L-Glutamine Supplementation Enhances Strength and Power of Knee Muscles and Improves Glycemia Control and Plasma Redox Balance in Exercising Elderly Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Glutamine Supplementation
2.3. Body Composition
2.4. Determination of Daily Physical Activity
2.5. Exercise Program
2.6. Isokinetic Strength Testing
2.7. Functional Fitness Tests
2.8. Biochemical Assays
2.8.1. Sample Collection
2.8.2. Determination of Plasma D-Fructosamine and Insulin Concentrations
2.8.3. Determination of Reduced/Oxidized Glutathione (GSH/GSSG)
2.8.4. Determination of Iron, Uric Acid, and Lipid Oxidation Indexes
2.9. Statistical Analysis
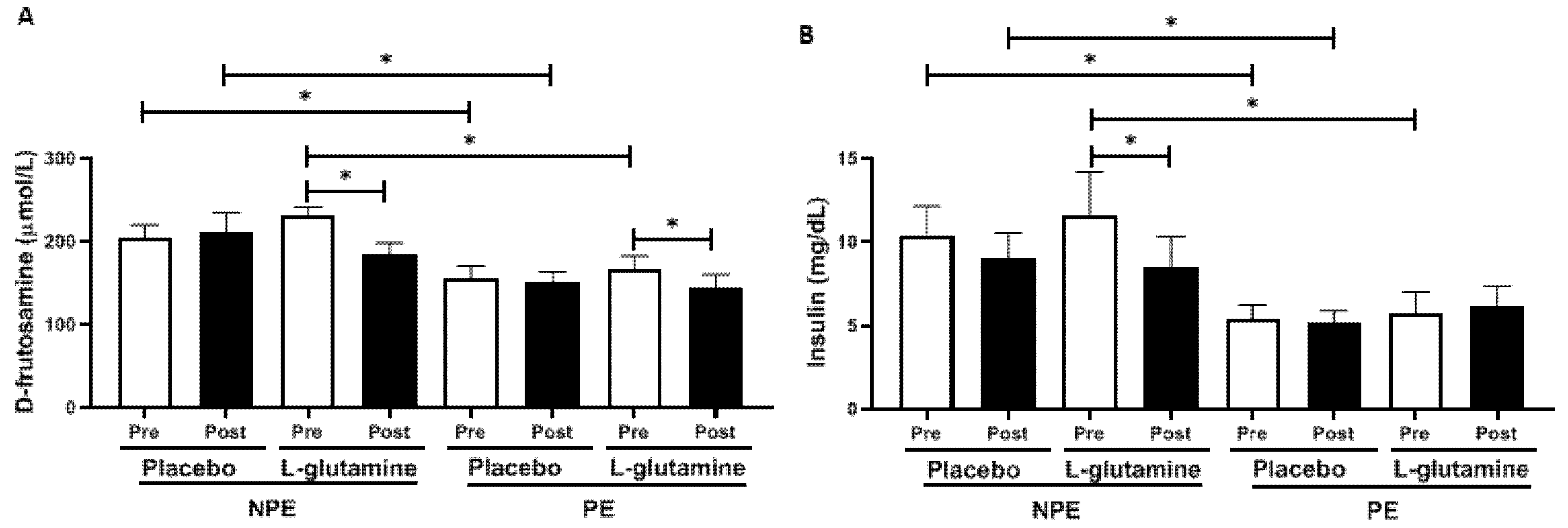
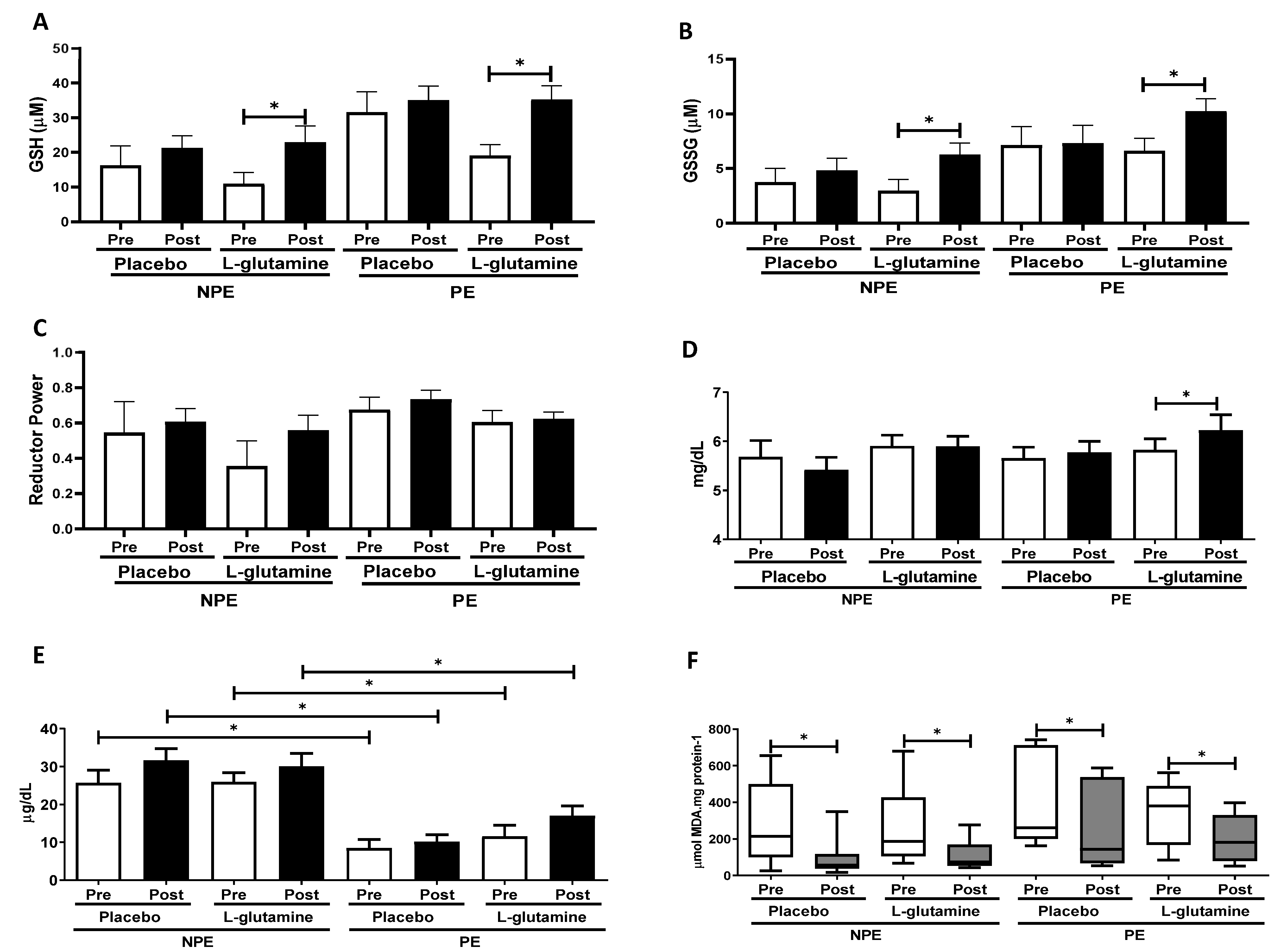
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American College of Sports Medicine; Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone-Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef] [PubMed]
- Bachi, A.L.; Suguri, V.M.; Ramos, L.R.; Mariano, M.; Vaisberg, M.; Lopes, J.D. Increased production of autoantibodies and specific antibodies in response to influenza virus vaccination in physically active older individuals. Results Immunol. 2013, 3, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A. Biology of aging: Paving the way for healthy aging. Exp. Gerontol. 2018, 107, 1–3. [Google Scholar] [CrossRef] [PubMed]
- United Nations, Department of Economic and Social Affairs, Population Division (2019). World Population Ageing 2019: Highlights (ST/ESA/SER.A/430). Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf (accessed on 29 January 2021).
- Dominguez, L.J.; Galioto, A.; Ferlisi, A.; Pineo, A.; Putignano, E.; Belvedere, M.; Costanza, G.; Barbagallo, M. Ageing, lifestyle modifications, and cardiovascular disease in developing countries. J. Nutr. Health Aging 2006, 10, 143–149. [Google Scholar]
- Jin, K. New perspectives on healthy aging. Prog. Neurobiol. 2017, 157, 1. [Google Scholar] [CrossRef]
- Cesari, M.; Araujo de Carvalho, I.; Amuthavalli Thiyagarajan, J.; Cooper, C.; Martin, F.C.; Reginster, J.Y.; Vellas, B.; Beard, J.R. Evidence for the Domains Supporting the Construct of Intrinsic Capacity. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1653–1660. [Google Scholar] [CrossRef]
- Charansonney, O.L. Physical activity and aging: A life-long story. Discov. Med. 2011, 12, 177–185. [Google Scholar]
- Pescatello, L.S.; Arena, R.; Riebe, D.; Thompson, P.D. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014; p. 456. ISBN 978-1-6091-3955-1. [Google Scholar]
- Witard, O.C.; Ball, D. The interaction between nutrition and exercise for promoting health and performance. Proc. Nutr. Soc. 2018, 77, 1–3. [Google Scholar] [CrossRef]
- Mora, J.C.; Valencia, W.M. Exercise and Older Adults. Clin. Geriatr. Med. 2018, 34, 145–162. [Google Scholar] [CrossRef]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef]
- Dickinson, J.M.; Volpi, E.; Rasmussen, B.B. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc. Sport Sci. Rev. 2013, 41, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Lagranha, C.J.; Doi, S.Q.; Sellitti, D.F.; Procopio, J.; Pithon-Curi, T.C.; Corless, M.; Newsholme, P. Molecular mechanisms of glutamine action. J. Cell Physiol. 2005, 204, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Morales, J.S.; Emanuele, E.; Pareja-Galeano, H.; Lucia, A. Supplements with purported effects on muscle mass and strength. Eur. J. Nutr. 2019, 58, 2983–3008. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.R.; de Vasconcelos, D.A.A.; Murata, G.M.; Fortes, M.A.S.; Marzuca-Nassr, G.N.; Levada-Pires, A.C.; Vitzel, K.F.; Abreu, P.; Scervino, M.V.M.; Hirabara, S.M.; et al. Glutamine supplementation versus functional overload in extensor digitorum longus muscle hypertrophy. PharmaNutrition 2020, 14, 100236. [Google Scholar] [CrossRef]
- de Vasconcelos, D.A.A.; Giesbertz, P.; de Souza, D.R.; Vitzel, K.F.; Abreu, P.; Marzuca-Nassr, G.N.; Fortes, M.A.S.; Murata, G.M.; Hirabara, S.M.; Curi, R.; et al. Oral L-glutamine pretreatment attenuates skeletal muscle atrophy induced by 24-h fasting in mice. J. Nutr. Biochem. 2019, 70, 202–214. [Google Scholar] [CrossRef]
- Lambertucci, A.C.; Lambertucci, R.H.; Hirabara, S.M.; Curi, R.; Moriscot, A.S.; Alba-Loureiro, T.C.; Guimarães-Ferreira, L.; Levada-Pires, A.C.; Vasconcelos, D.A.; Sellitti, D.F.; et al. Glutamine supplementation stimulates protein-synthetic and inhibits protein-degradative signaling pathways in skeletal muscle of diabetic rats. PLoS ONE 2012, 7, e50390. [Google Scholar] [CrossRef]
- de Vasconcelos, D.A.A.; Giesbertz, P.; Murata, G.M.; de Souza, D.R.; Fiamoncini, J.; Duque-Guimaraes, D.; Leandro, C.G.; Hirabara, S.M.; Daniel, H.; Curi, R.; et al. Myotube Protein Content Associates with Intracellular L-Glutamine Levels. Cell Physiol. Biochem. 2019, 53, 200–214. [Google Scholar] [CrossRef]
- Petry, É.R.; Dresch, D.F.; Carvalho, C.; Medeiros, P.C.; Rosa, T.G.; de Oliveira, C.M.; Martins, L.A.M.; Schemitt, E.; Bona, S.; Guma, F.C.R.; et al. Oral glutamine supplementation attenuates inflammation and oxidative stress-mediated skeletal muscle protein content degradation in immobilized rats: Role of 70 kDa heat shock protein. Free Radic. Biol. Med. 2019, 145, 87–102. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, T.R.B.; Antunes, P.d.C.; Rodriguez-Añez, C.R.; Mazo, G.Z.; Petroski, É.L. Reprodutibilidade e validade do Questionário Internacional de Atividade Física (IPAQ) em homens idosos (Portuguese). Rev. Bras. Med. Esporte 2007, 13, 11–16. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Rutkauskiene, L.; Lendraitiene, E. Batteries assessing functional fitness of older people: A brief review. J. Complex. Health Sci. 2019, 2, 64–69. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Leibovitz, B.E.; Tappel, A.L. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: Characterization and comparison with homogenates and microsomes. Free Radic. Biol. Med. 1988, 4, 155–161. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Fagerström, C.; Borglin, G. Mobility, functional ability and health-related quality of life among people of 60 years or older. Aging Clin Exp Res. 2010, 22, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Smolarek, A.d.C.; Ferreira, L.H.; Mascarenhas, L.P.; McAnulty, S.R.; Varela, K.D.; Dangui, M.C.; de Barros, M.P.; Utter, A.C.; Souza-Junior, T.P. The effects of strength training on cognitive performance in elderly women. Clin. Interv. Aging. 2016, 11, 749–754. [Google Scholar] [CrossRef]
- Bachi, A.L.L.; Barros, M.P.; Vieira, R.P.; Rocha, G.A.; de Andrade, P.B.M.; Victorino, A.B.; Ramos, L.R.; Gravina, C.F.; Lopes, J.D.; Vaisberg, M.; et al. Combined Exercise Training Performed by Elderly Women Reduces Redox Indexes and Proinflammatory Cytokines Related to Atherogenesis. Oxid. Med. Cell Longev. 2019, 6469213. [Google Scholar] [CrossRef]
- Hunter, G.R.; McCarthy, J.P.; Bamman, M.M. Effects of resistance training on older adults. Sports Med. 2004, 34, 329–348. [Google Scholar] [CrossRef]
- Badawi, Y.; Nishimune, H. Impairment Mechanisms and Intervention Approaches for Aged Human Neuromuscular Junctions. Front. Mol. Neurosci. 2020, 16, 568426. [Google Scholar] [CrossRef] [PubMed]
- Rikli, R.E.; Jessie-Jones, C. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist 2013, 53, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, L.B.; Santos, D.A.; Marques, E.A.; Mota, J. Criterion-referenced fitness standards for predicting physical independence into later life. Exp. Gerontol. 2015, 61, 142–146. [Google Scholar] [CrossRef]
- Regterschot, G.R.; Zhang, W.; Baldus, H.; Stevens, M.; Zijlstra, W. Test-retest reliability of sensor-based sit-to-stand measures in young and older adults. Gait Posture 2014, 40, 220–224. [Google Scholar] [CrossRef]
- Long, J.; Cai, T.; Huang, X.; Zhou, Y.; Kuang, J.; Wu, L. Reference value for the TUGT in healthy older people: A systematic review and meta-analysis. Geriatr. Nurs. 2020, 41, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y. Muscle Mass, Quality, and Composition Changes during Atrophy and Sarcopenia. Adv. Exp. Med. Biol. 2018, 1088, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.R.; Harber, M.P. Skeletal muscle hypertrophy after aerobic exercise training. Exerc. Sport Sci. Rev. 2014, 42, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.J.; Ryan, E.D.; Sobolewski, E.J.; Conchola, E.C.; Cramer, J.T. Age related differences in maximal and rapid torque characteristics of the leg extensors and flexors in young, middle-aged and old men. Exp. Gerontol. 2013, 48, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef] [PubMed]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin. J. Med. 2017, 84 (Suppl. 1), S15–S21. [Google Scholar] [CrossRef] [PubMed]
- Ostman, C.; Smart, N.A.; Morcos, D.; Duller, A.; Ridley, W.; Jewiss, D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2017, 16, 110. [Google Scholar] [CrossRef]
- Samocha-Bonet, D.; Chisholm, D.J.; Holst, J.J.; Greenfield, J.R. L-glutamine and whole protein restore first-phase insulin response and increase glucagon-like peptide-1 in type 2 diabetes patients. Nutrients 2015, 7, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef]
- Petry, É.R.; Cruzat, V.F.; Heck, T.G.; Homem de Bittencourt, P.I., Jr.; Tirapegui, J. L-glutamine supplementations enhance liver glutamine-glutathione axis and heat shock factor-1 expression in endurance-exercise trained rats. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 188–197. [Google Scholar] [CrossRef]
- Petry, E.R.; Cruzat, V.F.; Heck, T.G.; Leite, J.S.; Homem de Bittencourt, P.I., Jr.; Tirapegui, J. Alanyl-glutamine and glutamine plus alanine supplements improve skeletal redox status in trained rats: Involvement of heat shock protein pathways. Life Sci. 2014, 94, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Dohl, J.; Passos, M.E.P.; Foldi, J.; Chen, Y.; Pithon-Curi, T.; Curi, R.; Gorjao, R.; Deuster, P.A.; Yu, T. Glutamine depletion disrupts mitochondrial integrity and impairs C2C12 myoblast proliferation, differentiation, and the heat-shock response. Nutr. Res. 2020, 84, 42–52. [Google Scholar] [CrossRef]
- Thirupathi, A.; Pinho, R.A.; Chang, Y.Z. Physical exercise: An inducer of positive oxidative stress in skeletal muscle aging. Life Sci. 2020, 252, 117630. [Google Scholar] [CrossRef] [PubMed]
- Polotow, T.G.; Souza-Junior, T.P.; Sampaio, R.C.; Okuyama, A.R.; Ganini, D.; Vardaris, C.V.; Alves, R.C.; McAnulty, S.R.; Barros, M.P. Effect of 1 Repetition Maximum, 80% Repetition Maximum, and 50% Repetition Maximum Strength Exercise in Trained Individuals on Variations in Plasma Redox Biomarkers. J. Strength Cond. Res. 2017, 31, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Tuomainen, T.P.; Loft, S.; Nyyssönen, K.; Punnonen, K.; Salonen, J.T.; Poulsen, H.E. Body iron is a contributor to oxidative damage of DNA. Free Radic. Res. 2007, 41, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Souza-Junior, T.; Lorenço-Lima, L.; Ganini, D.; Vardaris, C.; Polotow, T.; Barros, M.P. Delayed uric Acid accumulation in plasma provides additional anti-oxidant protection against iron-triggered oxidative stress after a wingate test. Biol. Sport. 2014, 31, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, R.C.; Moura, N.R.; Barros, M.P.; Hatanaka, E.; Priviero, F.B.M.; de Moraes, C. Twice-weekly exercise training reduces oxidative stress and proinflammatory cytokine levels in elder women. Motriz 2019, 25, e101990. [Google Scholar] [CrossRef]
- Leite, J.S.; Raizel, R.; Hypólito, T.M.; Rosa, T.D.; Cruzat, V.F.; Tirapegui, J. L-Glutamine and L-alanine supplementation increase glutamine-glutathione axis and muscle HSP-27 in rats trained using a progressive high-intensity resistance exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Amores-Sánchez, M.I.; Medina, M.A. Glutamine, as a precursor of glutathione, and oxidative stress. Mol. Genet. Metab. 1999, 67, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Lian, G.; Gnanaprakasam, J.R.; Wang, T.; Wu, R.; Chen, X.; Liu, L.; Shen, Y.; Yang, M.; Yang, J.; Chen, Y.; et al. Glutathione de novo synthesis but not recycling process coordinates with glutamine catabolism to control redox homeostasis and directs murine T cell differentiation. eLife 2018, 7, e36158. [Google Scholar] [CrossRef]
- Inzitari, M.; Doets, E.; Bartali, B.; Benetou, V.; Di Bari, M.; Visser, M.; Volpato, S.; Gambassi, G.; Topinkova, E.; De Groot, L.; et al. International Association of Gerontology and Geriatrics (IAGG) Task Force For Nutrition In The Elderly. Nutrition in the age-related disablement process. J. Nutr. Health Aging 2011, 15, 599–604. [Google Scholar] [CrossRef]


| Pre | Exercise Intervention | Post | |
|---|---|---|---|
| Before supplementation period | 30 days (between sampling) | After supplementation period | |
| Blood sampling | X | X | |
| Isokinetic Test | X | X | |
| Gln or placebo supplementation | X |
| Characteristics | NPE (n = 23) | PE (n = 21) | p Value |
|---|---|---|---|
| Age (year) | 68.6 ± 4.5 | 69.8 ± 4.8 | >0.05 |
| Height (cm) | 155 ± 6.1 | 155 ± 6.5 | >0.05 |
| Weight (kg) | 64.1 ± 8.1 | 59.6 ± 10.5 | >0.05 |
| Body mass index (kg/m2) | 26.5 ± 3.4 | 24.6 ± 3.1 | >0.05 |
| Total body fat (%) | 40.3 ± 7.3 | 43.8 ± 6.8 | >0.05 |
| ASM/height (kg/m) | 4.4 ± 0.6 | 6.1 ± 0.3 | >0.05 |
| Clinical conditions | |||
| (based on medications use) | |||
| Diabetes mellitus, n(%) | 3(13) | 2(10) | >0.05 |
| Dyslipidemia, n(%) | 6(26) | 6(29) | >0.05 |
| Hypertension, n(%) | 9(39) | 8(38) | >0.05 |
| Coronary heart diseases, n(%) | 1(4) | 1(5) | >0.05 |
| Osteoarthritis, n(%) | 4(17) | 0(0) * | <0.05 |
| Depression, n(%) | 6(26) | 0(0) * | <0.05 |
| Hypothyroidism, n(%) | 4(17) | 3(14) | >0.05 |
| IPAQ | |||
| Low level | 497 ± 83 | 886 ± 175 * | <0.05 |
| Moderate level | 413 ± 112 | 1006 ± 263 * | <0.05 |
| High level | 318 ± 70 | 409 ± 121 | >0.05 |
| Sitting time (min/wk) | 1745 ± 242 | 1169 ± 90 * | <0.05 |
| Functional Fitness Tests | |||
| TUGT a (s) | 8.4 ± 1.0 | 6.8 ± 1.2 * | <0.05 |
| 5X ST b (s) | 11.0 ± 2.3 | 11.0 ± 1.5 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amirato, G.R.; Borges, J.O.; Marques, D.L.; Santos, J.M.B.; Santos, C.A.F.; Andrade, M.S.; Furtado, G.E.; Rossi, M.; Luis, L.N.; Zambonatto, R.F.; et al. L-Glutamine Supplementation Enhances Strength and Power of Knee Muscles and Improves Glycemia Control and Plasma Redox Balance in Exercising Elderly Women. Nutrients 2021, 13, 1025. https://doi.org/10.3390/nu13031025
Amirato GR, Borges JO, Marques DL, Santos JMB, Santos CAF, Andrade MS, Furtado GE, Rossi M, Luis LN, Zambonatto RF, et al. L-Glutamine Supplementation Enhances Strength and Power of Knee Muscles and Improves Glycemia Control and Plasma Redox Balance in Exercising Elderly Women. Nutrients. 2021; 13(3):1025. https://doi.org/10.3390/nu13031025
Chicago/Turabian StyleAmirato, Gislene R., Juliana O. Borges, Daniella L. Marques, Juliana M. B. Santos, Carlos A. F. Santos, Marilia S. Andrade, Guilherme E. Furtado, Marcelo Rossi, Lais N. Luis, Raquel F. Zambonatto, and et al. 2021. "L-Glutamine Supplementation Enhances Strength and Power of Knee Muscles and Improves Glycemia Control and Plasma Redox Balance in Exercising Elderly Women" Nutrients 13, no. 3: 1025. https://doi.org/10.3390/nu13031025
APA StyleAmirato, G. R., Borges, J. O., Marques, D. L., Santos, J. M. B., Santos, C. A. F., Andrade, M. S., Furtado, G. E., Rossi, M., Luis, L. N., Zambonatto, R. F., Silva, E. B. d., Poma, S. O., Almeida, M. M. d., Pelaquim, R. L., Santos-Oliveira, L. C. d., Diniz, V. L. S., Passos, M. E. P., Levada-Pires, A. C., Gorjão, R., ... Pithon-Curi, T. C. (2021). L-Glutamine Supplementation Enhances Strength and Power of Knee Muscles and Improves Glycemia Control and Plasma Redox Balance in Exercising Elderly Women. Nutrients, 13(3), 1025. https://doi.org/10.3390/nu13031025







