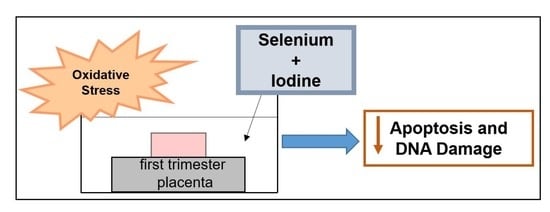
Effect of Selenium and Iodine on Oxidative Stress in the First Trimester Human Placenta Explants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Placenta Explant Tissue Culture
2.2. Micronutrient Uptake
2.3. Assessment of Proliferation, Apoptosis and DNA Damage
2.4. Statistical Analyses
3. Results
3.1. Confirmation of Micronutrient Uptake within the Treated First Trimester Placenta Explants
3.2. Apoptosis and DNA Damage Induction by Menadione and Antimycin
3.3. Effect of Selenium, Iodine and Copper Supplementation on Proliferation
3.4. Effect of Selenium, Iodine, and Copper Supplementation on Apoptosis
3.5. Effect of Selenium, Iodine and Copper Supplementation on DNA Damage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grieger, J.A.; Clifton, V.L. A review of the impact of dietary intakes in human pregnancy on infant birthweight. Nutrients 2014, 7, 153–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahir, M.J.; Haapala, J.L.; Foster, L.P.; Duncan, K.M.; Teague, A.M.; Kharbanda, E.O.; McGovern, P.M.; Whitaker, K.M.; Rasmussen, K.M.; Fields, D.A.; et al. Higher maternal diet quality during pregnancy and lactation is associated with lower infant weight-for-length, body fat percent, and fat mass in early postnatal life. Nutrients 2019, 11, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Grzeskowiak, L.E.; Dekker, G.A.; Roberts, C.T. Early pregnancy maternal trace mineral status and the association with adverse pregnancy outcome in a cohort of Australian women. J. Trace Elem. Med. Biol. 2018, 46, 103–109. [Google Scholar] [CrossRef]
- Wilson, R.L.; Leviton, A.J.; Leemaqz, S.Y.; Anderson, P.H.; Grieger, J.A.; Grzeskowiak, L.E.; Verburg, P.E.; McCowan, L.; Dekker, G.A.; Bianco-Miotto, T.; et al. Vitamin D levels in an Australian and New Zealand cohort and the association with pregnancy outcome. BMC Pregnancy Childbirth 2018, 18, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistry, H.D.; Wilson, V.; Ramsay, M.M.; Symonds, M.E.; Broughton Pipkin, F. Reduced selenium concentrations and glutathione peroxidase activity in preeclamptic pregnancies. Hypertension 2008, 52, 881–888. [Google Scholar] [CrossRef] [Green Version]
- Molnar, J.; Garamvolgyi, Z.; Herold, M.; Adanyi, N.; Somogyi, A.; Rigo, J., Jr. Serum selenium concentrations correlate significantly with inflammatory biomarker high-sensitive crp levels in hungarian gestational diabetic and healthy pregnant women at mid-pregnancy. Biol. Trace Elem. Res. 2008, 121, 16–22. [Google Scholar] [CrossRef]
- Charoenratana, C.; Leelapat, P.; Traisrisilp, K.; Tongsong, T. Maternal iodine insufficiency and adverse pregnancy outcomes. Matern. Child Nutr. 2016, 12, 680–687. [Google Scholar] [CrossRef]
- Neiger, R. Long-term effects of pregnancy complications on maternal health: A review. J. Clin. Med. 2017, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Pisaneschi, S.; Boldrini, A.; Genazzani, A.R.; Coceani, F.; Simoncini, T. Feto-placental vascular dysfunction as a prenatal determinant of adult cardiovascular disease. Intern. Emerg. Med. 2013, 8 (Suppl. 1), S41–S45. [Google Scholar] [CrossRef]
- Leeson, P. Long term cardiovascular outcomes for mother and child. Pregnancy Hypertens. 2013, 3, 60–61. [Google Scholar] [CrossRef]
- Falco, M.L.; Sivanathan, J.; Laoreti, A.; Thilaganathan, B.; Khalil, A. Placental histopathology associated with pre-eclampsia: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2017, 50, 295–301. [Google Scholar] [CrossRef]
- Roberts, J.M.; Escudero, C. The placenta in preeclampsia. Pregnancy Hypertens. 2012, 2, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Wiktor, H.; Kankofer, M.; Schmerold, I.; Dadak, A.; Lopucki, M.; Niedermüller, H. Oxidative DNA damage in placentas from normal and pre-eclamptic pregnancies. Virchows Arch. 2004, 445, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, A.; Watanabe, K.; Mori, T.; Kimura, C.; Shinohara, K.; Wakatsuki, A. Placental oxidative DNA damage and its repair in preeclamptic women with fetal growth restriction. Placenta 2011, 32, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, R.E.; Kaufmann, W.K.; Paules, R.S. Oxidative stress and cell cycle checkpoint function. Free Radic. Biol. Med. 2000, 28, 1387–1404. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, N.; Matsuo, H.; Murakoshi, H.; Laoag-Fernandez, J.B.; Samoto, T.; Maruo, T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am. J. Obstet. Gynecol. 2002, 186, 158–166. [Google Scholar] [CrossRef]
- Mondola, P.; Damiano, S.; Sasso, A.; Santillo, M. The Cu, Zn superoxide dismutase: Not only a dismutase enzyme. Front. Physiol. 2016, 7, 594. [Google Scholar] [CrossRef] [Green Version]
- Friedmann Angeli, J.P.; Conrad, M. Selenium and GPX4, a vital symbiosis. Free Radic. Biol. Med. 2018, 127, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Rayman, M.P.; Bath, S.C.; Westaway, J.; Williams, P.; Mao, J.; Vanderlelie, J.J.; Perkins, A.V.; Redman, C.W. Selenium status in u.K. Pregnant women and its relationship with hypertensive conditions of pregnancy. Br. J. Nutr. 2015, 113, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Tara, F.; Rayman, M.P.; Boskabadi, H.; Ghayour-Mobarhan, M.; Sahebkar, A.; Yazarlu, O.; Ouladan, S.; Tavallaie, S.; Azimi-Nezhad, M.; Shakeri, M.T.; et al. Selenium supplementation and premature (pre-labour) rupture of membranes: A randomised double-blind placebo-controlled trial. J. Obstet. Gynaecol. 2010, 30, 30–34. [Google Scholar] [CrossRef]
- Rayman, M.P.; Searle, E.; Kelly, L.; Johnsen, S.; Bodman-Smith, K.; Bath, S.C.; Mao, J.; Redman, C.W. Effect of selenium on markers of risk of pre-eclampsia in UK pregnant women: A randomised, controlled pilot trial. Br. J. Nutr. 2014, 112, 99–111. [Google Scholar] [CrossRef]
- Borekci, B.; Gulaboglu, M.; Gul, M. Iodine and magnesium levels in maternal and umbilical cord blood of preeclamptic and normal pregnant women. Biol. Trace Elem. Res. 2009, 129, 1–8. [Google Scholar] [CrossRef]
- Cuellar-Rufino, S.; Navarro-Meza, M.; Garcia-Solis, P.; Xochihua-Rosas, I.; Arroyo-Helguera, O. Iodine levels are associated with oxidative stress and antioxidant status in pregnant women with hypertensive disease. Nutr. Hosp. 2017, 34, 661–666. [Google Scholar] [CrossRef]
- Dillon, J.C.; Milliez, J. Reproductive failure in women living in iodine deficient areas of West Africa. BJOG 2000, 107, 631–636. [Google Scholar] [CrossRef]
- Gulaboglu, M.; Borekci, B.; Halici, Z. Placental tissue iodine level and blood magnesium concentration in pre-eclamptic and normal pregnancy. Int. J. Gynaecol. Obstet. 2007, 98, 100–104. [Google Scholar] [CrossRef]
- Vidal, Z.E.; Rufino, S.C.; Tlaxcalteco, E.H.; Trejo, C.H.; Campos, R.M.; Meza, M.N.; Rodriguez, R.C.; Arroyo-Helguera, O. Oxidative stress increased in pregnant women with iodine deficiency. Biol. Trace Elem. Res. 2014, 157, 211–217. [Google Scholar] [CrossRef]
- Habibi, N.; Jankovic-Karasoulos, T.; Leemaqz, S.Y.-L.; Francois, M.; Zhou, S.J.; Leifert, W.R.; Perkins, A.V.; Roberts, C.T.; Bianco-Miotto, T. Effect of iodine and selenium on proliferation, viability, and oxidative stress in HTR-8/SVneo placental cells. Biol. Trace Elem. Res. 2021, 199, 1332–1344. [Google Scholar] [CrossRef]
- Fontecave, M.; Pierre, J.-L. Oxidations by copper metalloenzymes and some biomimetic approaches. Coord. Chem. Rev. 1998, 170, 125–140. [Google Scholar] [CrossRef]
- Pan, Z.; Cui, T.; Chen, W.; Gao, S.; Pearce, E.N.; Wang, W.; Chen, Y.; Guo, W.; Tan, L.; Shen, J.; et al. Serum iodine concentration in pregnant women and its association with urinary iodine concentration and thyroid function. Clin. Endocrinol. (Oxf.) 2019, 90, 711–718. [Google Scholar] [CrossRef]
- Trigona, W.L.; Mullarky, I.K.; Cao, Y.; Sordillo, L.M. Thioredoxin reductase regulates the induction of haem oxygenase-1 expression in aortic endothelial cells. Biochem. J. 2006, 394, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H. Selenium as an essential micronutrient: Roles in cell cycle and apoptosis. Molecules 2009, 14, 1263–1278. [Google Scholar] [CrossRef] [Green Version]
- Chao, H.X.; Poovey, C.E.; Privette, A.A.; Grant, G.D.; Chao, H.Y.; Cook, J.G.; Purvis, J.E. Orchestration of DNA damage checkpoint dynamics across the human cell cycle. Cell Syst. 2017, 5, 445–459.e5. [Google Scholar] [CrossRef] [Green Version]
- Muri, J.; Heer, S.; Matsushita, M.; Pohlmeier, L.; Tortola, L.; Fuhrer, T.; Conrad, M.; Zamboni, N.; Kisielow, J.; Kopf, M. The thioredoxin-1 system is essential for fueling DNA synthesis during t-cell metabolic reprogramming and proliferation. Nat. Commun. 2018, 9, 1851. [Google Scholar] [CrossRef]
- Matsushita, M.; Freigang, S.; Schneider, C.; Conrad, M.; Bornkamm, G.W.; Kopf, M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 2015, 212, 555–568. [Google Scholar] [CrossRef] [Green Version]
- Selenius, M.; Hedman, M.; Brodin, D.; Gandin, V.; Rigobello, M.P.; Flygare, J.; Marzano, C.; Bindoli, A.; Brodin, O.; Bjornstedt, M.; et al. Effects of redox modulation by inhibition of thioredoxin reductase on radiosensitivity and gene expression. J. Cell. Mol. Med. 2012, 16, 1593–1605. [Google Scholar] [CrossRef]
- Olivo-Vidal, Z.E.; Rodriguez, R.C.; Arroyo-Helguera, O. Iodine affects differentiation and migration process in trophoblastic cells. Biol. Trace Elem. Res. 2016, 169, 180–188. [Google Scholar] [CrossRef]
- Smyth, P.P. Role of iodine in antioxidant defence in thyroid and breast disease. Biofactors 2003, 19, 121–130. [Google Scholar] [CrossRef]
- Winkler, R.; Griebenow, S.; Wonisch, W. Effect of iodide on total antioxidant status of human serum. Cell Biochem. Funct. 2000, 18, 143–146. [Google Scholar] [CrossRef]
- Mugesh, G.; Klotz, L.O.; du Mont, W.W.; Becker, K.; Sies, H. Selenenyl iodide: A new substrate for mammalian thioredoxin reductase. Org. Biomol. Chem. 2003, 1, 2848–2852. [Google Scholar] [CrossRef]
- Cottone, S.; Mule, G.; Nardi, E.; Vadala, A.; Guarneri, M.; Briolotta, C.; Arsena, R.; Palermo, A.; Riccobene, R.; Cerasola, G. Relation of c-reactive protein to oxidative stress and to endothelial activation in essential hypertension. Am. J. Hypertens. 2006, 19, 313–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohsoonthorn, V.; Qiu, C.; Williams, M.A. Maternal serum c-reactive protein concentrations in early pregnancy and subsequent risk of preterm delivery. Clin. Biochem. 2007, 40, 330–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cebesoy, F.B.; Balat, O.; Dikensoy, E.; Kalayci, H.; Ibar, Y. CA-125 and CRP are elevated in preeclampsia. Hypertens. Pregnancy 2009, 28, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.L.; Zhang, J.G.; Wang, X.C.; Chen, J. Excessive copper induces the production of reactive oxygen species, which is mediated by phospholipase d, nicotinamide adenine dinucleotide phosphate oxidase and antioxidant systems. J. Integr. Plant Biol. 2008, 50, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.E.; Soria-Castro, E.; Lans, V.G.; Ontiveros, E.M.; Mejía, B.I.; Hernandez, H.J.; García, R.B.; Herrera, V.; Pérez-Torres, I. Analysis of oxidative stress enzymes and structural and functional proteins on human aortic tissue from different aortopathies. Oxid. Med. Cell. Longev. 2014, 2014, 760694. [Google Scholar] [CrossRef] [Green Version]





| Antibody | Dilution | Target Species | CAT # | Company | Diluent | Antigen Retrieval |
|---|---|---|---|---|---|---|
| Ki67 | 1/100 | Rabbit | ab16667 | Abcam® | 5% Goat serum | Citrate buffer (10 mM Citric acid; pH 6.0; 10 min boiling in microwave, Sixth Sense, Whirlpool, VIC, Australia) |
| cleaved caspase-3 | 1/100 | Rabbit | CST.9661L | Cell Signalling Technology® | 5% Goat serum | Citrate buffer (10 mM Citric acid; pH 6.0; 10 min boiling in microwave) |
| 8-hydroxy-2’-deoxyguanosine | 1/200 | Mouse | ab48508 | Abcam® | 5% Goat serum | Citrate buffer (10 mM Citric acid; pH 6.0; 10 min boiling in microwave) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habibi, N.; Labrinidis, A.; Leemaqz, S.Y.-L.; Jankovic-Karasoulos, T.; McCullough, D.; Grieger, J.A.; Gilbert, S.; Ricciardelli, C.; Zhou, S.J.; Perkins, A.V.; et al. Effect of Selenium and Iodine on Oxidative Stress in the First Trimester Human Placenta Explants. Nutrients 2021, 13, 800. https://doi.org/10.3390/nu13030800
Habibi N, Labrinidis A, Leemaqz SY-L, Jankovic-Karasoulos T, McCullough D, Grieger JA, Gilbert S, Ricciardelli C, Zhou SJ, Perkins AV, et al. Effect of Selenium and Iodine on Oxidative Stress in the First Trimester Human Placenta Explants. Nutrients. 2021; 13(3):800. https://doi.org/10.3390/nu13030800
Chicago/Turabian StyleHabibi, Nahal, Agatha Labrinidis, Shalem Yiner-Lee Leemaqz, Tanja Jankovic-Karasoulos, Dylan McCullough, Jessica A. Grieger, Sarah Gilbert, Carmela Ricciardelli, Shao Jia Zhou, Anthony V. Perkins, and et al. 2021. "Effect of Selenium and Iodine on Oxidative Stress in the First Trimester Human Placenta Explants" Nutrients 13, no. 3: 800. https://doi.org/10.3390/nu13030800
APA StyleHabibi, N., Labrinidis, A., Leemaqz, S. Y.-L., Jankovic-Karasoulos, T., McCullough, D., Grieger, J. A., Gilbert, S., Ricciardelli, C., Zhou, S. J., Perkins, A. V., Roberts, C. T., & Bianco-Miotto, T. (2021). Effect of Selenium and Iodine on Oxidative Stress in the First Trimester Human Placenta Explants. Nutrients, 13(3), 800. https://doi.org/10.3390/nu13030800