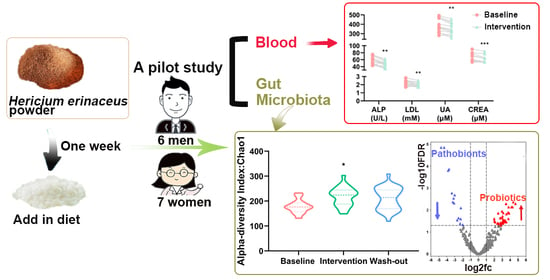
Influence of Short-Term Consumption of Hericium erinaceus on Serum Biochemical Markers and the Changes of the Gut Microbiota: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
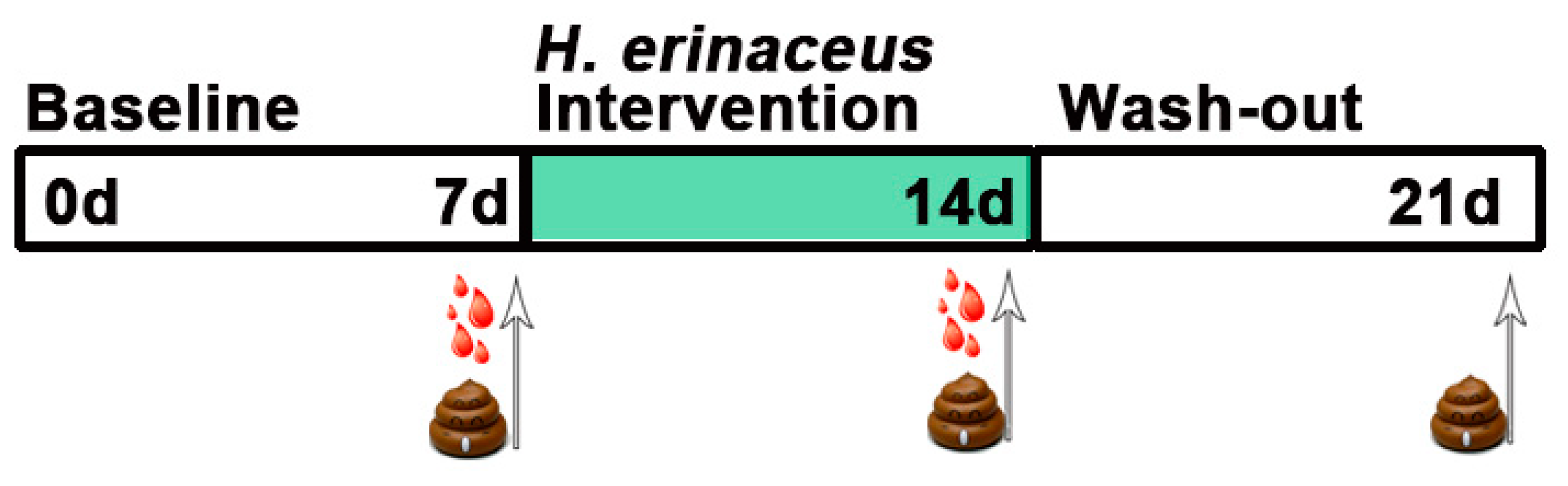
2.2. Study Design
2.3. Assessment of Samples
2.4. Statistical Analysis
3. Results
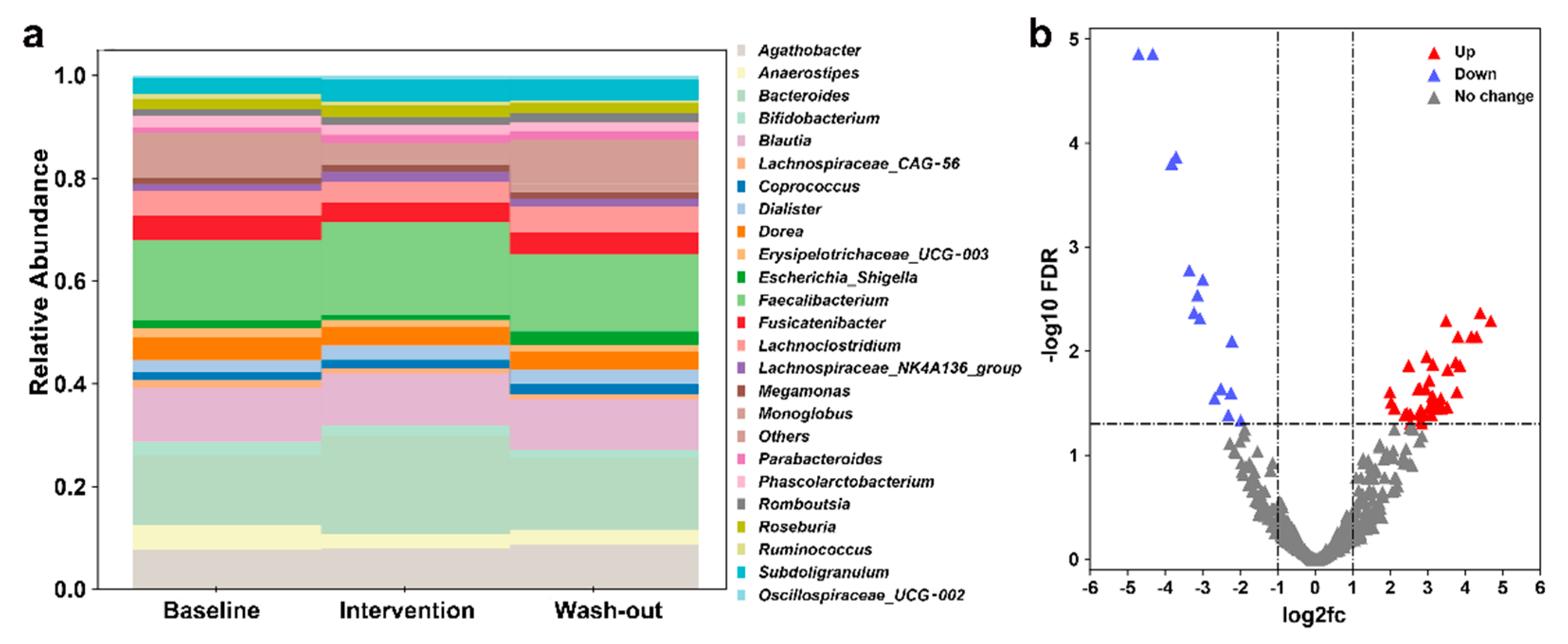
3.1. Effect of H. erinaceus Powder on the Composition of Gut Microbiota
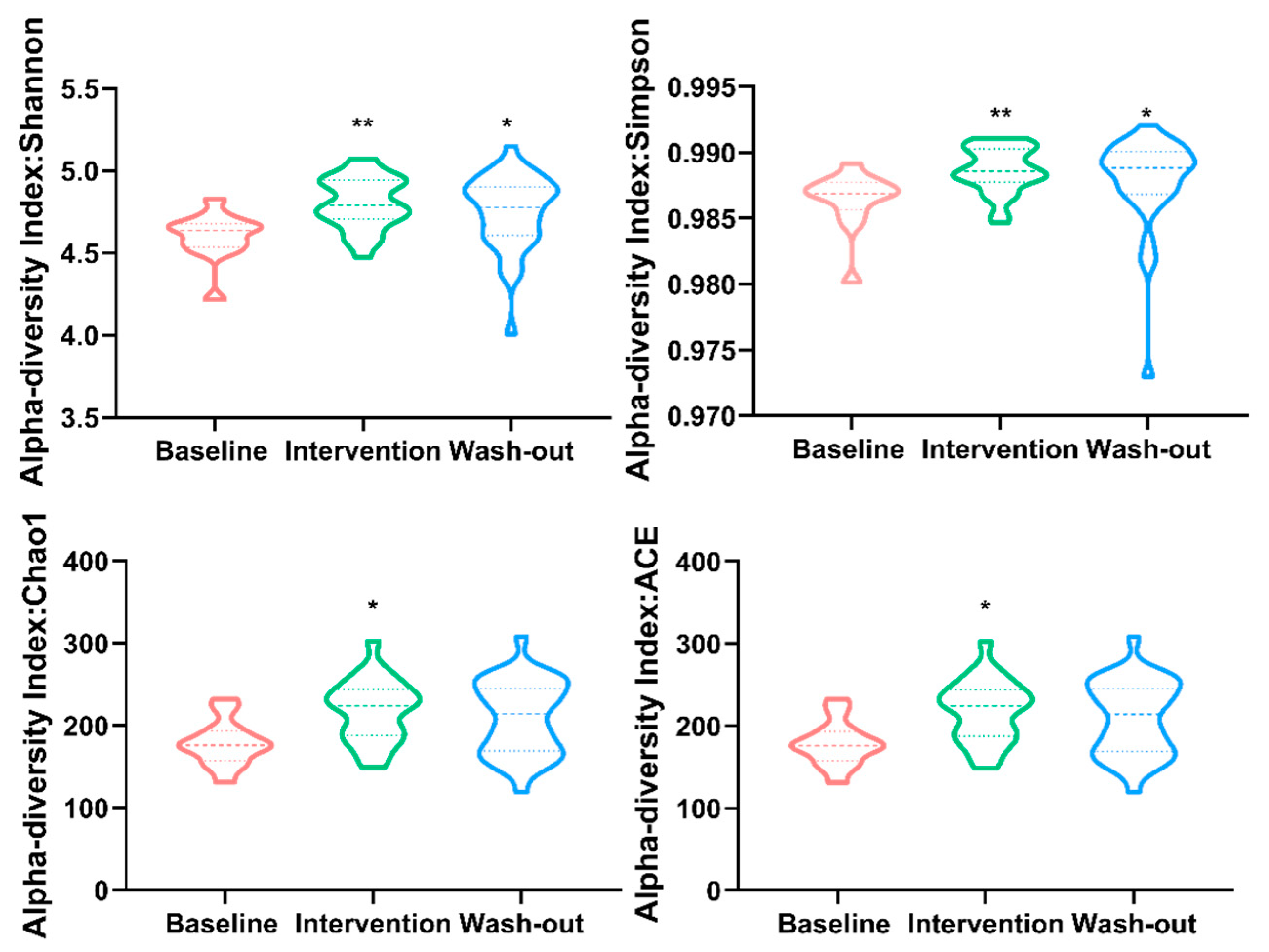
3.2. Effect of H. erinaceus Powder on the Diversity of Gut Microbiota
3.3. Effect of H. erinaceus Powder on Blood Parameters related to Metabolic Diseases
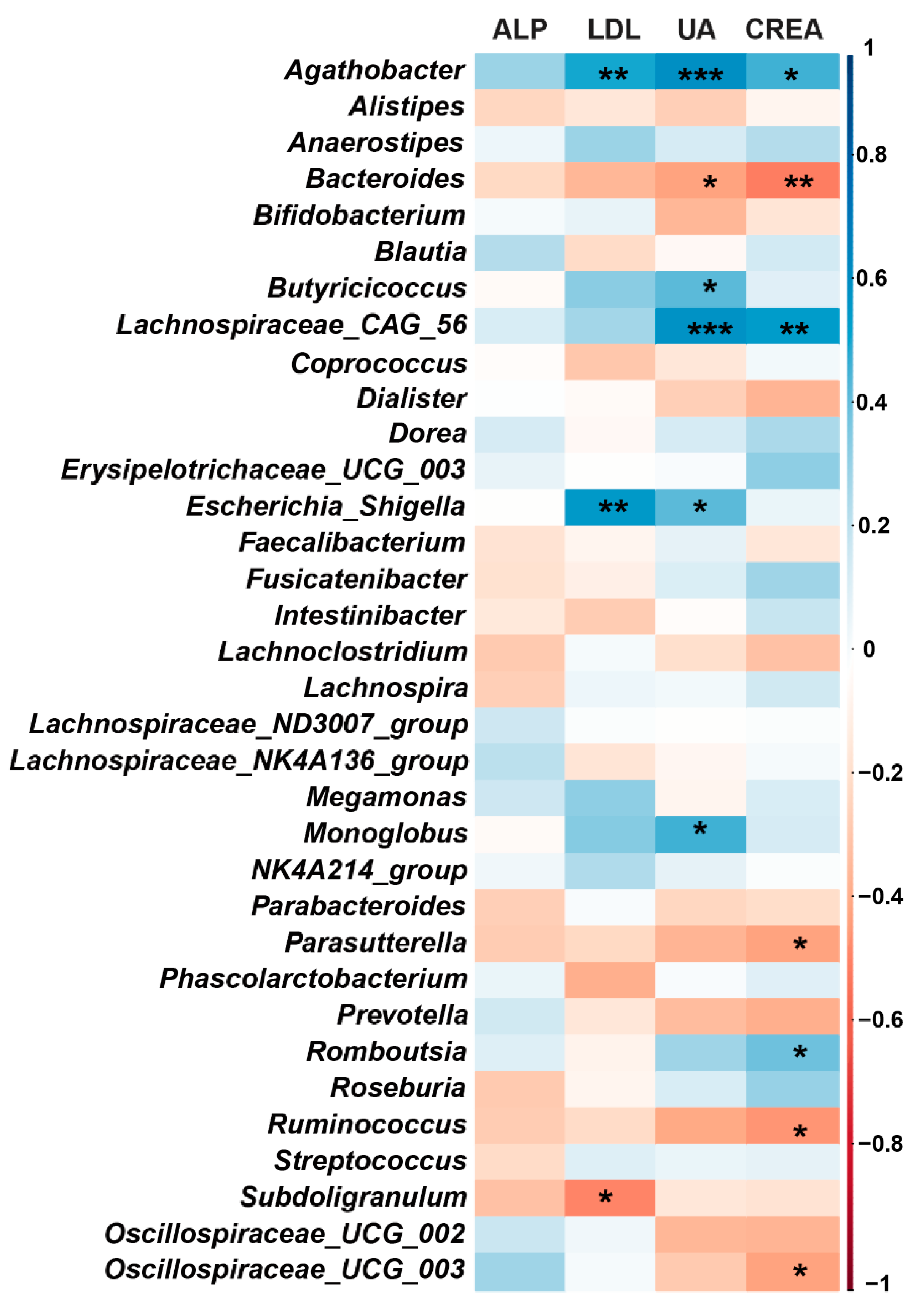
3.4. Correlation between Gut Microbiota and Blood Biochemical Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adebowale, T.O.; Yao, K.; Oso, A.O. Major cereal carbohydrates in relation to intestinal health of monogastric animals: A review. Anim. Nutr. 2019, 5, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.; Bäckhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gerard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Chou, C.J. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010, 24, 4948. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Heinken, A.; Thiele, I. Systems biology of host-microbe metabolomics. Wiley Interdiscip. Rev. Syst. Biol. Med. 2015, 7, 195–219. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480. [Google Scholar] [CrossRef]
- Nemet, I.; Saha, P.P.; Gupta, N.; Zhu, W.F.; Romano, K.A.; Skye, S.M.; Cajka, T.; Mohan, M.L.; Li, L.; Wu, Y.P.; et al. A Cardiovascular Disease-Linked Gut Microbial Metabolite Acts via Adrenergic Receptors. Cell 2020, 180, 86. [Google Scholar] [CrossRef]
- Kim, S.P.; Kang, M.Y.; Choi, Y.H.; Kim, J.H.; Nam, S.H.; Friedman, M. Mechanism of Hericium erinaceus (Yamabushitake) mushroom-induced apoptosis of U937 human monocytic leukemia cells. Food Funct 2011, 2, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Inatomi, S.; Ouchi, K.; Azumi, Y.; Tuchida, T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: A double-blind placebo-controlled clinical trial. Phytother. Res. 2009, 23, 367–372. [Google Scholar] [CrossRef]
- Vigna, L.; Morelli, F.; Agnelli, G.M.; Napolitano, F.; Ratto, D.; Occhinegro, A.; Di Iorio, C.; Savino, E.; Girometta, C.; Brandalise, F.; et al. Hericium erinaceus Improves Mood and Sleep Disorders in Patients Affected by Overweight or Obesity: Could Circulating Pro-BDNF and BDNF Be Potential Biomarkers? Evid. Based Complement Alternat. Med. 2019, 2019, 7861297. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, X.; Tang, X.; Li, H.; Yizhen, X.; Chen, D. Auxiliary antitumor effects of fungal proteins from Hericium erinaceus by target on the gut microbiota. J. Food Sci. 2020, 85, 1872–1890. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The long-term stability of the human gut microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Z.-M.; Guo-Hua, X.U.; Zhao, H.; Shi, J.S.; Zheng-Hong, X.U. Component Analysis of Hericium Erinaceus Mycelia. Food Res. Dev. 2015, 36, 95–97. (in Chinese). [Google Scholar]
- Li, J.; Lei, R.H.; Li, X.; Xiong, F.X.; Zhang, Q.Y.; Zhou, Y.; Yang, S.M.; Chang, Y.A.; Chen, K.; Gu, W.H.; et al. The antihyperlipidemic effects of fullerenol nanoparticles via adjusting the gut microbiota in vivo. Part Fibre Toxicol. 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581. [Google Scholar] [CrossRef]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J.G. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef]
- Bowerman, K.L.; Rehman, S.F.; Vaughan, A.; Lachner, N.; Budden, K.F.; Kim, R.Y.; Wood, D.L.A.; Gellatly, S.L.; Shukla, S.D.; Wood, L.G.; et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat. Commun. 2020, 11, 5886. [Google Scholar] [CrossRef]
- Hartman, K.; van der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.C.; Schlaeppi, K. Correction to: Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2020, 8, 66. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.Y.; Xia, J.G. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Diling, C.; Xin, Y.; Chaoqun, Z.; Jian, Y.; Xiaocui, T.; Jun, C.; Ou, S.; Yizhen, X. Hericium erinaceus Extracts from relieve inflammatory bowel disease by regulating immunity and gut microbiota. Oncotarget 2017, 8, 85838–85857. [Google Scholar] [CrossRef]
- Chen, D.L.; Zheng, C.Q.; Yang, J.; Li, J.; Su, J.Y.; Xie, Y.Z.; Lai, G.X. Immunomodulatory Activities of a Fungal Protein Extracted from Hericium erinaceus through Regulating the Gut Microbiota. Front Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Ren, Y.L.; Geng, Y.; Du, Y.; Li, W.; Lu, Z.M.; Xu, H.Y.; Xu, G.H.; Shi, J.S.; Xu, Z.H. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018, 57, 67–76. [Google Scholar] [CrossRef]
- Shao, S.; Wang, D.D.; Zheng, W.; Li, X.Y.; Zhang, H.; Zhao, D.Q.; Wang, M.X. A unique polysaccharide from Hericium erinaceus mycelium ameliorates acetic acid-induced ulcerative colitis rats by modulating the composition of the gut microbiota, short chain fatty acids levels and GPR41/43 respectors. Int. Immunopharmacol. 2019, 71, 411–422. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, H.; Zhu, E.; Li, J.; Wang, Q.; Zhou, W.; Qin, T.; Wu, X.; Wu, B.; Huang, Y. Hericium erinaceus polysaccharide facilitates restoration of injured intestinal mucosal immunity in Muscovy duck reovirus-infected Muscovy ducklings. Int. J. Biol. Macromol. 2018, 107, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.S.; Fung, M.L.; Wong, K.H.; Lim, L.W. Therapeutic Potential of Hericium erinaceus for Depressive Disorder. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Bastiaanssen, T.F.S.; Cussotto, S.; Claesson, M.J.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Gutted! Unraveling the Role of the Microbiome in Major Depressive Disorder. Harvard Rev. Psychiat. 2020, 28, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.L.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Wilmanski, T.; Rappaport, N.; Earls, J.C.; Magis, A.T.; Manor, O.; Lovejoy, J.; Omenn, G.S.; Hood, L.; Gibbons, S.M.; Price, N.D. Blood metabolome predicts gut microbiome alpha-diversity in humans. Nat. Biotechnol. 2019, 37, 1217–1228. [Google Scholar] [CrossRef]
- Hu, S.H.; Li, A.; Huang, T.T.; Lai, J.B.; Li, J.J.; Sublette, M.E.; Lu, H.F.; Lu, Q.Q.; Du, Y.L.; Hu, Z.Y.; et al. Gut Microbiota Changes in Patients with Bipolar Depression. Adv. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104. [Google Scholar] [CrossRef]
- Yi, Y.; Shen, L.; Shi, W.; Xia, F.; Zhang, H.; Wang, Y.; Zhang, J.; Wang, Y.; Sun, X.; Zhang, Z.; et al. Gut Microbiome Components Predict Response to Neoadjuvant Chemoradiotherapy in Patients with Locally Advanced Rectal Cancer: A Prospective, Longitudinal Study. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Wang, Z.; Ang, K.Y.; Huang, S.; Hou, Q.; Su, X.; Qiao, J.; Zheng, Y.; Wang, L.; et al. Intestinal Microbiota Distinguish Gout Patients from Healthy Humans. Sci. Rep. 2016, 6, 20602. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Jiang, Y.; Tan, A.; Ye, J.; Xian, X.; Xie, Y.; Wang, Q.; Yao, Z.; Mo, Z. 16S rRNA gene sequencing reveals altered composition of gut microbiota in individuals with kidney stones. Urolithiasis 2018, 46, 503–514. [Google Scholar] [CrossRef]
- Solé, C.; Guilly, S.; Da Silva, K.; Llopis, M.; Le-Chatelier, E.; Huelin, P.; Carol, M.; Moreira, R.; Fabrellas, N.; De Prada, G.; et al. Alterations in Gut Microbiome in Cirrhosis as Assessed by Quantitative Metagenomics: Relationship With Acute-on-Chronic Liver Failure and Prognosis. Gastroenterology 2021, 160, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Freidin, M.; Stalteri, M.; Wells, P.; Lachance, G.; Baleanu, A.; Bowyer, R.; Kurilshikov, A.; Zhernakova, A.; Steves, C.; Williams, F. An association between chronic widespread pain and the gut microbiome. Rheumatology 2020. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Leth, M.L.; Ejby, M.; Workman, C.; Ewald, D.A.; Pedersen, S.S.; Sternberg, C.; Bahl, M.I.; Licht, T.R.; Aachmann, F.L.; Westereng, B.; et al. Differential bacterial capture and transport preferences facilitate co-growth on dietary xylan in the human gut. Nat. Microbiol. 2018, 3, 570–580. [Google Scholar] [CrossRef]
- Lammers, W.J.; van Buuren, H.R.; Hirschfield, G.M.; Janssen, H.L.A.; Invernizzi, P.; Mason, A.L.; Ponsioen, C.Y.; Floreani, A.; Corpechot, C.; Mayo, M.J.; et al. Levels of Alkaline Phosphatase and Bilirubin Are Surrogate End Points of Outcomes of Patients With Primary Biliary Cirrhosis: An International Follow-up Study. Gastroenterology 2014, 147, 1338. [Google Scholar] [CrossRef]
- Bates, J.M.; Akerlund, J.; Mittge, E.; Guillemin, K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2007, 2, 371–382. [Google Scholar] [CrossRef]
- Perez, C.F.M.; Harms, M.H.; Lindor, K.D.; van Buuren, H.R.; Hirschfield, G.M.; Corpechot, C.; van der Meer, A.J.; Feld, J.J.; Gulamhusein, A.; Lammers, W.J.; et al. Goals of Treatment for Improved Survival in Primary Biliary Cholangitis: Treatment Target Should Be Bilirubin Within the Normal Range and Normalization of Alkaline Phosphatase. Am. J. Gastroenterol. 2020, 115, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, R.; Hoogeveen, R.M.; Langsted, A.; Stiekema, L.C.A.; Verweij, S.L.; Hovingh, G.K.; Wareham, N.J.; Khaw, K.T.; Boekholdt, S.M.; Nordestgaard, B.G.; et al. Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low-density lipoprotein cholesterol levels in a primary prevention setting. Eur. Heart J. 2018, 39, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Lu, D.; Huang, Z.M.; Xiao, L. Comment on Pilemann-Lyberg et al. Uric Acid Is an Independent Risk Factor for Decline in Kidney Function, Cardiovascular Events, and Mortality in Patients With Type 1 Diabetes. Diabetes Care 2019;42:1088–1094. Diabetes Care 2019, 42, E187. [Google Scholar] [CrossRef] [PubMed]
- Bakker, S.J.L. Chronic Kidney Disease Defining clinical cut-offs for albumin: Creatinine ratio. Nat. Rev. Nephrol. 2013, 9, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Yang, S.T.; Li, S.H.; Zhao, L.; Hao, Y.L.; Qin, J.J.; Zhang, L.; Zhang, C.Y.; Bian, W.J.; Zuo, L.; et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131. [Google Scholar] [CrossRef] [PubMed]

| Blood Routine Index | Baseline (n = 13) | Intervention (n = 13) | p-Value |
|---|---|---|---|
| Red blood cell (109/L) | 4.848 ± 0.311 | 4.802 ± 0.316 | 0.077 |
| Leukocyte (109/L) | 6.522 ± 0.923 | 6.495 ± 1.106 | 0.883 |
| Hemoglobin (g/L) | 144.333 ± 11.448 | 143.250 ± 11.959 | 0.286 |
| Platelet (109/L) | 247.308 ± 35.569 | 251.231 ± 54.104 | 0.564 |
| Lymphocyte (109/L) | 2.465 ± 0.576 | 2.244 ± 0.417 | 0.052 |
| Monocytes (109/L) | 0.382 ± 0.061 | 0.389 ± 0.061 | 0.615 |
| Neutrophil (109/L) | 3.508 ± 0.613 | 3.705 ± 0.892 | 0.106 |
| Eosinophil (109/L) | 0.150 ± 0.114 | 0.143 ± 0.096 | 0.590 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.-Q.; Geng, Y.; Guan, Q.; Ren, Y.; Guo, L.; Lv, Q.; Lu, Z.-M.; Shi, J.-S.; Xu, Z.-H. Influence of Short-Term Consumption of Hericium erinaceus on Serum Biochemical Markers and the Changes of the Gut Microbiota: A Pilot Study. Nutrients 2021, 13, 1008. https://doi.org/10.3390/nu13031008
Xie X-Q, Geng Y, Guan Q, Ren Y, Guo L, Lv Q, Lu Z-M, Shi J-S, Xu Z-H. Influence of Short-Term Consumption of Hericium erinaceus on Serum Biochemical Markers and the Changes of the Gut Microbiota: A Pilot Study. Nutrients. 2021; 13(3):1008. https://doi.org/10.3390/nu13031008
Chicago/Turabian StyleXie, Xiao-Qian, Yan Geng, Qijie Guan, Yilin Ren, Lin Guo, Qiqi Lv, Zhen-Ming Lu, Jin-Song Shi, and Zheng-Hong Xu. 2021. "Influence of Short-Term Consumption of Hericium erinaceus on Serum Biochemical Markers and the Changes of the Gut Microbiota: A Pilot Study" Nutrients 13, no. 3: 1008. https://doi.org/10.3390/nu13031008
APA StyleXie, X.-Q., Geng, Y., Guan, Q., Ren, Y., Guo, L., Lv, Q., Lu, Z.-M., Shi, J.-S., & Xu, Z.-H. (2021). Influence of Short-Term Consumption of Hericium erinaceus on Serum Biochemical Markers and the Changes of the Gut Microbiota: A Pilot Study. Nutrients, 13(3), 1008. https://doi.org/10.3390/nu13031008