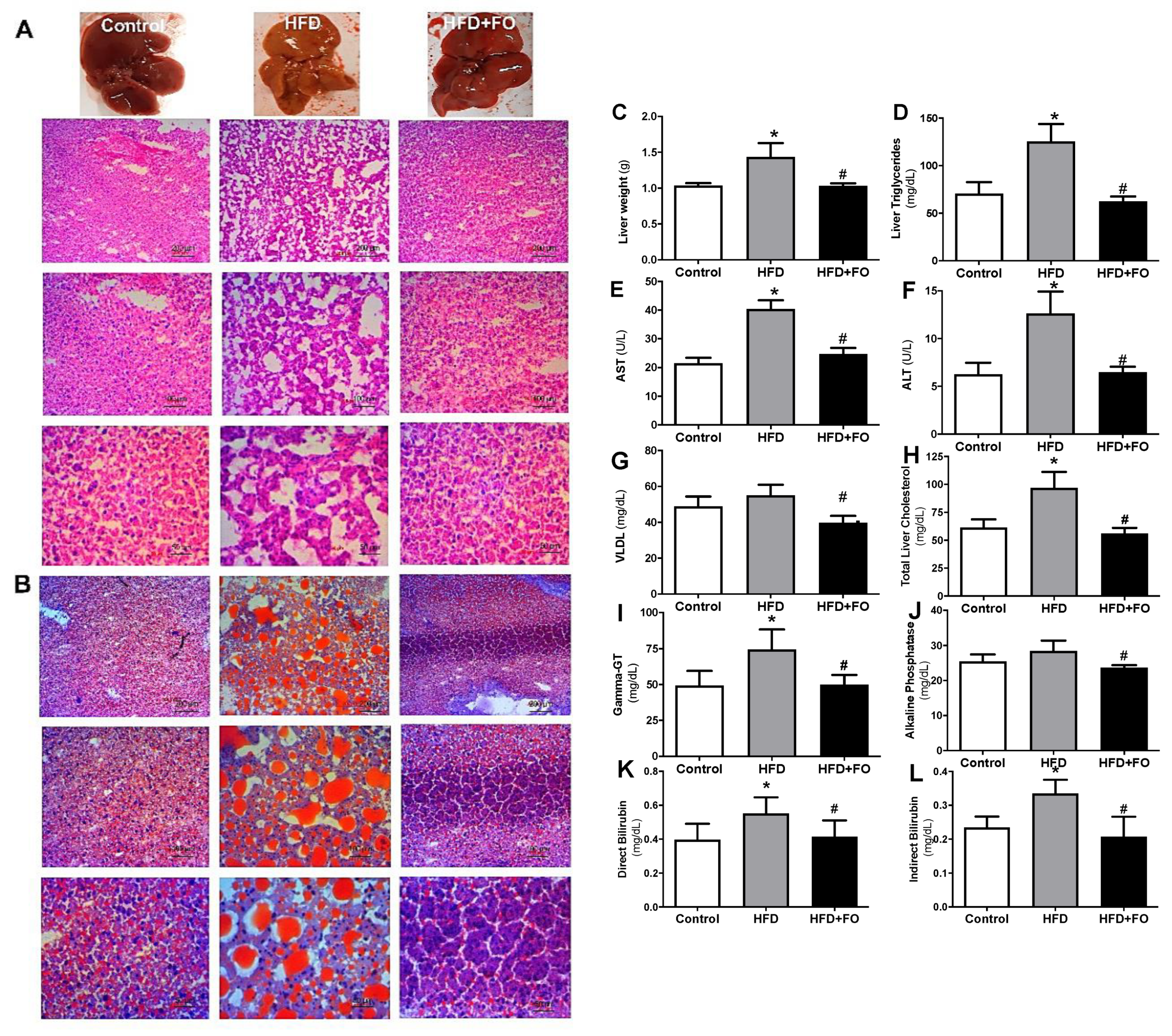
3.3. Effects of a High-Fat Diet and Treatment with Fish Oil on Nonalcoholic Steatohepatitis (NASH)
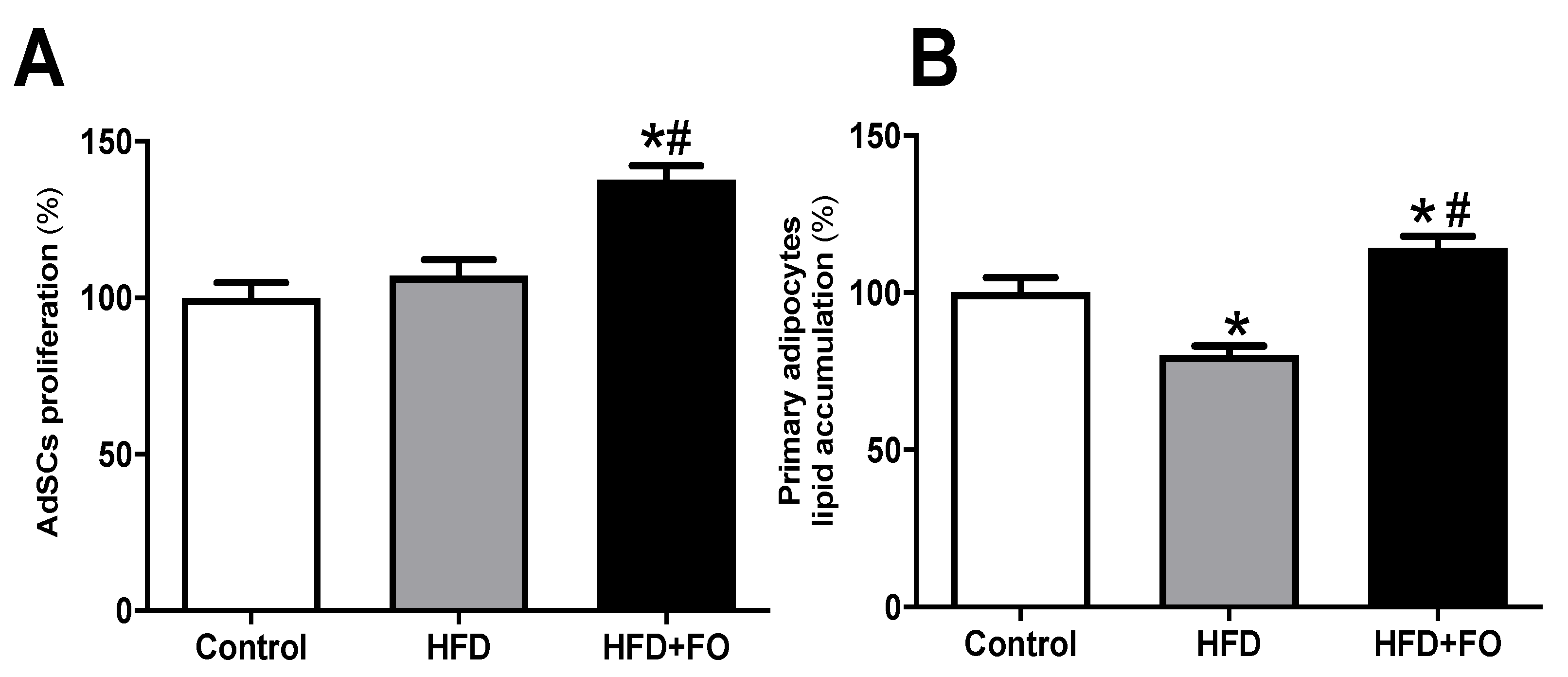
3.4. Effects of FO Treatment on Proliferation and Differentiation of AdSCs Isolated from Obese Mice
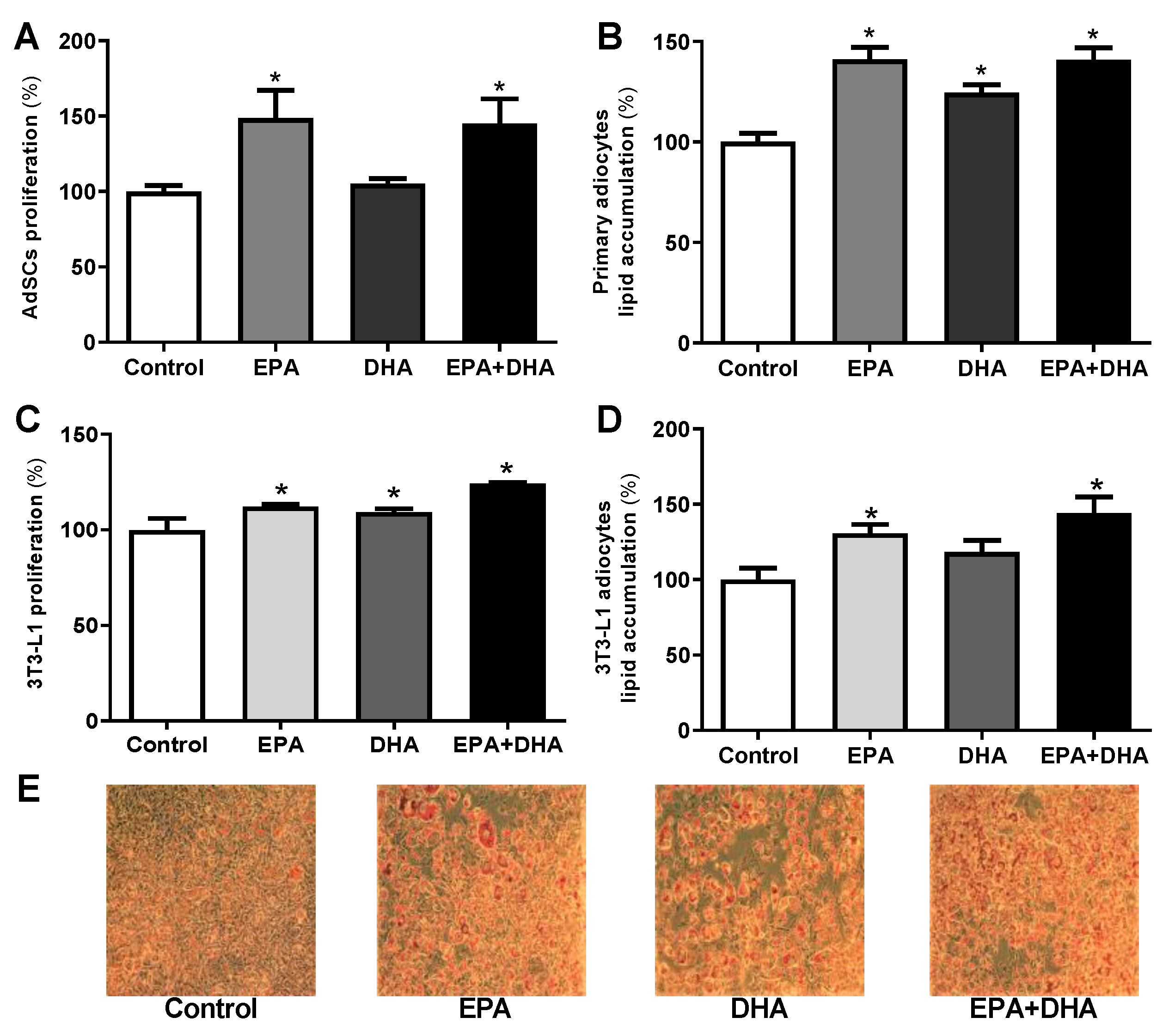
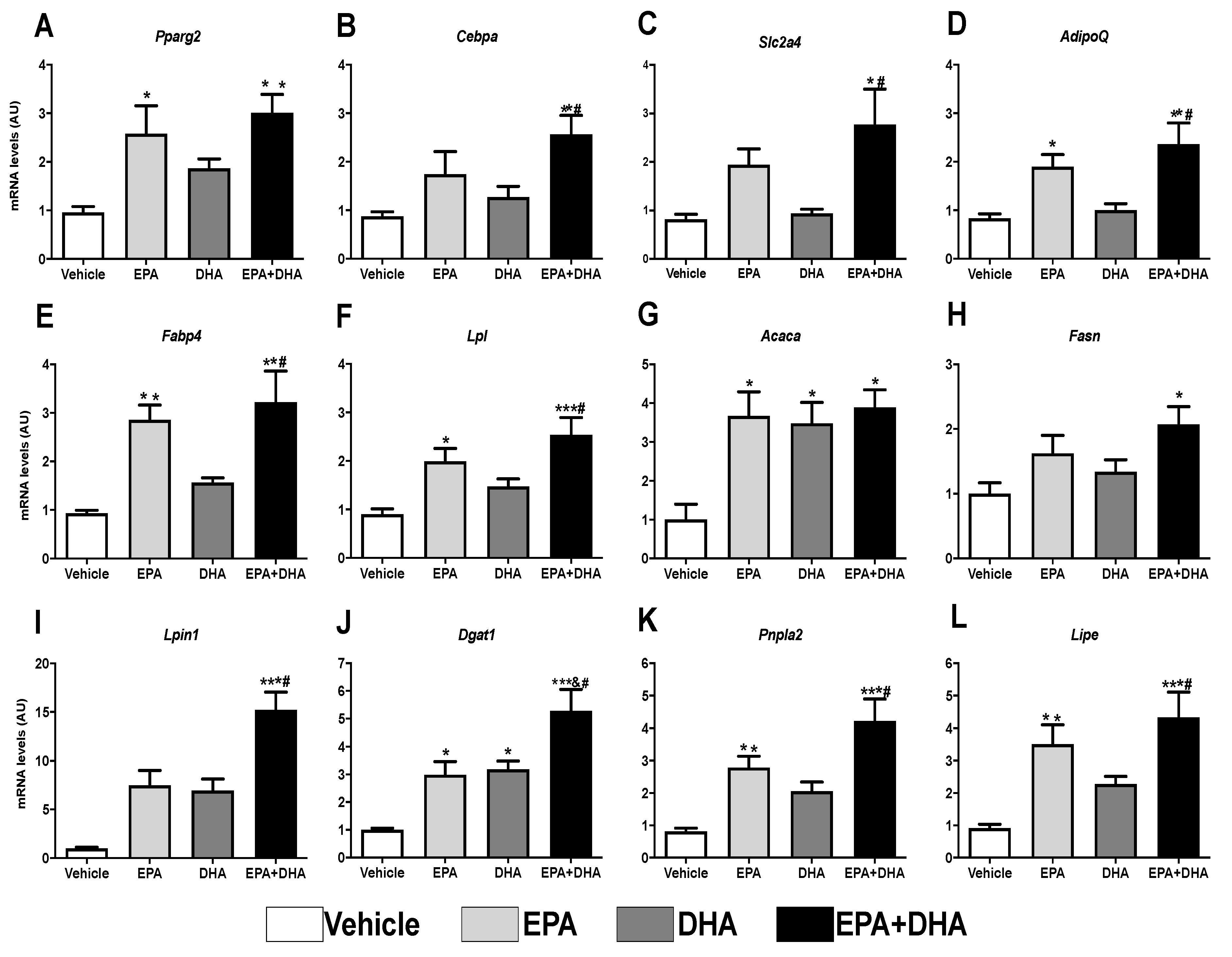
3.5. Effects of In Vitro n-3 PUFA Treatment on Proliferation, Differentiation, and Gene Expression of Markers of Adipogenesis on Both AdSCs and 3T3-L1 Pre-Adipocytes
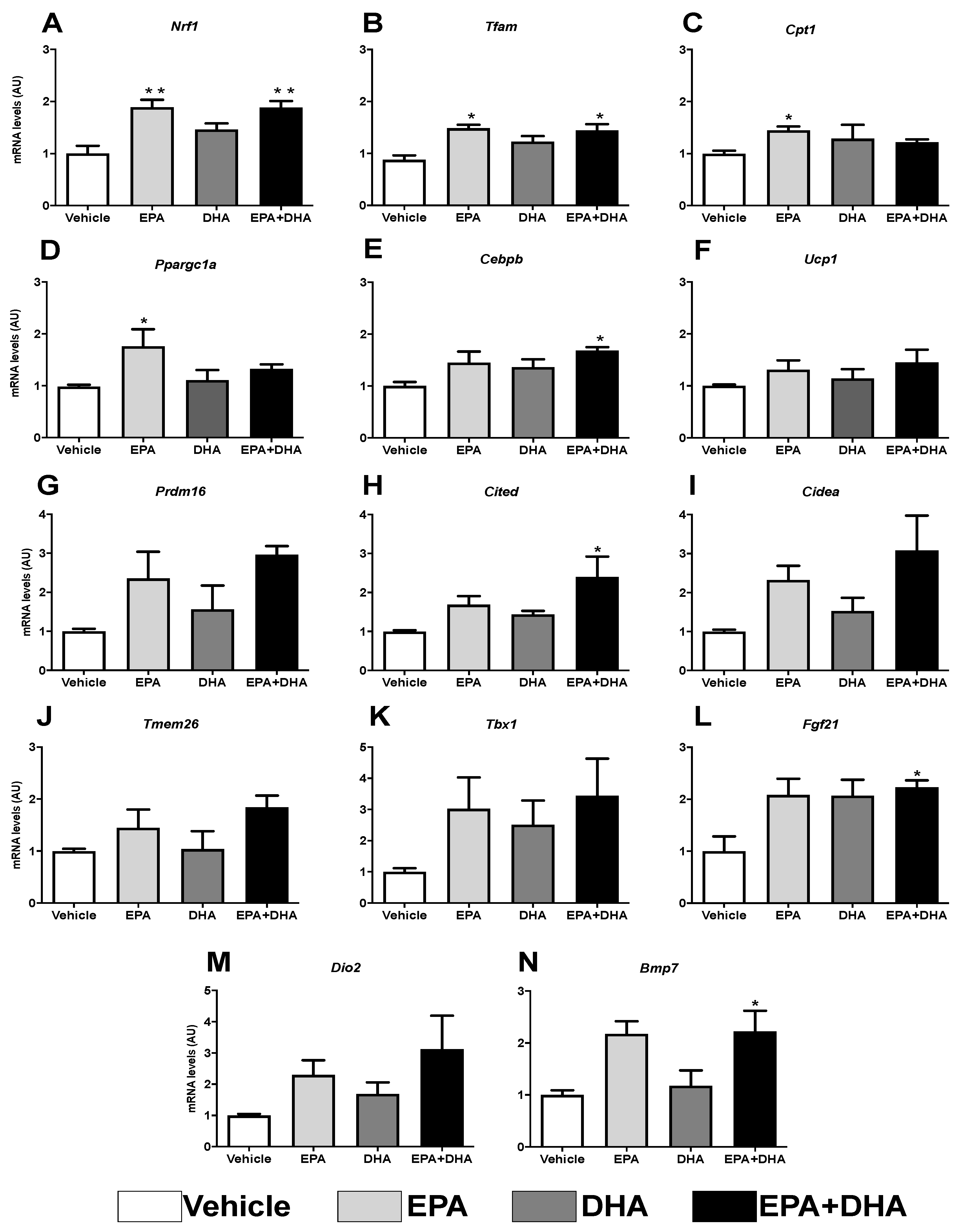
3.6. Analysis of Gene Expression of Beige Adipocyte Markers after Browning Induction in 3T3-L1 Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chechi, K.; Nedergaard, J.; Richard, D. Brown adipose tissue as an anti-obesity tissue in humans. Obes. Rev. 2014, 15, 92–106. [Google Scholar] [CrossRef]
- González-Muniesa, P.; Mártinez-González, M.A.; Hu, F.B.; Després, J.P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Prim. 2017, 3, 1–18. [Google Scholar] [CrossRef]
- Albuquerque, D.; Stice, E.; Rodríguez-López, R.; Manco, L.; Nóbrega, C. Current review of genetics of human obesity: From molecular mechanisms to an evolutionary perspective. Mol. Genet. Genom. 2015, 290, 1191–1221. [Google Scholar] [CrossRef]
- Than, N.N.; Newsome, P.N. A concise review of non-alcoholic fatty liver disease. Atherosclerosis 2015, 239, 192–202. [Google Scholar] [CrossRef]
- Xiang, Z.; Chen, Y.; Ma, K.; Ye, Y.; Zheng, L.; Li, Y.; Jin, X. The role of ursodeoxycholic acid in non-alcoholic steatohepatitis: A systematic review. BMC Gastroenterol. 2013, 13, 140. [Google Scholar] [CrossRef]
- Pagadala, M.; Kasumov, T.; McCullough, A.J.; Zein, N.N.; Kirwan, J.P. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol. Metab. 2012, 23, 365–371. [Google Scholar] [CrossRef]
- Angelico, F.; Del Ben, M.; Conti, R.; Francioso, S.; Feole, K.; Fiorello, S.; Cavallo, M.G.; Zalunardo, B.; Lirussi, F.; Alessandri, C. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2005, 90, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 21 January 2021).
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef]
- Mendez-Sanchez, N.; Cruz-Ramon, V.C.; Ramirez-Perez, O.L.; Hwang, J.P.; Barranco-Fragoso, B.; Cordova-Gallardo, J. New aspects of lipotoxicity in nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2018, 19, 2034. [Google Scholar] [CrossRef]
- Da Cunha de Sá, R.D.C.; Crisma, A.R.; Cruz, M.M.; Martins, A.R.; Masi, L.N.; do Amaral, C.L.; Curi, R.; Alonso-Vale, M.I.C. Fish oil prevents changes induced by a high-fat diet on metabolism and adipokine secretion in mice subcutaneous and visceral adipocytes. J. Physiol. 2016, 594, 6301–6317. [Google Scholar] [CrossRef]
- Da Cunha de Sá, R.D.C.; Cruz, M.M.; de Farias, T.M.; da Silva, V.S.; de Jesus Simão, J.; Telles, M.M.; Alonso-Vale, M.I.C. Fish oil reverses metabolic syndrome, adipocyte dysfunction, and altered adipokines secretion triggered by high-fat diet-induced obesity. Physiol. Rep. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Berná, G.; Romero-Gomez, M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management. Liver Int. 2020, 40, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milanović, M.; Milić, N.; Luzza, F.; Giuffrè, A.M. Olive oil antioxidants and non-alcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Boccuto, L.; Federico, A.; Dallio, M.; Loguercio, C.; Di Renzo, L.; De Lorenzo, A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int. J. Environ. Res. Public Health 2019, 16, 3011. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Couet, C.; Delarue, J.; Ritz, P.; Antoine, J.M.; Lamisse, F. Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int. J. Obes. 1997, 21, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Do Amaral, C.L.; Milagro, F.I.; Curi, R.; Martínez, J.A. DNA methylation pattern in overweight women under an energy-restricted diet supplemented with fish oil. BioMed Res. Int. 2014, 2014, 675021. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, G.; David, H. Quantitative determination of serum triglycerides by the use of enzymes. Clin. Chem. 1973, 19, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, A.; Cicerano, U.; Gallotta, G.; Gnasso, A.; Lamenza, F.; Rubba, P.; Mancini, M. Prevalence of peripheral arterial disease and related risk factors in elderly institutionalized subjects. Gerontology 1992, 38, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Grillo, F.; Izzo, C.; Mazzotti, G.; Murador, E. Improved method for determination of high-density-lipoprotein cholesterol II. Enzymic determination of cholesterol in high-density lipoprotein fractions with a sensitive reagent. Clin. Chem. 1981, 27, 375–379. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008; ISBN 0443102791. [Google Scholar]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008, pdb-prot4986. [Google Scholar] [CrossRef] [PubMed]
- Catta-Preta, M.; Mendonca, L.S.; Fraulob-Aquino, J.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. A critical analysis of three quantitative methods of assessment of hepatic steatosis in liver biopsies. Virchows Arch. 2011, 459, 477. [Google Scholar] [CrossRef]
- Rodbell, M. Metabolism of Isolated Fat Cells: I. Effects of Hormones on Glucose metabolism and lipolysis. J. Biol. Chem. 1964, 239, 375–380. [Google Scholar] [CrossRef]
- Seo, J.-H.; Moon, H.-S.; Kim, I.-Y.; Guo, D.-D.; Lee, H.-G.; Choi, Y.-J.; Cho, C.-S. PEGylated conjugated linoleic acid stimulation of apoptosis via a p53-mediated signaling pathway in MCF-7 breast cancer cells. Eur. J. Pharm. Biopharm. 2008, 70, 621–626. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Vishvanath, L.; Busbuso, N.C.; Hepler, C.; Shan, B.; Sharma, A.X.; Chen, S.; Yu, X.; An, Y.A.; Zhu, Y. De novo adipocyte differentiation from Pdgfrβ+ preadipocytes protects against pathologic visceral adipose expansion in obesity. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Hammarstedt, A.; Hedjazifar, S.; Smith, U. Restricted adipogenesis in hypertrophic obesity: The role of WISP2, WNT, and BMP4. Diabetes 2013, 62, 2997–3004. [Google Scholar] [CrossRef] [PubMed]
- Arner, P.; Arner, E.; Hammarstedt, A.; Smith, U. Genetic predisposition for Type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PLoS ONE 2011, 6, e18284. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.-A.; Pellmé, F.; Hammarstedt, A.; Sandqvist, M.; Brekke, H.; Caidahl, K.; Forsberg, M.; Volkmann, R.; Carvalho, E.; Funahashi, T. A novel cellular marker of insulin resistance and early atherosclerosis in humans is related to impaired fat cell differentiation and low adiponectin. FASEB J. 2003, 17, 1434–1440. [Google Scholar] [CrossRef]
- Newmark, H.L.; Lipkin, M. Colonic Hyperplasia and Hyperproliferation Induced in Rodents by a Nutritional Stress Diet Containing 4 Factors of The Western Human Diet: High Fat and Phosphate, Low Calcium and Vitamin D. In Calcium, Vitamin D, and Prevention of Colon Cancer; CRC Press: Boca Raton, FL, USA, 2018; pp. 145–157. [Google Scholar]
- Tabarés Seisdedos, R. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Ikemoto, S.; Takahashi, M.; Tsunoda, N.; Maruyama, K.; Itakura, H.; Ezaki, O. High-fat diet-induced hyperglycemia and obesity in mice: Differential effects of dietary oils. Metabolism 1996, 45, 1539–1546. [Google Scholar] [CrossRef]
- Rendina-Ruedy, E.; Smith, B.J. Methodological considerations when studying the skeletal response to glucose intolerance using the diet-induced obesity model. Bonekey Rep. 2016, 5, 845. [Google Scholar] [CrossRef]
- Foulds, C.E.; Treviño, L.S.; York, B.; Walker, C.L. Endocrine-disrupting chemicals and fatty liver disease. Nat. Rev. Endocrinol. 2017, 13, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Chen, G.; Pan, M.; Zhang, J.; He, W.; Liu, Y.; Nian, X.; Sheng, L.; Xu, B. High fat diet-induced oxidative stress blocks hepatocyte nuclear factor 4α and leads to hepatic steatosis in mice. J. Cell. Physiol. 2018, 233, 4770–4782. [Google Scholar] [CrossRef]
- Kersten, S. Integrated physiology and systems biology of PPARα. Mol. Metab. 2014, 3, 354–371. [Google Scholar] [CrossRef]
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, F.; Zhou, R.; Zhu, Z.; Xie, M. PPARα/γ antagonists reverse the ameliorative effects of osthole on hepatic lipid metabolism and inflammatory response in steatohepatitic rats. Inflammopharmacology 2018, 26, 425–433. [Google Scholar] [CrossRef]
- Scorletti, E.; Byrne, C.D. Omega-3 fatty acids and non-alcoholic fatty liver disease: Evidence of efficacy and mechanism of action. Mol. Aspects Med. 2018, 64, 135–146. [Google Scholar] [CrossRef]
- Casula, M.; Soranna, D.; Catapano, A.L.; Corrao, G. Long-term effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: A meta-analysis of randomized, double blind, placebo controlled trials. Atheroscler. Suppl. 2013, 14, 243–251. [Google Scholar] [CrossRef]
- Meyer, B.J.; Onyiaodike, C.C.; Brown, E.A.; Jordan, F.; Murray, H.; Nibbs, R.J.B.; Sattar, N.; Lyall, H.; Nelson, S.M.; Freeman, D.J. Maternal plasma DHA levels increase prior to 29 days post-LH surge in women undergoing frozen embryo transfer: A prospective, observational study of human pregnancy. J. Clin. Endocrinol. Metab. 2016, 101, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Gibson, R.A. Long-chain polyunsaturated fatty acid requirements during pregnancy and lactation. Am. J. Clin. Nutr. 2000, 71, 307S–311S. [Google Scholar] [CrossRef]
- Sinn, N.; Milte, C.; Howe, P.R.C. Oiling the brain: A review of randomized controlled trials of omega-3 fatty acids in psychopathology across the lifespan. Nutrients 2010, 2, 128–170. [Google Scholar] [CrossRef] [PubMed]
- Flachs, P.; Rossmeisl, M.; Kopecky, J. The effect of n-3 fatty acids on glucose homeostasis and insulin sensitivity. Physiol. Res. 2014, 63, S93. [Google Scholar] [CrossRef]
- Duvall, M.G.; Levy, B.D. DHA-and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef]
- Parletta, N.; Milte, C.M.; Meyer, B.J. Nutritional modulation of cognitive function and mental health. J. Nutr. Biochem. 2013, 24, 725–743. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Health benefits of n-3 polyunsaturated fatty acids: Eicosapentaenoic acid and docosahexaenoic acid. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 65, pp. 211–222. ISBN 1043-4526. [Google Scholar]
- Schakarowski, F.B.; Padoin, A.V.; Mottin, C.C.; Castro, E.K. De Percepção de risco da cirurgia bariátrica em pacientes com diferentes comorbidades associadas à obesidade. Trends Psychol. 2018, 26, 339–346. [Google Scholar] [CrossRef]
- Patel, D. Pharmacotherapy for the management of obesity. Metabolism 2015, 64, 1376–1385. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Courcoulas, A.P. Bariatric surgery for obesity and metabolic conditions in adults. BMJ 2014, 349, g3961. [Google Scholar] [CrossRef] [PubMed]
- Raynor, H.A.; Champagne, C.M. Position of the Academy of Nutrition and Dietetics: Interventions for the Treatment of Overweight and Obesity in Adults. J. Acad. Nutr. Diet. 2016, 116, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; De Angelis, M.H.; Schürmann, A. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140. [Google Scholar] [CrossRef]
- Duivenvoorde, L.P.M.; van Schothorst, E.M.; Swarts, H.M.; Kuda, O.; Steenbergh, E.; Termeulen, S.; Kopecky, J.; Keijer, J. A difference in fatty acid composition of isocaloric high-fat diets alters metabolic flexibility in male C57BL/6JOlaHsd mice. PLoS ONE 2015, 10, e0128515. [Google Scholar] [CrossRef]
- Bertrand, C.; Pignalosa, A.; Wanecq, E.; Rancoule, C.; Batut, A.; Deleruyelle, S.; Lionetti, L.; Valet, P.; Castan-Laurell, I. Effects of Dietary Eicosapentaenoic Acid (EPA) Supplementation in High-Fat Fed Mice on Lipid Metabolism and Apelin/APJ System in Skeletal Muscle. PLoS ONE 2013, 8, e78874. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Kawano, H.; Notsu, T.; Ohta, M.; Nakakuki, M.; Mizuguchi, K.; Itoh, M.; Suganami, T.; Ogawa, Y. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet–induced obesity: Importance of hepatic lipogenesis. Diabetes 2010, 59, 2495–2504. [Google Scholar] [CrossRef]
- Jeffery, E.; Church, C.D.; Holtrup, B.; Colman, L.; Rodeheffer, M.S. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat. Cell Biol. 2015, 17, 376–385. [Google Scholar] [CrossRef]
- Jernås, M.; Palming, J.; Sjöholm, K.; Jennische, E.; Svensson, P.-A.; Gabrielsson, B.G.; Levin, M.; Sjögren, A.; Rudemo, M.; Lystig, T.C. Separation of human adipocytes by size: Hypertrophic fat cells display distinct gene expression. FASEB J. 2006, 20, 1540–1542. [Google Scholar] [CrossRef]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P.S. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, J.; Osborne, O.; Sasik, R.; Schenk, S.; Chen, A.; Chung, H.; Murphy, A.; Watkins, S.M.; Quehenberger, O. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 2014, 157, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Hernandez-Morante, J.J.; Lujan, J.; Tebar, F.J.; Zamora, S. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int. J. Obes. 2006, 30, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Holland, W.L.; Sun, K.; Park, J.; Spurgin, S.B.; Lin, Y.; Askew, G.R.; Simcox, J.A.; McClain, D.A.; Li, C. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 2012, 18, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinelli, V.; Carobbio, S.; Vidal-Puig, A. Adipose tissue plasticity: How fat depots respond differently to pathophysiological cues. Diabetologia 2016, 59, 1075–1088. [Google Scholar] [CrossRef]
- Rodeheffer, M.S.; Birsoy, K.; Friedman, J.M. Identification of white adipocyte progenitor cells in vivo. Cell 2008, 135, 240–249. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Giralt, M. Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 2017, 13, 26. [Google Scholar] [CrossRef]
- Contador, D.; Ezquer, F.; Espinosa, M.; Arango-Rodriguez, M.; Puebla, C.; Sobrevia, L.; Conget, P. Featured Article: Dexamethasone and rosiglitazone are sufficient and necessary for producing functional adipocytes from mesenchymal stem cells. Exp. Biol. Med. 2015, 240, 1235–1246. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- Cohen, P.; Levy, J.D.; Zhang, Y.; Frontini, A.; Kolodin, D.P.; Svensson, K.J.; Lo, J.C.; Zeng, X.; Ye, L.; Khandekar, M.J. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014, 156, 304–316. [Google Scholar] [CrossRef]
- Tzameli, I.; Fang, H.; Ollero, M.; Shi, H.; Hamm, J.K.; Kievit, P.; Hollenberg, A.N.; Flier, J.S. Regulated production of a peroxisome proliferator-activated receptor-γ ligand during an early phase of adipocyte differentiation in 3T3-L1 adipocytes. J. Biol. Chem. 2004, 279, 36093–36102. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.; Petersen, R.K.; Kristiansen, K. Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2005, 1740, 266–286. [Google Scholar] [CrossRef] [PubMed]
- Mater, M.K.; Pan, D.; Bergen, W.G.; Jump, D.B. Arachidonic acid inhibits lipogenic gene expression in 3T3-L1 adipocytes through a prostanoid pathway. J. Lipid Res. 1998, 39, 1327–1334. [Google Scholar] [CrossRef]

| Gene | 5′ Primer (5′-3′)—Sense | 3′ Primer (5′-3′)—Antisense |
|---|---|---|
| 36B4 | TAAAGACTGGAGACAAGGTG | GTGTACTCAGTCTCCACAGA |
| 18S | GAGAGGGAGCCTGAGAAAC | GGCCTGCTTTGAACACTC |
| Lipe | GGGAGGGCCTCAGCGTTCTCACA | ATAGCACGGAGCTGGGTGAGGG |
| Fabp4 | AAGGTGAAGAGCATCATAACCCT | TCACGCCTTTCATAACACATTCC |
| Slc2a4 | CATTCCCTGGTTCATTGTGG | GAAGACGTAAGGACCCATAGC |
| AdipoQ | GCAGAGATGGCACTCCTGGA | CCCTTCAGCTCCTGTCATTCC |
| Cebpa | CGCAAGAGCCGAGATAAAGC | CAGTTCACGGCTCAGCTGTTC |
| Pnpla2 | GGTCCTCTGCATCCCTCCTT | CTGTCCTGAGGGAGATGTC |
| Lpl | GGCCAGATTCATCAACTGGAT | GCTCCAAGGCTGTACCCTAAG |
| Fasn | AGAGGCTTGTGCTGACTTCC | GTGGCTTCGGCGATGAGAG |
| Acaca | GAGAGGGGTCAAGTCCTTCC | ACATCCACTTCCACACACGA |
| Dgat1 | AGAGGTGTTGGGAGGATCTG | GTCTGAGTGGGTGGCAGGT |
| Lpin1 | TGATGTGGTGTTCAGTGTCACT | TCGTTGACCCAGTGCAGGTA |
| Nrf1 | CGCAGCACCTTTGGAGAA | CCCGACCTGTGGAATACTTG |
| Pparg2 | GCATCAGGCTTCCACTATGGA | AAGGCACTTCTGAAACCGACA |
| Cebpb | GCAAGAGCCGCGACAAG | GGCTCGGGCAGCTGCTT |
| Ppargc1a | ATCTACTGCCTGGGGACCTT | ATGTGTCGCCTTCTTGCTCT |
| Tfam | GGAATGTGGAGCGTGCTAAAA | TGCTGGAAAAACACTTCGGAATA |
| Ucp1 | CACCTTCCCGCTGGACACT | CCCTAGGACACCTTTATACCTAATGG |
| Prdm16 | CCACCAGCGAGGACTTCAC | GGAGGACTCTCGTAGCTCGAA |
| Cidea | ATCACAACTGGCCTGGTTACG | TACTACCCGGTGTCCATTTCT |
| Cited1 | CGCTTCGTCCGTACCTCAGCT | CAGCTGGGCCTGTTGGTCTC |
| Tmem26 | GAAACCAGTATTGCAGCACCC | CCAGACCGGTTCACATACCA |
| Tbx1 | CGAATGTTCCCCACGTTCCA | GTGTACTCGGCCAGGTGTAG |
| Fgf21 | CGTCTGCCTCAGAAGGACTC | TCTACCATGCTCAGGGGGTC |
| Dio2 | AATTATGCCTCGGAGAAGACCG | GGCAGTTGCCTAGTGAAAGGT |
| Pparalfa | TCGGACTCGGTCTTCTTGAT | TCTTCCCAAAGCTCCTTCAA |
| Bmp7 | CCTGTCCATCTTAGGGTTGC | GCCTTGTAGGGGTAGGAGAAG |
| Srebf1 | CACTCCCTCTGATGCTACGG | CTTGTTTGCGATGTCTCCAG |
| Cpt1 | TGTCCAAGTATCTGGCAGTCG | CATAGCCGTCATCAGCAACC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antraco, V.J.; Hirata, B.K.S.; de Jesus Simão, J.; Cruz, M.M.; da Silva, V.S.; da Cunha de Sá, R.D.C.; Abdala, F.M.; Armelin-Correa, L.; Alonso-Vale, M.I.C. Omega-3 Polyunsaturated Fatty Acids Prevent Nonalcoholic Steatohepatitis (NASH) and Stimulate Adipogenesis. Nutrients 2021, 13, 622. https://doi.org/10.3390/nu13020622
Antraco VJ, Hirata BKS, de Jesus Simão J, Cruz MM, da Silva VS, da Cunha de Sá RDC, Abdala FM, Armelin-Correa L, Alonso-Vale MIC. Omega-3 Polyunsaturated Fatty Acids Prevent Nonalcoholic Steatohepatitis (NASH) and Stimulate Adipogenesis. Nutrients. 2021; 13(2):622. https://doi.org/10.3390/nu13020622
Chicago/Turabian StyleAntraco, Vitor Jacó, Bruna Kelly Sousa Hirata, Jussara de Jesus Simão, Maysa Mariana Cruz, Viviane Simões da Silva, Roberta Dourado Cavalcante da Cunha de Sá, Fernanda Miranda Abdala, Lucia Armelin-Correa, and Maria Isabel Cardoso Alonso-Vale. 2021. "Omega-3 Polyunsaturated Fatty Acids Prevent Nonalcoholic Steatohepatitis (NASH) and Stimulate Adipogenesis" Nutrients 13, no. 2: 622. https://doi.org/10.3390/nu13020622
APA StyleAntraco, V. J., Hirata, B. K. S., de Jesus Simão, J., Cruz, M. M., da Silva, V. S., da Cunha de Sá, R. D. C., Abdala, F. M., Armelin-Correa, L., & Alonso-Vale, M. I. C. (2021). Omega-3 Polyunsaturated Fatty Acids Prevent Nonalcoholic Steatohepatitis (NASH) and Stimulate Adipogenesis. Nutrients, 13(2), 622. https://doi.org/10.3390/nu13020622








