Effects of Vitamin D Supplementation on CD4+ T Cell Subsets and mTOR Signaling Pathway in High-Fat-Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. T Cell Isolation and Culture
2.3. Serum 25(OH)D Concentration Measurement
2.4. Flow Cytometric Analysis
2.5. Quantification of Cytokine Production
2.6. Western Blot Analysis
2.7. RNA Extraction and Quantitative Real-Time PCR
2.8. Statistical Analysis
3. Results
3.1. Body Weight, Weight Change, Adipose Tissue Weight, and Food Intake
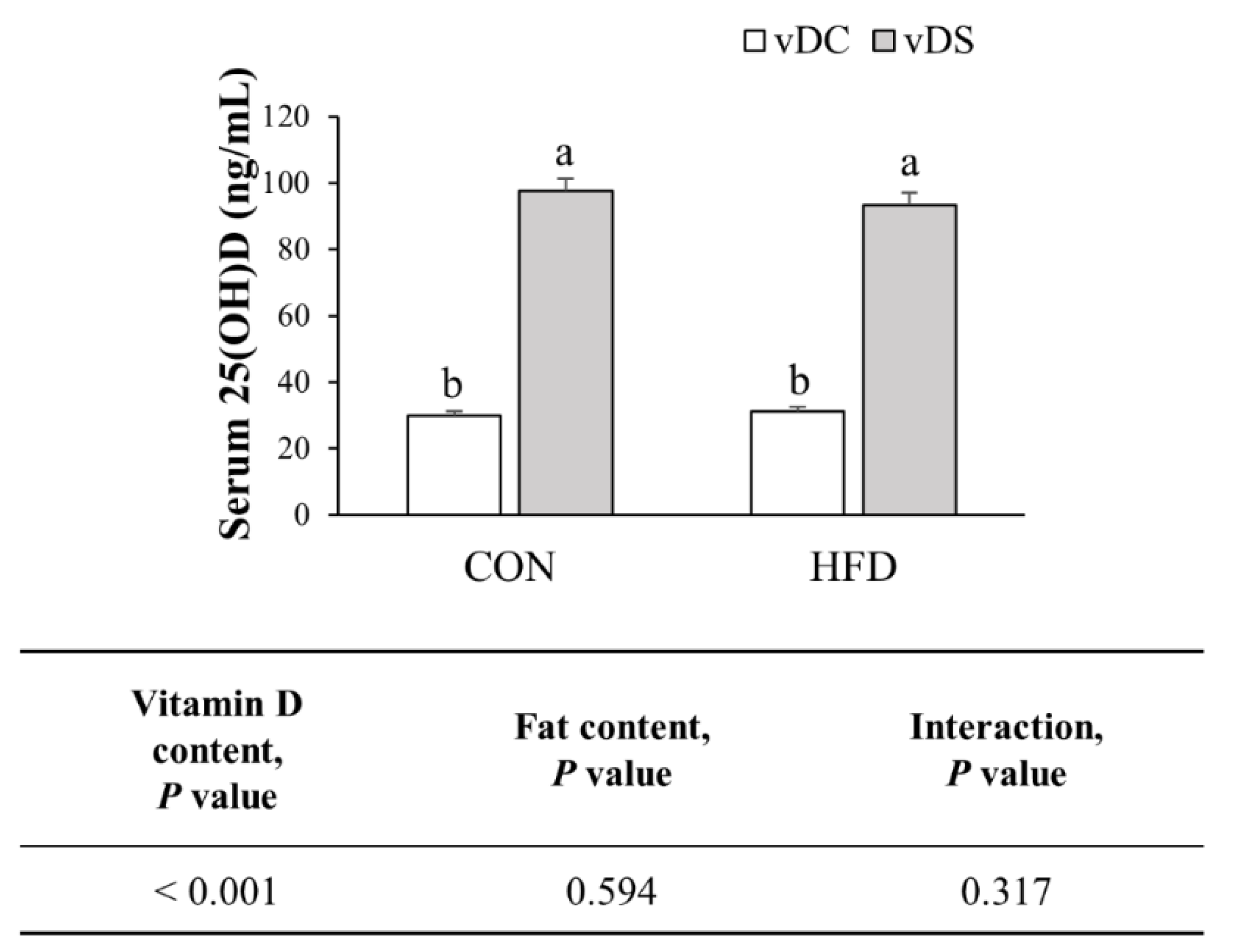
3.2. Serum 25(OH)D Concentration
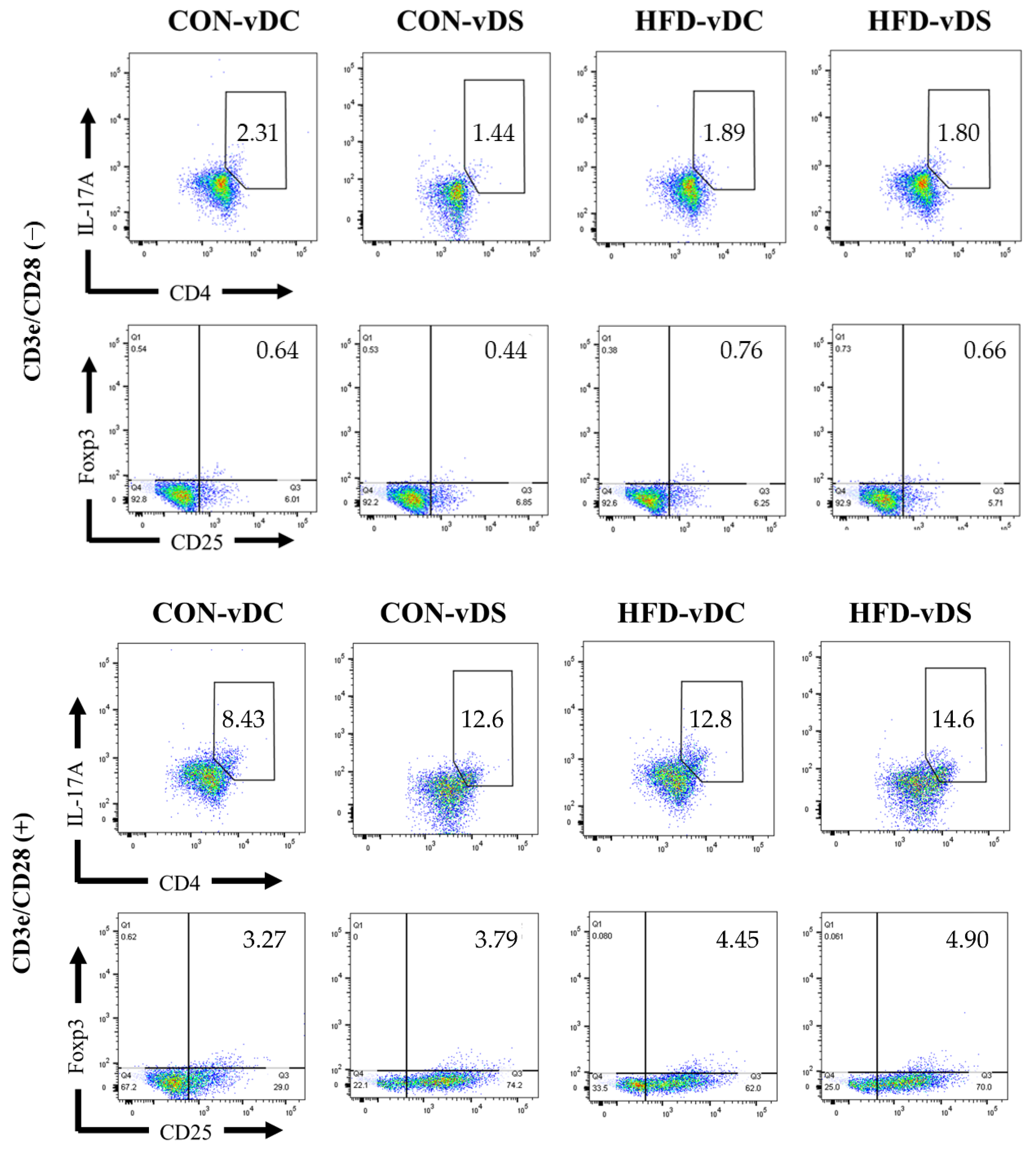
3.3. Population of CD4+IL-17+ T cells and CD4+CD25+Foxp3+ T Cells
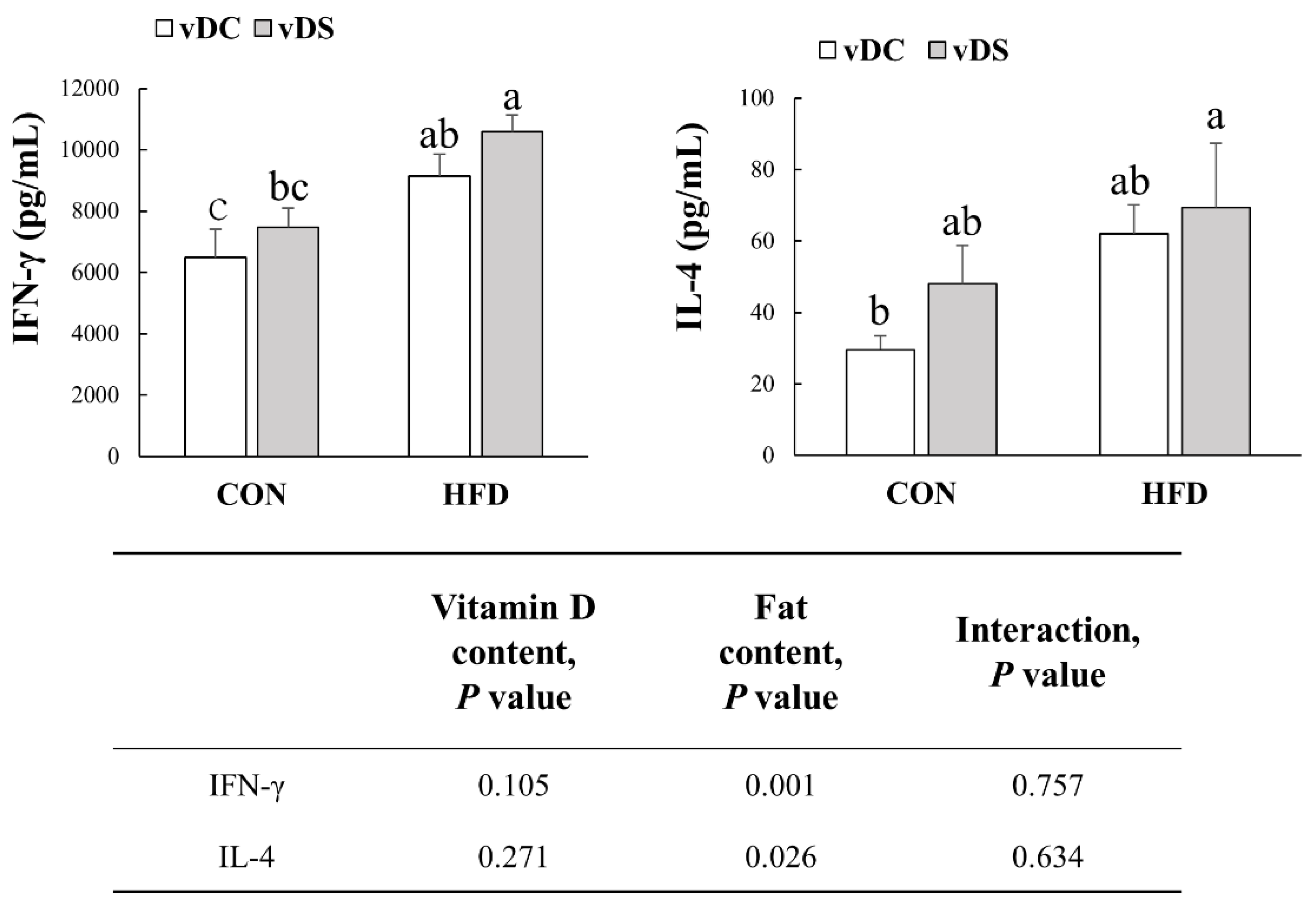
3.4. Production of IFN-γ and IL-4 by T-Cells
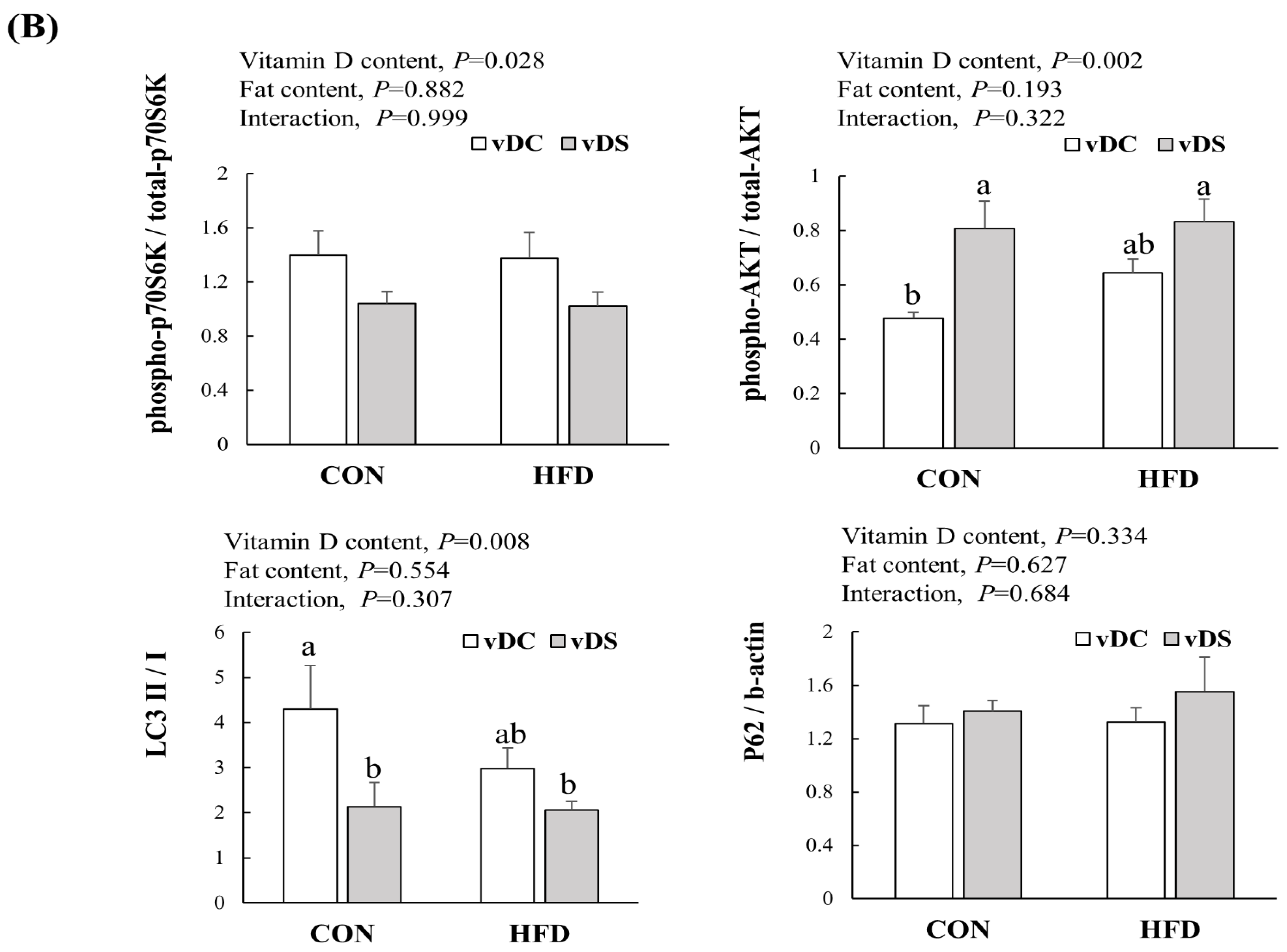
3.5. Expression of Proteins Related to mTOR Pathway in T Cells
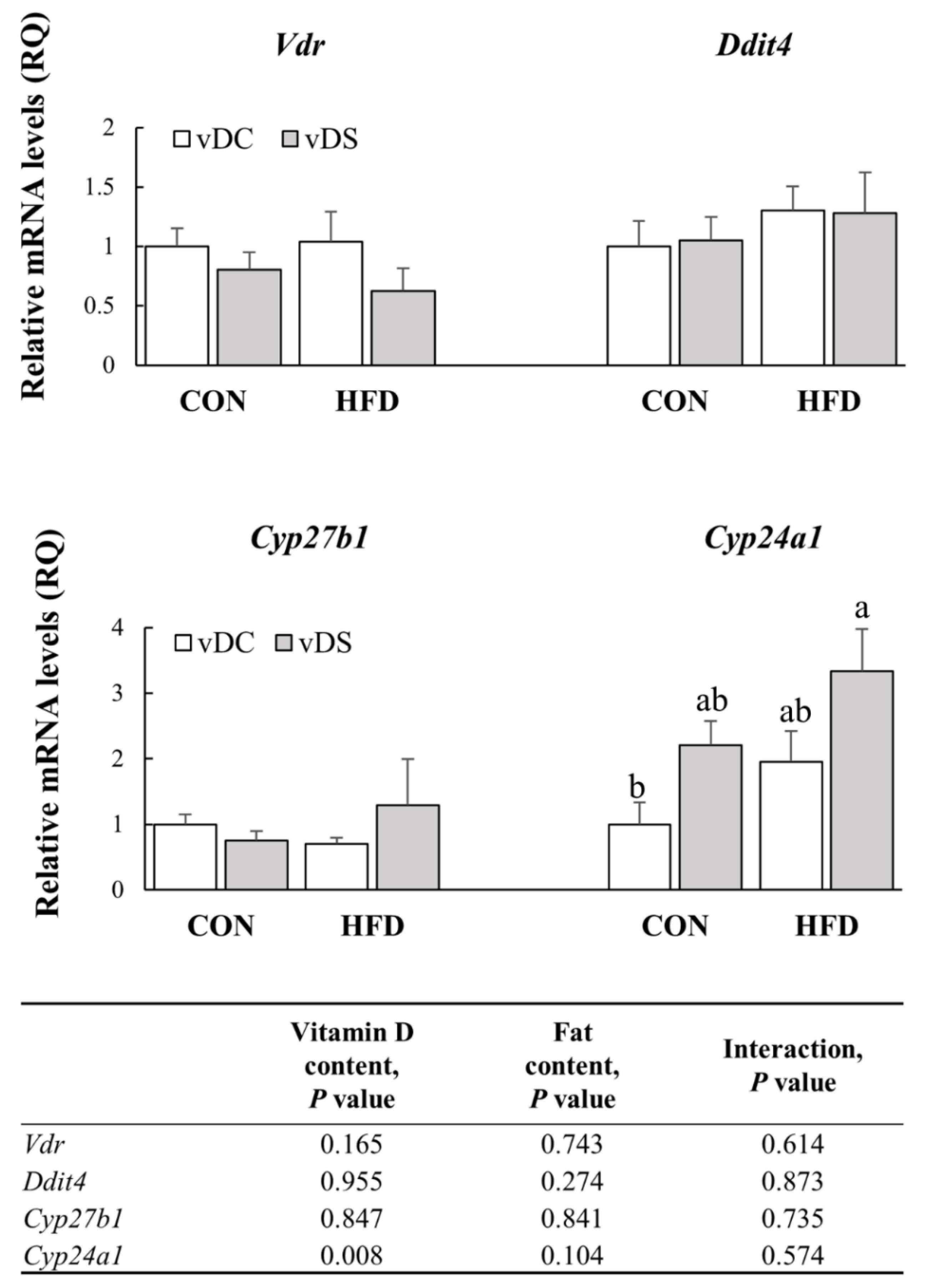
3.6. The mRNA Levels of Genes Involved in Vitamin D Metabolism in T Cells
3.7. The mRNA Levels of Genes Involved in Th1 and Th2 Differentiation
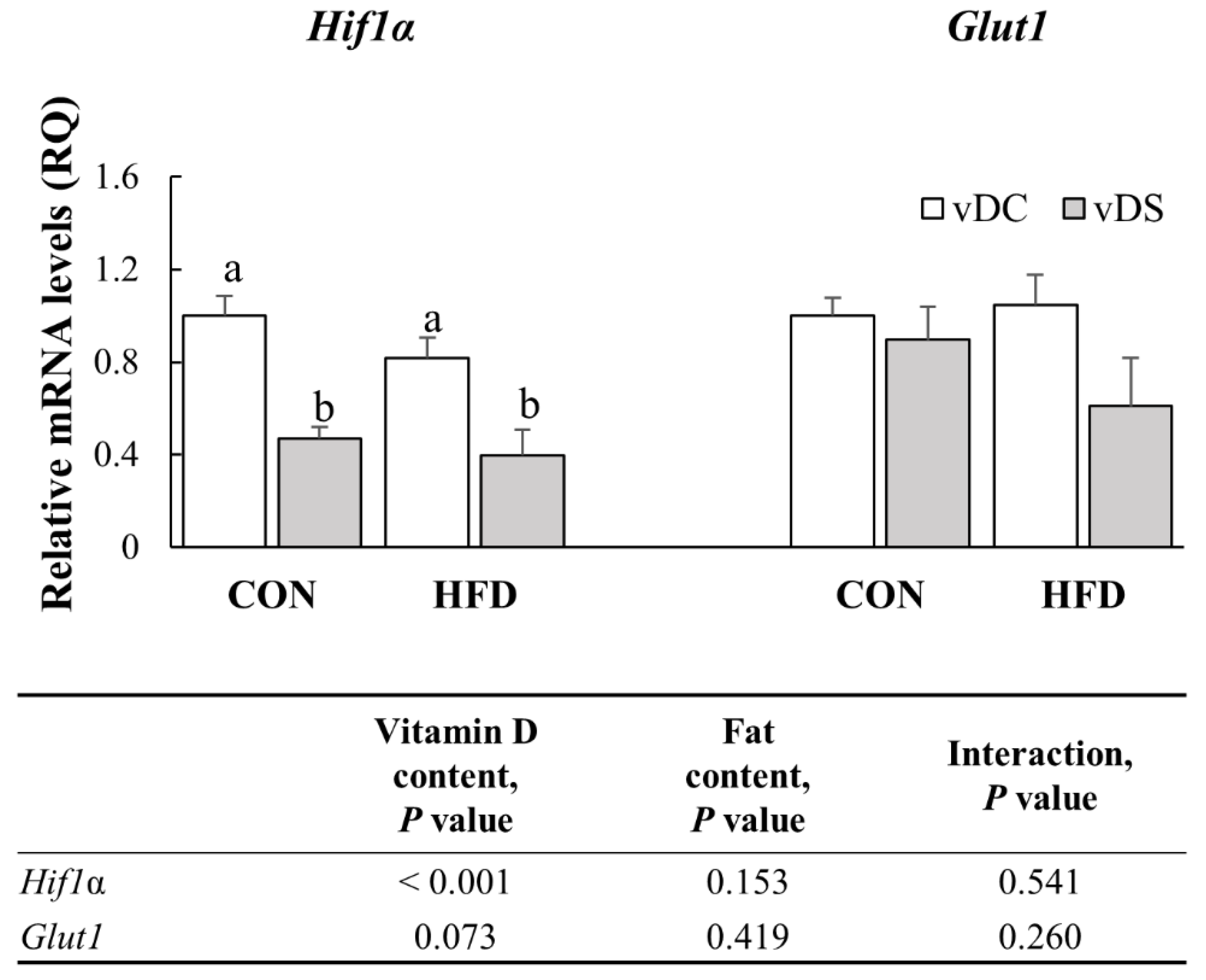
3.8. The mRNA Levels of Genes Involved in Glycolytic Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.; Paul, W.E. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol. Rev. 2010, 238, 247–262. [Google Scholar] [CrossRef]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [Green Version]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 2012, 12, 325–338. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Kole, T.P.; Zheng, Y.; Zarek, P.E.; Matthews, K.L.; Xiao, B.; Worley, P.F.; Kozma, S.C.; Powell, J.D. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009, 30, 832–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurebayashi, Y.; Nagai, S.; Ikejiri, A.; Ohtani, M.; Ichiyama, K.; Baba, Y.; Yamada, T.; Egami, S.; Hoshii, T.; Hirao, A.; et al. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORγ. Cell Rep. 2012, 1, 360–373. [Google Scholar] [CrossRef] [Green Version]
- Delgoffe, G.M.; Pollizzi, K.N.; Waickman, A.T.; Heikamp, E.; Meyers, D.J.; Horton, M.R.; Xiao, B.; Worley, P.F.; Powell, J.D. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 2011, 12, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Gudapati, P.; Dragovic, S.; Spencer, C.; Joyce, S.; Killeen, N.; Magnuson, M.A.; Boothby, M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 2010, 32, 743–753. [Google Scholar] [CrossRef] [Green Version]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bantug, G.R.; Galluzzi, L.; Kroemer, G.; Hess, C. The spectrum of T cell metabolism in health and disease. Nat. Rev. Immunol. 2018, 18, 19–34. [Google Scholar] [CrossRef]
- Shi, L.Z.; Wang, R.; Huang, G.; Vogel, P.; Neale, G.; Green, D.R.; Chi, H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011, 208, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Macintyre, A.N.; Gerriets, V.A.; Nichols, A.G.; Michalek, R.D.; Rudolph, M.C.; Deoliveira, D.; Anderson, S.M.; Abel, E.D.; Chen, B.J.; Hale, L.P.; et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014, 20, 61–72. [Google Scholar] [CrossRef] [Green Version]
- De Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touch, S.; Clément, K.; André, S. T Cell Populations and Functions Are Altered in Human Obesity and Type 2 Diabetes. Curr. Diabetes Rep. 2017, 17, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Chen, F.; Wang, J.; Zeng, Z.; Yang, Q.; Shao, S. Th17 and Treg lymphocytes in obesity and Type 2 diabetic patients. Clin. Immunol. 2018, 197, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Deiuliis, J.; Shah, Z.; Shah, N.; Needleman, B.; Mikami, D.; Narula, V.; Perry, K.; Hazey, J.; Kampfrath, T.; Kollengode, M.; et al. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS ONE 2011, 6, e16376. [Google Scholar] [CrossRef] [Green Version]
- Zúñiga, L.A.; Shen, W.J.; Joyce-Shaikh, B.; Pyatnova, E.A.; Richards, A.G.; Thom, C.; Andrade, S.M.; Cua, D.J.; Kraemer, F.B.; Butcher, E.C. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J. Immunol. 2010, 185, 6947–6959. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wu, Y.; Wang, L. Fat-resident Tregs: An emerging guard protecting from obesity-associated metabolic disorders. Obes. Rev. 2013, 14, 568–578. [Google Scholar] [CrossRef]
- Duffaut, C.; Zakaroff-Girard, A.; Bourlier, V.; Decaunes, P.; Maumus, M.; Chiotasso, P.; Sengenès, C.; Lafontan, M.; Galitzky, J.; Bouloumié, A. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1608–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Womack, J.; Tien, P.C.; Feldman, J.; Shin, J.H.; Fennie, K.; Anastos, K.; Cohen, M.H.; Bacon, M.C.; Minkoff, H. Obesity and immune cell counts in women. Metab. Clin. Exp. 2007, 56, 998–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalmas, E.; Venteclef, N.; Caer, C.; Poitou, C.; Cremer, I.; Aron-Wisnewsky, J.; Lacroix-Desmazes, S.; Bayry, J.; Kaveri, S.V.; Clément, K.; et al. T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: Relevance to obesity and type 2 diabetes. Diabetes 2014, 63, 1966–1977. [Google Scholar] [CrossRef] [Green Version]
- Fabbrini, E.; Cella, M.; McCartney, S.A.; Fuchs, A.; Abumrad, N.A.; Pietka, T.A.; Chen, Z.; Finck, B.N.; Han, D.H.; Magkos, F.; et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology 2013, 145, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Tang, D.; Yi, S.; Li, W.; Wu, C.; Lu, Y.; Hou, X.; Song, J.; Lin, P.; Chen, L.; et al. Elevated peripheral frequencies of Th22 cells: A novel potent participant in obesity and type 2 diabetes. PLoS ONE 2014, 9, e85770. [Google Scholar] [CrossRef]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.M.; Brandhorst, G.; Czepluch, F.; Lankeit, M.; Eberle, C.; Herzberg, S.; Faustin, V.; Riggert, J.; Oellerich, M.; Hasenfuss, G.; et al. Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity 2013, 21, 461–468. [Google Scholar] [CrossRef]
- Travers, R.L.; Motta, A.C.; Betts, J.A.; Bouloumié, A.; Thompson, D. The impact of adiposity on adipose tissue-resident lymphocyte activation in humans. Int. J. Obes. 2015, 39, 762–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Procaccini, C.; De Rosa, V.; Galgani, M.; Carbone, F.; Cassano, S.; Greco, D.; Qian, K.; Auvinen, P.; Calì, G.; Stallone, G.; et al. Leptin-induced mTOR activation defines a specific molecular and transcriptional signature controlling CD4+ effector T cell responses. J. Immunol. 2012, 189, 2941–2953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahon, B.D.; Wittke, A.; Weaver, V.; Cantorna, M.T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell. Biochem. 2003, 89, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.T.; Lee, Y.K.; Maynard, C.L.; Oliver, J.R.; Bikle, D.D.; Jetten, A.M.; Weaver, C.T. Lineage-specific effects of 1,25-dihydroxyvitamin D(3) on the development of effector CD4 T cells. J. Biol. Chem. 2011, 286, 997–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korf, H.; Wenes, M.; Stijlemans, B.; Takiishi, T.; Robert, S.; Miani, M.; Eizirik, D.L.; Gysemans, C.; Mathieu, C. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology 2012, 217, 1292–1300. [Google Scholar] [CrossRef]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.; O’Garra, A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichler, J.; Gerstmayr, M.; Szépfalusi, Z.; Urbanek, R.; Peterlik, M.; Willheim, M. 1 alpha,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatric Res. 2002, 52, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Lisse, T.S.; Liu, T.; Irmler, M.; Beckers, J.; Chen, H.; Adams, J.S.; Hewison, M. Gene targeting by the vitamin D response element binding protein reveals a role for vitamin D in osteoblast mTOR signaling. FASEB J. 2011, 25, 937–947. [Google Scholar] [CrossRef] [Green Version]
- O’Kelly, J.; Uskokovic, M.; Lemp, N.; Vadgama, J.; Koeffler, H.P. Novel Gemini-vitamin D3 analog inhibits tumor cell growth and modulates the Akt/mTOR signaling pathway. J. Steroid Biochem. Mol. Biol. 2006, 100, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Earthman, C.P.; Beckman, L.M.; Masodkar, K.; Sibley, S.D. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: Considerations and implications. Int. J. Obes. 2012, 36, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Vilarrasa, N.; Maravall, J.; Estepa, A.; Sánchez, R.; Masdevall, C.; Navarro, M.A.; Alía, P.; Soler, J.; Gómez, J.M. Low 25-hydroxyvitamin D concentrations in obese women: Their clinical significance and relationship with anthropometric and body composition variables. J. Endocrinol. Investig. 2007, 30, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santos, M.; Costa, P.R.; Assis, A.M.; Santos, C.A.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Kull, M.; Kallikorm, R.; Lember, M. Body mass index determines sunbathing habits: Implications on vitamin D levels. Intern. Med. J. 2009, 39, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Daly, R.M.; Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Sikaris, K.A.; Zimmet, P.Z.; Ebeling, P.R.; Shaw, J.E. Prevalence of vitamin D deficiency and its determinants in Australian adults aged 25 years and older: A national, population-based study. Clin. Endocrinol. 2012, 77, 26–35. [Google Scholar] [CrossRef]
- Olson, M.L.; Maalouf, N.M.; Oden, J.D.; White, P.C.; Hutchison, M.R. Vitamin D deficiency in obese children and its relationship to glucose homeostasis. J. Clin. Endocrinol. Metab. 2012, 97, 279–285. [Google Scholar] [CrossRef]
- Park, C.Y.; Shin, Y.; Kim, J.H.; Zhu, S.; Jung, Y.S.; Han, S.N. Effects of high fat diet-induced obesity on vitamin D metabolism and tissue distribution in vitamin D deficient or supplemented mice. Nutr. Metab. 2020, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Foltyn, M.; Luger, A.L.; Lorenz, N.I.; Sauer, B.; Mittelbronn, M.; Harter, P.N.; Steinbach, J.P.; Ronellenfitsch, M.W. The physiological mTOR complex 1 inhibitor DDIT4 mediates therapy resistance in glioblastoma. Br. J. Cancer 2019, 120, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Zeitelhofer, M.; Adzemovic, M.Z.; Gomez-Cabrero, D.; Bergman, P.; Hochmeister, S.; N’Diaye, M.; Paulson, A.; Ruhrmann, S.; Almgren, M.; Tegnér, J.N.; et al. Functional genomics analysis of vitamin D effects on CD4+ T cells in vivo in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2017, 114, E1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarbassov, D.D.; Ali, S.M.; Sengupta, S.; Sheen, J.H.; Hsu, P.P.; Bagley, A.F.; Markhard, A.L.; Sabatini, D.M. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 2006, 22, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Lamming, D.W.; Ye, L.; Katajisto, P.; Goncalves, M.D.; Saitoh, M.; Stevens, D.M.; Davis, J.G.; Salmon, A.B.; Richardson, A.; Ahima, R.S.; et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 2012, 335, 1638–1643. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, J.T.; Rodgers, J.T.; Arlow, D.H.; Vazquez, F.; Mootha, V.K.; Puigserver, P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 2007, 450, 736–740. [Google Scholar] [CrossRef]
- Fraenkel, M.; Ketzinel-Gilad, M.; Ariav, Y.; Pappo, O.; Karaca, M.; Castel, J.; Berthault, M.F.; Magnan, C.; Cerasi, E.; Kaiser, N.; et al. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes 2008, 57, 945–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houde, V.P.; Brûlé, S.; Festuccia, W.T.; Blanchard, P.G.; Bellmann, K.; Deshaies, Y.; Marette, A. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes 2010, 59, 1338–1348. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, K.H.; Arriola Apelo, S.I.; Yu, D.; Brinkman, J.A.; Velarde, M.C.; Syed, F.A.; Liao, C.Y.; Baar, E.L.; Carbajal, K.A.; Sherman, D.S.; et al. A novel rapamycin analog is highly selective for mTORC1 in vivo. Nat. Commun. 2019, 10, 3194. [Google Scholar] [CrossRef]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef] [Green Version]
- Surendar, J.; Frohberger, S.J.; Karunakaran, I.; Schmitt, V.; Stamminger, W.; Neumann, A.L.; Wilhelm, C.; Hoerauf, A.; Hübner, M.P. Adiponectin Limits IFN-γ and IL-17 Producing CD4 T Cells in Obesity by Restraining Cell Intrinsic Glycolysis. Front. Immunol. 2019, 10, 2555. [Google Scholar] [CrossRef] [PubMed]
- Winer, S.; Paltser, G.; Chan, Y.; Tsui, H.; Engleman, E.; Winer, D.; Dosch, H.M. Obesity predisposes to Th17 bias. Eur. J. Immunol. 2009, 39, 2629–2635. [Google Scholar] [CrossRef]
- Luck, H.; Tsai, S.; Chung, J.; Clemente-Casares, X.; Ghazarian, M.; Revelo, X.S.; Lei, H.; Luk, C.T.; Shi, S.Y.; Surendra, A.; et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015, 21, 527–542. [Google Scholar] [CrossRef] [Green Version]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.K.; Lee, S.H.; Jhun, J.Y.; Byun, J.K.; Jeong, J.H.; Lee, S.Y.; Kim, J.K.; Choi, J.Y.; Cho, M.L. Metformin Prevents Fatty Liver and Improves Balance of White/Brown Adipose in an Obesity Mouse Model by Inducing FGF21. Mediat. Inflamm. 2016, 2016, 5813030. [Google Scholar] [CrossRef]
- Kang, K.Y.; Kim, Y.K.; Yi, H.; Kim, J.; Jung, H.R.; Kim, I.J.; Cho, J.H.; Park, S.H.; Kim, H.Y.; Ju, J.H. Metformin downregulates Th17 cells differentiation and attenuates murine autoimmune arthritis. Int. Immunopharmacol. 2013, 16, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou-Athanassiou, I.; Athanassiou, P.; Gkountouvas, A.; Kaldrymides, P. Vitamin D and glycemic control in diabetes mellitus type 2. Ther. Adv. Endocrinol. Metab. 2013, 4, 122–128. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Luan, J.; Cooper, A.; Boucher, B.J.; Wareham, N.J. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: The Medical Research Council Ely Prospective Study 1990–2000. Diabetes 2008, 57, 2619–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.; Pantalena, L.C.; Liu, X.K.; Gaffen, S.L.; Liu, H.; Rohowsky-Kochan, C.; Ichiyama, K.; Yoshimura, A.; Steinman, L.; Christakos, S.; et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol. Cell. Biol. 2011, 31, 3653–3669. [Google Scholar] [CrossRef] [Green Version]
- Unger, W.W.; Laban, S.; Kleijwegt, F.S.; van der Slik, A.R.; Roep, B.O. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: Differential role for PD-L1. Eur. J. Immunol. 2009, 39, 3147–3159. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleet, J.C.; Gliniak, C.; Zhang, Z.; Xue, Y.; Smith, K.B.; McCreedy, R.; Adedokun, S.A. Serum Metabolite Profiles and Target Tissue Gene Expression Define the Effect of Cholecalciferol Intake on Calcium Metabolism in Rats and Mice. J. Nutr. 2008, 138, 1114–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Shrestha, S.; Zeng, H.; Karmaus, P.W.; Neale, G.; Vogel, P.; Guertin, D.A.; Lamb, R.F.; Chi, H. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity 2013, 39, 1043–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollizzi, K.N.; Patel, C.H.; Sun, I.H.; Oh, M.H.; Waickman, A.T.; Wen, J.; Delgoffe, G.M.; Powell, J.D. mTORC1 and mTORC2 selectively regulate CD8⁺ T cell differentiation. J. Clin. Investig. 2015, 125, 2090–2108. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, L.A.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef] [Green Version]
- Lips, P. Relative value of 25(OH)D and 1,25(OH)2D measurements. J. Bone Miner. Res. 2007, 22, 1668–1671. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [Green Version]
- Van Etten, E.; Stoffels, K.; Gysemans, C.; Mathieu, C.; Overbergh, L. Regulation of vitamin D homeostasis: Implications for the immune system. Nutr. Rev. 2008, 66, S125–S134. [Google Scholar] [CrossRef]
- Kongsbak, M.; von Essen, M.R.; Boding, L.; Levring, T.B.; Schjerling, P.; Lauritsen, J.P.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Geisler, C. Vitamin D up-regulates the vitamin D receptor by protecting it from proteasomal degradation in human CD4+ T cells. PLoS ONE 2014, 9, e96695. [Google Scholar] [CrossRef] [Green Version]


| CON (10% kcal fat) | HFD (45% kcal Fat) | |||
|---|---|---|---|---|
| vDC | vDS | vDC | vDS | |
| (948 IU/kg Diet) | (9477 IU/kg Diet) | (1165 IU/kg Diet) | (11652 IU/kg Diet) | |
| Casein, 30 Mesh (g) | 200 | 200 | 200 | 200 |
| L-Cystine (g) | 3 | 3 | 3 | 3 |
| Corn Starch (g) | 452.2 | 452.2 | 72.8 | 72.8 |
| Maltodextrin 10 (g) | 75 | 75 | 100 | 100 |
| Sucrose (g) | 172.8 | 172.8 | 172.8 | 172.8 |
| Cellulose, BW200 (g) | 50 | 50 | 50 | 50 |
| Soybean Oil (g) | 25 | 25 | 25 | 25 |
| Lard (g) | 20 | 20 | 177.5 | 177.5 |
| Mineral Mix (g) 1 | 10 | 10 | 10 | 10 |
| DiCalcium Phosphate (g) | 13 | 13 | 13 | 13 |
| Calcium Carbonate (g) | 5.5 | 5.5 | 5.5 | 5.5 |
| Potassium Citrate (g) | 16.5 | 16.5 | 16.5 | 16.5 |
| Vitamin Mix (g) 2 | 10 | 10 | 10 | 10 |
| Vitamin D3 (g), | 0 | 0.09 | 0 | 0.09 |
| (100,000 IU/g) | ||||
| Choline Bitartrate (g) | 2 | 2 | 2 | 2 |
| FD&C Yellow Dye No. 5 (g) | 0.04 | 0 | 0 | 0.025 |
| FD&C Red Dye No. 40 (g) | 0.01 | 0 | 0.05 | 0 |
| FD&C Blue Dye No. 1 (g) | 0 | 0.5 | 0 | 0.025 |
| Total (g) | 1055.05 | 1055.14 | 858.15 | 858.24 |
| kcal/g diet | 3.85 | 3.84 | 4.73 | 4.73 |
| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| Vdr | 5’-GGGATGATGGGTAGGTTGTG-3’ | 5’-GGAAGAGGGTAGAGGGCAGA-3’ |
| Ddit4 | 5’-TGTAACCAGGGACCAAGGAA-3’ | 5’-GTGTGTGGAGCAAGGCAAG-3’ |
| Cyp27b1 | 5’-GACGATGTTGGCTGTCTTCC-3’ | 5’-ATCTCTTCCCTTCGGCTTTG-3’ |
| Cyp24a1 | 5’-TCCCTGAGTAATGGGCTTTG-3’ | 5’-CACGGTAGGCTGCTGAGATT-3’ |
| T-bet | 5’-AATCGACAACAACCCCTTTG-3’ | 5’-AACTGTGTTCCCGAGGTGTC-3’ |
| Gata3 | 5’-GAACCGCCCCTTATCAAG-3’ | 5’-CAGGATGTCCCTGCTCTCCTT-3’ |
| Hif1α | 5’-CTTGAAAAAGGGAGCCATCA-3’ | 5’-ACAGCCTCACCAGACAGAGC-3’ |
| Glut1 | 5’-CTGGACCTCAAACTTCATTGTGGG-3’ | 5’-GGGTGTCTTGTCACTTTGGCTGG-3’ |
| Gapdh | 5’-GGAGAAACCTGCCAAGTA-3’ | 5’-AAGAGTGGGAGTTGCTGTTG-3’ |
| CON | HFD | Vitamin D Content, p-Value | Fat Content, p-Value | Interaction, p-Value | |||
|---|---|---|---|---|---|---|---|
| vDC | vDS | vDC | vDS | ||||
| Body weight at week 0 (g) | 23.4 ± 0.4 | 23.7 ± 0.3 | 23.7 ± 0.3 | 23.9 ± 0.2 | 0.342 | 0.481 | 0.910 |
| Body weight at week 12 (g) | 31.5 ± 0.6 b | 31.4 ± 0.7 b | 41.3 ± 0.8 a | 42.5 ± 0.8 a | 0.428 | <0.001 | 0.344 |
| Weight gain (g) | 8.1 ± 0.6 b | 7.7 ± 0.8 b | 17.6 ± 0.8 a | 18.7 ± 0.8 a | 0.616 | <0.001 | 0.340 |
| WAT weight (g) | 3.5 ± 0.4 b | 3.1 ± 0.3 b | 7.4 ± 0.3 a | 7.5 ± 0.3 a | 0.567 | <0.001 | 0.433 |
| Average food intake (g/day) | 2.6 ± 0.03 a,b | 2.6 ± 0.03 a | 2.5 ± 0.03 b | 2.6 ± 0.03 a | 0.001 | 0.078 | 0.491 |
| Average energy Intake (kcal/day) | 9.8 ± 0.1 c | 10.1 ± 0.1 c | 11.7 ± 0.1 b | 12.3 ± 0.1 a | 0.001 | <0.001 | 0.275 |
| Average vitamin D intake (IU/day) | 2.4 ± 0.03 d | 24.9 ± 0.3 b | 2.9 ± 0.03 c | 30.3 ± 0.3 a | <0.001 | <0.001 | <0.001 |
| CON | HFD | Vitamin D Content, p-Value | Fat Content, p-Value | Interaction, p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| vDC | vDS | vDC | vDS | |||||
| CD3e CD28 (−) | CD4+IL-17+ T cells (%) | 2.16 ± 0.37 | 1.89 ± 0.18 | 1.69 ± 0.36 | 2.00 ± 0.29 | 0.952 | 0.575 | 0.370 |
| CD4+CD25+Foxp3+ T cells (%) | 0.66 ± 0.05 a,b | 0.74 ± 0.11 a | 0.44 ± 0.07 b | 0.71 ± 0.08 a | 0.037 | 0.118 | 0.226 | |
| CD4+IL-17+ T cells / CD4+CD25+Foxp3+ T cells (ratio) | 3.25 ± 0.50 | 2.96 ± 0.64 | 4.65 ± 1.43 | 3.29 ± 0.74 | 0.380 | 0.360 | 0.568 | |
| CD3e CD28 (+) | CD4+IL-17+ T cells (%) | 9.83 ± 0.97 b | 13.44 ± 1.68 a | 12.80 ± 0.71 a,b | 14.53 ± 1.09 a | 0.026 | 0.084 | 0.412 |
| CD4+CD25+Foxp3+ T cells (%) | 3.65 ± 0.46 b | 4.30 ± 0.43 a,b | 4.68 ± 0.30 a,b | 5.06 ± 0.35 a | 0.164 | 0.022 | 0.550 | |
| CD4+IL-17+ T cells / CD4+CD25+Foxp3+ T cells (ratio) | 2.78 ± 0.15 | 3.28 ± 0.51 | 2.87 ± 0.35 | 2.96 ± 0.30 | 0.383 | 0.739 | 0.555 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, J.H.; Cho, D.H.; Lee, G.Y.; Kang, M.S.; Kim, S.J.; Han, S.N. Effects of Vitamin D Supplementation on CD4+ T Cell Subsets and mTOR Signaling Pathway in High-Fat-Diet-Induced Obese Mice. Nutrients 2021, 13, 796. https://doi.org/10.3390/nu13030796
An JH, Cho DH, Lee GY, Kang MS, Kim SJ, Han SN. Effects of Vitamin D Supplementation on CD4+ T Cell Subsets and mTOR Signaling Pathway in High-Fat-Diet-Induced Obese Mice. Nutrients. 2021; 13(3):796. https://doi.org/10.3390/nu13030796
Chicago/Turabian StyleAn, Jeong Hee, Da Hye Cho, Ga Young Lee, Min Su Kang, So Jeong Kim, and Sung Nim Han. 2021. "Effects of Vitamin D Supplementation on CD4+ T Cell Subsets and mTOR Signaling Pathway in High-Fat-Diet-Induced Obese Mice" Nutrients 13, no. 3: 796. https://doi.org/10.3390/nu13030796
APA StyleAn, J. H., Cho, D. H., Lee, G. Y., Kang, M. S., Kim, S. J., & Han, S. N. (2021). Effects of Vitamin D Supplementation on CD4+ T Cell Subsets and mTOR Signaling Pathway in High-Fat-Diet-Induced Obese Mice. Nutrients, 13(3), 796. https://doi.org/10.3390/nu13030796






