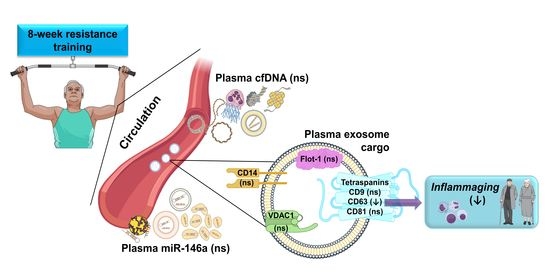
Resistance Training Diminishes the Expression of Exosome CD63 Protein without Modification of Plasma miR-146a-5p and cfDNA in the Elderly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects Characteristics
2.2. Maximal Strength Assessment
2.3. Resistance Exercise Training
2.4. Blood Sampling
2.5. miRNA Extraction, Reverse Transcription, and RT-PCR
2.6. cfDNA Assay
2.7. Exosome Isolation
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Anthropometric and Strength Measurements of the Study Participants
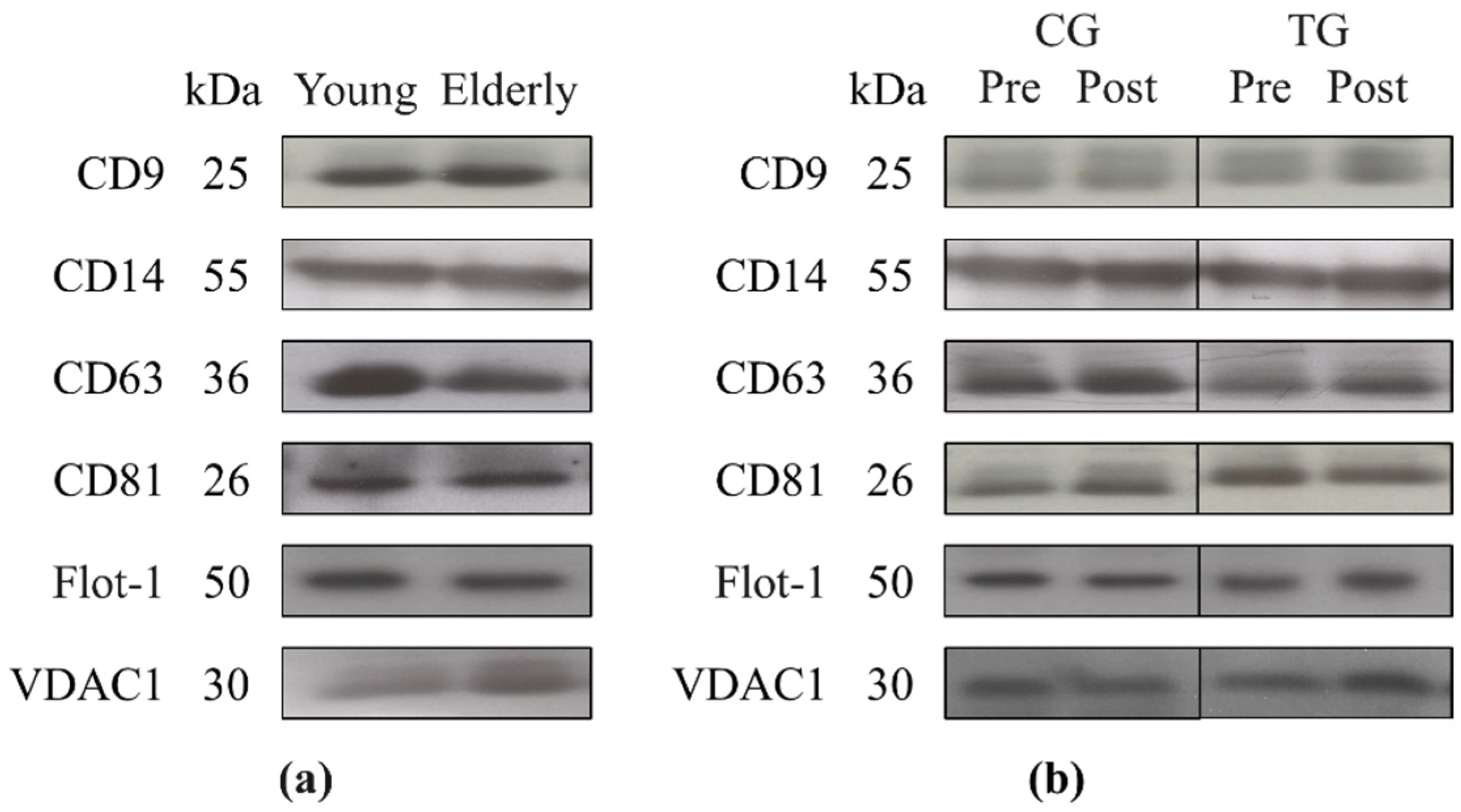
3.2. Plasma Levels of miR-146, cfDNA, Total Exosome Protein Content, and Exosome Cargo at Baseline
3.3. Plasma Levels of miR-146a and cfDNA and Exosome Markers Following an 8-Week Resistance Training
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.; Kappei, D.; Kumar, A.P.; Sethi, G. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, H.Z.; Liu, D.P. The four layers of aging. Cell Syst. 2015, 1, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.; Wang, W.; Su, D.M. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun. Ageing 2020, 17, 2. [Google Scholar] [CrossRef] [Green Version]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef] [Green Version]
- Rippo, M.R.; Olivieri, F.; Monsurrò, V.; Prattichizzo, F.; Albertini, M.C.; Procopio, A.D. MitomiRs in human inflamm-aging: A hypothesis involving miR-181a, miR-34a and miR-146a. Exp. Gerontol. 2014, 56, 154–163. [Google Scholar] [CrossRef]
- Olivieri, F.; Lazzarini, R.; Recchioni, R.; Marcheselli, F.; Rippo, M.R.; Di Nuzzo, S.; Albertini, M.C.; Graciotti, L.; Babini, L.; Mariotti, S.; et al. MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age 2013, 35, 1157–1172. [Google Scholar] [CrossRef] [Green Version]
- Olivieri, F.; Albertini, M.C.; Orciani, M.; Ceka, A.; Cricca, M.; Procopio, A.D.; Bonafè, M. DNA damage response (DDR) and senescence: Shuttled inflamma-miRNAs on the stage of inflamm-aging. Oncotarget 2015, 6, 35509–35521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostyuk, S.V.; Porokhovnik, L.N.; Ershova, E.S.; Malinovskaya, E.M.; Konkova, M.S.; Kameneva, L.V.; Dolgikh, O.A.; Veiko, V.P.; Pisarev, V.M.; Martynov, A.V.; et al. Changes of KEAP1/NRF2 and IKB/NF-κB expression levels induced by cell-free DNA in different cell types. Oxid. Med. Cell. Longev. 2018, 2018, 1052413. [Google Scholar] [CrossRef] [Green Version]
- Jylhävä, J.; Kotipelto, T.; Raitala, A.; Jylhä, M.; Hervonen, A.; Hurme, M. Aging is associated with quantitative and qualitative changes in circulating cell-free DNA: The Vitality 90+ study. Mech. Ageing Dev. 2011, 132, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Jylhävä, J.; Nevalainen, T.; Marttila, S.; Jylhä, M.; Hervonen, A.; Hurme, M. Characterization of the role of distinct plasma cell-free DNA species in age-associated inflammation and frailty. Aging Cell. 2013, 12, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Bronkhorst, A.J.; Ungerer, V.; Holdenrieder, S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol. Detect. Quantif. 2019, 17, 100087. [Google Scholar] [CrossRef] [PubMed]
- Chelobanov, B.P.; Laktionov, P.P.; Vlasov, V.V. Proteins involved in binding and cellular uptake of nucleic acids. Biochemistry 2006, 71, 583–596. [Google Scholar] [CrossRef]
- Thierry, A.R.; El Messaoudi, S.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, M.R.; Jiang, C.; Krzyzanowski, G.D.; Ryan, W.L. New evidence that a large proportion of human blood plasma cell-free DNA is localized in exosomes. PLoS ONE 2017, 12, e0183915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eitan, E.; Green, J.; Bodogai, M.; Mode, N.A.; Bæk, R.; Jørgensen, M.M.; Freeman, D.W.; Witwer, K.W.; Zonderman, A.B.; Biragyn, A.; et al. Age-related changes in plasma extracellular vesicle characteristics and internalization by leukocytes. Sci. Rep. 2017, 7, 1342. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Miguelez, P.; Fernandez-Gonzalo, R.; Almar, M.; Mejías, Y.; Rivas, A.; de Paz, J.A.; Cuevas, M.J.; González-Gallego, J. Role of Toll-like receptor 2 and 4 signaling pathways on the inflammatory response to resistance training in elderly subjects. Age 2014, 36, 9734. [Google Scholar] [CrossRef] [Green Version]
- Whitham, M.; Febbraio, M.A. The ever-expanding myokinome: Discovery challenges and therapeutic implications. Nat. Rev. Drug Discov. 2016, 15, 719–729. [Google Scholar] [CrossRef]
- Estébanez, B.; Rodriguez-Miguelez, P.; Fernandez-Gonzalo, R.; González-Gallego, J.; Cuevas, M.J. Beneficial effect of physical exercise on telomere length and aging, and genetics of aging-associated noncommunicable diseases. In Sports, Exercise, and Nutritional Genomics; Barh, D., Ahmetov, I.I., Eds.; Academic Press: London, UK, 2019; pp. 509–538. [Google Scholar]
- Li, Y.; Han, C.; Wang, J.; Zhou, J.; Liang, C.; Ranganna, K.; Song, Y.H. Exosomes mediate the beneficial effects of exercise. Adv. Exp. Med. Biol. 2017, 1000, 333–353. [Google Scholar] [CrossRef]
- Safdar, A.; Tarnopolsky, M.A. Exosomes as mediators of the systemic adaptations to endurance exercise. Cold Spring Harb. Perspect. Med. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Bertoldi, K.; Cechinel, L.R.; Schallenberger, B.; Corssac, G.B.; Davies, S.; Guerreiro, I.C.K.; Belló-Klein, A.; Araujo, A.S.R.; Siqueira, I.R. Circulating extracellular vesicles in the aging process: Impact of aerobic exercise. Mol. Cell. Biochem. 2018, 440, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef]
- Li, G.; Liu, H.; Ma, C.; Chen, Y.; Wang, J.; Yang, Y. Exosomes are the novel players involved in the beneficial effects of exercise on type 2 diabetes. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Bei, Y.; Xu, T.; Lv, D.; Yu, P.; Xu, J.; Che, L.; Das, A.; Tigges, J.; Toxavidis, V.; Ghiran, I.; et al. Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic Res. Cardiol. 2017, 112, 38. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Qin, X.; Hu, Y.; Zhang, X.; Li, G.; Wu, J.; Li, J.; Sha, J.; Chen, J.; Xia, J.; et al. Longterm exercise-derived exosomal miR-342-5p: A novel exerkine for cardioprotection. Circ. Res. 2019, 124, 1386–1400. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, J.; Liu, H.; Chen, Y.; Ma, X.; Chen, S.; Bihl, J.I.; Yang, Y.I. Moderate exercise enhances endothelial progenitor cell exosomes release and function. Med. Sci. Sports Exerc. 2018, 50, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Wang, L.; Peng, Z.; Liao, Y.; Li, D.; Yang, X.; Nuessler, A.K.; Liu, L.; Bao, W.; Yang, W. The mechanisms and treatments for sarcopenia: Could exosomes be a perspective research strategy in the future? J. Cachexia Sarcopenia Muscle 2020, 11, 348–365. [Google Scholar] [CrossRef] [Green Version]
- Annibalini, G.; Contarelli, S.; Lucertini, F.; Guescini, M.; Maggio, S.; Ceccaroli, P.; Gervasi, M.; Ferri Marini, C.; Fardetti, F.; Grassi, E.; et al. Muscle and systemic molecular responses to a single flywheel based iso-inertial training session in resistance-trained men. Front. Physiol. 2019, 10, 554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, P.; Kalani, A.; Medina, I.; Familtseva, A.; Tyagi, S.C. Cardiosome mediated regulation of MMP9 in diabetic heart: Role of mir29b and mir455 in exercise. J. Cell. Mol. Med. 2015, 19, 2153–2161. [Google Scholar] [CrossRef] [Green Version]
- Frühbeis, C.; Helmig, S.; Tug, S.; Simon, P.; Krämer-Albers, E.M. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J. Extracell Vesicles 2015, 4, 28239. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, G.P.; Porto, W.F.; Palu, C.C.; Pereira, L.M.; Petriz, B.; Almeida, J.A.; Viana, J.; Filho, N.N.A.; Franco, O.L.; Pereira, R.W. Effects of acute aerobic exercise on rats serum extracellular vesicles diameter, concentration and small RNAs content. Front. Physiol. 2018, 9, 532. [Google Scholar] [CrossRef] [Green Version]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018, 27, 237–251.e234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barone, R.; Macaluso, F.; Sangiorgi, C.; Campanella, C.; Marino Gammazza, A.; Moresi, V.; Coletti, D.; Conway de Macario, E.; Macario, A.J.; Cappello, F.; et al. Skeletal muscle Heat shock protein 60 increases after endurance training and induces peroxisome proliferator-activated receptor gamma coactivator 1 α1 expression. Sci. Rep. 2016, 6, 19781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmig, S.; Frühbeis, C.; Krämer-Albers, E.M.; Simon, P.; Tug, S. Release of bulk cell free DNA during physical exercise occurs independent of extracellular vesicles. Eur. J. Appl. Physiol. 2015, 115, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Tosevska, A.; Franzke, B.; Hofmann, M.; Vierheilig, I.; Schober-Halper, B.; Oesen, S.; Neubauer, O.; Wessner, B.; Wagner, K.H. Circulating cell-free DNA, telomere length and bilirubin in the Vienna Active Ageing Study: Exploratory analysis of a randomized, controlled trial. Sci. Rep. 2016, 6, 38084. [Google Scholar] [CrossRef] [Green Version]
- Morais Junior, G.S.; Souza, V.C.; Machado-Silva, W.; Henriques, A.D.; Melo Alves, A.; Barbosa Morais, D.; Nóbrega, O.T.; Brito, C.J.; Dos Santos Silva, R.J. Acute strength training promotes responses in whole blood circulating levels of miR-146a among older adults with type 2 diabetes mellitus. Clin. Interv. Aging 2017, 12, 1443–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portilla-Cueto, K.; Medina-Pérez, C.; Romero-Pérez, E.M.; Hernández-Murúa, J.A.; Oliveira, C.E.P.; de Souza-Teixeira, F.; González-Bernal, J.J.; Vila-Chã, C.; de Paz, J.A. Reference values for isometric, dynamic, and asymmetry leg extension strength in patients with multiple sclerosis. Int. J. Environ. Res. Public Health 2020, 17, 8083. [Google Scholar] [CrossRef]
- Gearhart, R.F.; Lagally, K.M.; Riechman, S.E.; Andrews, R.D.; Robertson, R.J. Strength tracking using the OMNI resistance exercise scale in older men and women. J. Strength Cond. Res. 2009, 23, 1011–1015. [Google Scholar] [CrossRef]
- Goldshtein, H.; Hausmann, M.J.; Douvdevani, A. A rapid direct fluorescent assay for cell-free DNA quantification in biological fluids. Ann. Clin. Biochem. 2009, 46, 488–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, D.; Scott, G.K.; Schokrpur, S.; Patil, C.K.; Orjalo, A.V.; Rodier, F.; Lithgow, G.J.; Campisi, J. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging 2009, 1, 402–411. [Google Scholar] [CrossRef]
- Quan, X.; Ji, Y.; Zhang, C.; Guo, X.; Zhang, Y.; Jia, S.; Ma, W.; Fan, Y.; Wang, C. Circulating miR-146a may be a potential biomarker of coronary heart disease in patients with subclinical hypothyroidism. Cell. Physiol. Biochem. 2018, 45, 226–236. [Google Scholar] [CrossRef]
- Guo, N.; Zhou, Q.; Huang, X.; Yu, J.; Han, Q.; Nong, B.; Xiong, Y.; Liang, P.; Li, J.; Feng, M.; et al. Identification of differentially expressed circulating exosomal lncRNAs in IgA nephropathy patients. BMC Immunol. 2020, 21, 16. [Google Scholar] [CrossRef]
- Russo, A.; Bartolini, D.; Mensà, E.; Torquato, P.; Albertini, M.C.; Olivieri, F.; Testa, R.; Rossi, S.; Piroddi, M.; Cruciani, G.; et al. Physical activity modulates the overexpression of the inflammatory miR-146a-5p in obese patients. IUBMB Life 2018, 70, 1012–1022. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, R.F.; Markworth, J.F.; Aasen, K.M.M.; Zeng, N.; Cameron-Smith, D.; Mitchell, C.J. Acute resistance exercise modulates microRNA expression profiles: Combined tissue and circulatory targeted analyses. PLoS ONE 2017, 12, e0181594. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y.; Wang, H.; Li, H.; Liu, S.; Chen, J.; Ying, H. miR-146a-5p acts as a negative regulator of TGF-β signaling in skeletal muscle after acute contusion. Acta Biochim. Biophys. Sin. 2017, 49, 628–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stortz, J.A.; Hawkins, R.B.; Holden, D.C.; Raymond, S.L.; Wang, Z.; Brakenridge, S.C.; Cuschieri, J.; Moore, F.A.; Maier, R.V.; Moldawer, L.L.; et al. Cell-free nuclear, but not mitochondrial, DNA concentrations correlate with the early host inflammatory response after severe trauma. Sci. Rep. 2019, 9, 13648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- Ferrandi, P.J.; Fico, B.G.; Whitehurst, M.; Zourdos, M.C.; Bao, F.; Dodge, K.M.; Rodriguez, A.L.; Pena, G.; Huang, C.J. Acute high-intensity interval exercise induces comparable levels of circulating cell-free DNA and Interleukin-6 in obese and normal-weight individuals. Life Sci. 2018, 202, 161–166. [Google Scholar] [CrossRef]
- Andreatta, M.V.; Curty, V.M.; Coutinho, J.V.S.; Santos, M.A.; Vassallo, P.F.; de Sousa, N.F.; Barauna, V.G. Cell-free DNA as an earlier predictor of exercise-induced performance decrement related to muscle damage. Int. J. Sports Physiol. Perform. 2018, 13, 953–956. [Google Scholar] [CrossRef]
- Borghesan, M.; Fafián-Labora, J.; Eleftheriadou, O.; Carpintero-Fernández, P.; Paez-Ribes, M.; Vizcay-Barrena, G.; Swisa, A.; Kolodkin-Gal, D.; Ximénez-Embún, P.; Lowe, R.; et al. Small extracellular vesicles are key regulators of non-cell autonomous intercellular communication in senescence via the interferon protein IFITM3. Cell Rep. 2019, 27, 3956–3971.e3956. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, B.D.; Paine, M.S.; Brooks, A.M.; McCubrey, J.A.; Renegar, R.H.; Wang, R.; Terrian, D.M. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008, 68, 7864–7871. [Google Scholar] [CrossRef] [Green Version]
- Takasugi, M. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.H.; Wilson, D.R.; Clement, C.C.; Rathod, S.; Cherry, C.; Powell, B.; Lee, Z.; Khalil, A.M.; Green, J.J.; Campisi, J.; et al. Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Dallas, S.L. Exosomes and extracellular RNA in muscle and bone aging and crosstalk. Curr. Osteoporos. Rep. 2019, 17, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Estébanez, B.; Jiménez-Pavón, D.; Huang, C.J.; Cuevas, M.J.; González-Gallego, J. Effects of exercise on exosome release and cargo in in vivo and ex vivo models: A systematic review. J. Cell. Physiol. 2020. [Google Scholar] [CrossRef]
- Gomes de Andrade, G.; Reck Cechinel, L.; Bertoldi, K.; Galvão, F.; Valdeci Worm, P.; Rodrigues Siqueira, I. The aging process alters IL-1β and CD63 levels differently in extracellular vesicles obtained from the plasma and cerebrospinal fluid. Neuroimmunomodulation 2018, 25, 18–22. [Google Scholar] [CrossRef]
- Brahmer, A.; Neuberger, E.; Esch-Heisser, L.; Haller, N.; Jorgensen, M.M.; Baek, R.; Möbius, W.; Simon, P.; Krämer-Albers, E.M. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J. Extracell Vesicles 2019, 8, 1615820. [Google Scholar] [CrossRef]
- Logozzi, M.; De Milito, A.; Lugini, L.; Borghi, M.; Calabrò, L.; Spada, M.; Perdicchio, M.; Marino, M.L.; Federici, C.; Iessi, E.; et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS ONE 2009, 4, e5219. [Google Scholar] [CrossRef] [Green Version]
- Chettimada, S.; Lorenz, D.R.; Misra, V.; Dillon, S.T.; Reeves, R.K.; Manickam, C.; Morgello, S.; Kirk, G.D.; Mehta, S.H.; Gabuzda, D. Exosome markers associated with immune activation and oxidative stress in HIV patients on antiretroviral therapy. Sci. Rep. 2018, 8, 7227. [Google Scholar] [CrossRef]
- Kabashima, K.; Nakashima, C.; Nonomura, Y.; Otsuka, A.; Cardamone, C.; Parente, R.; De Feo, G.; Triggiani, M. Biomarkers for evaluation of mast cell and basophil activation. Immunol. Rev. 2018, 282, 114–120. [Google Scholar] [CrossRef]
- Yeung, L.; Hickey, M.J.; Wright, M.D. The many and varied roles of tetraspanins in immune cell recruitment and migration. Front. Immunol. 2018, 9, 1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Young (N = 12) | CG (N = 10) | TG (N = 28) | p-Value # | |
|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | ||
| Age (years) | 22.3 ± 2.1 | 73.6 ± 0.8 | 72.6 ± 0.4 | 0.209 |
| Height (cm) | 176.4 ± 1.7 | 155.6 ± 3.2 | 163.3 ± 2.3 | 0.088 |
| Weight (kg) | 78.6 ± 2.4 | 69.8 ± 4,4 | 73.9 ± 3.1 | 0.516 |
| BMI (kg/m2) | 24.1 ± 1.5 | 28.9 ± 1.9 | 27.4 ± 0.8 | 0.406 |
| CG (% Change) | TG (% Change) | p-Value | |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||
| 1RM bench press seated (kg) | −11.744 ± 4.415 | 32.672 ± 4.463 | <0.001 * |
| 1RM leg extension (kg) | 14.343 ± 7.585 | 25.189 ± 3.021 | 0.040 * |
| Young (N = 12) | Elderly (N = 38) | p-Value | |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||
| miR-146a-5p (log10-scale) | 3.948 ± 0.482 | 4.3151 ± 0.214 | 0.618 |
| cfDNA (ng/mL) | 2277.079 ± 63.658 | 2443.279 ± 56.015 | 0.114 |
| Total exosome protein (µg/µL) | 5.022 ± 0.382 | 6.000 ± 0.328 | 0.140 |
| CD9 (O.D.) | 0.904 ± 0.179 | 1.049 ± 0.088 | 0.262 |
| CD14 (O.D.) | 1.850 ± 0.597 | 1.582 ± 0.164 | 0.486 |
| CD63 (O.D.) | 1.690 ± 0.218 | 1.499 ± 0.341 | 0.014 * |
| CD81 (O.D.) | 1.311 ± 0.295 | 1.101 ± 0.089 | 0.856 |
| Flot-1 (O.D.) | 1.879 ± 0.399 | 1.633 ± 0.365 | 0.125 |
| VDAC1 (O.D.) | 0.958 ± 0.152 | 1.530 ± 0.184 | 0.078 |
| CG (N = 10) | TG (N = 28) | p Value | |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||
| miR-146a (log10-scale) | 4.728 ± 0.224 | 4.150 ± 0.280 | 0.416 |
| cfDNA (ng/mL) | 2416.217 ± 156.234 | 2451.978 ± 56.499 | 0.376 |
| Total exosome protein (µg/µL) | 6.593 ± 0.587 | 5.789 ± 0.391 | 0.317 |
| CD9 (O.D.) | 1.053 ± 0.209 | 1.048 ± 0.095 | 0.777 |
| CD14 (O.D.) | 1.481 ± 0.230 | 1.623 ± 0.214 | 0.728 |
| CD63 (O.D.) | 1.397 ± 0.299 | 1.092 ± 0.097 | 0.584 |
| CD81 (O.D.) | 1.027 ± 0.156 | 1.128 ± 0.108 | 0.507 |
| Flot-1 (O.D.) | 2.207 ± 0.884 | 1.420 ± 0.383 | 0.608 |
| VDAC1 (O.D.) | 1.151 ± 0.268 | 1.685 ± 0.230 | 0.151 |
| CG (% Change) | TG (% Change) | p-Value | |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||
| miR-146a-5p (log10-scale) | −2.681 ± 9.184 | 13.945 ± 8.659 | 0.360 |
| cfDNA (ng/mL) | −1.370 ± 1.431 | −3.057 ± 1.159 | 0.396 |
| Total exosome protein (µg/µL) | −6.663 ± 8.173 | −3.990 ± 8.173 | 0.947 |
| CD9 (O.D.) | 26.147 ± 15.547 | 17.668 ± 9.393 | 0.571 |
| CD14 (O.D.) | −2.586 ± 13.333 | 103.368 ± 71.834 | 0.192 |
| CD63 (O.D.) | 42.541 ± 14.044 | 6.786 ± 5.524 | 0.027 * |
| CD81 (O.D.) | 4.681 ± 7.292 | 5.985 ± 6.481 | 0.842 |
| Flot-1 (O.D.) | 49.712 ± 31.691 | 31.018 ± 12.679 | 0.784 |
| VDAC1 (O.D.) | 0.551 ± 11.800 | 34.428 ± 18.493 | 0.433 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estébanez, B.; Visavadiya, N.P.; de Paz, J.A.; Whitehurst, M.; Cuevas, M.J.; González-Gallego, J.; Huang, C.-J. Resistance Training Diminishes the Expression of Exosome CD63 Protein without Modification of Plasma miR-146a-5p and cfDNA in the Elderly. Nutrients 2021, 13, 665. https://doi.org/10.3390/nu13020665
Estébanez B, Visavadiya NP, de Paz JA, Whitehurst M, Cuevas MJ, González-Gallego J, Huang C-J. Resistance Training Diminishes the Expression of Exosome CD63 Protein without Modification of Plasma miR-146a-5p and cfDNA in the Elderly. Nutrients. 2021; 13(2):665. https://doi.org/10.3390/nu13020665
Chicago/Turabian StyleEstébanez, Brisamar, Nishant P. Visavadiya, José A. de Paz, Michael Whitehurst, María J. Cuevas, Javier González-Gallego, and Chun-Jung Huang. 2021. "Resistance Training Diminishes the Expression of Exosome CD63 Protein without Modification of Plasma miR-146a-5p and cfDNA in the Elderly" Nutrients 13, no. 2: 665. https://doi.org/10.3390/nu13020665
APA StyleEstébanez, B., Visavadiya, N. P., de Paz, J. A., Whitehurst, M., Cuevas, M. J., González-Gallego, J., & Huang, C.-J. (2021). Resistance Training Diminishes the Expression of Exosome CD63 Protein without Modification of Plasma miR-146a-5p and cfDNA in the Elderly. Nutrients, 13(2), 665. https://doi.org/10.3390/nu13020665