Lactoferrin and Immunoglobulin Concentrations in Milk of Gestational Diabetic Mothers
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment of Breastfeeding Mothers
2.2. Milk Collection
2.3. Sample Pre-Treatment for Analysis
2.4. Determination of Lactoferrin Concentration
2.5. Determination of SIgA, IgG, and IgM Concentrations
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
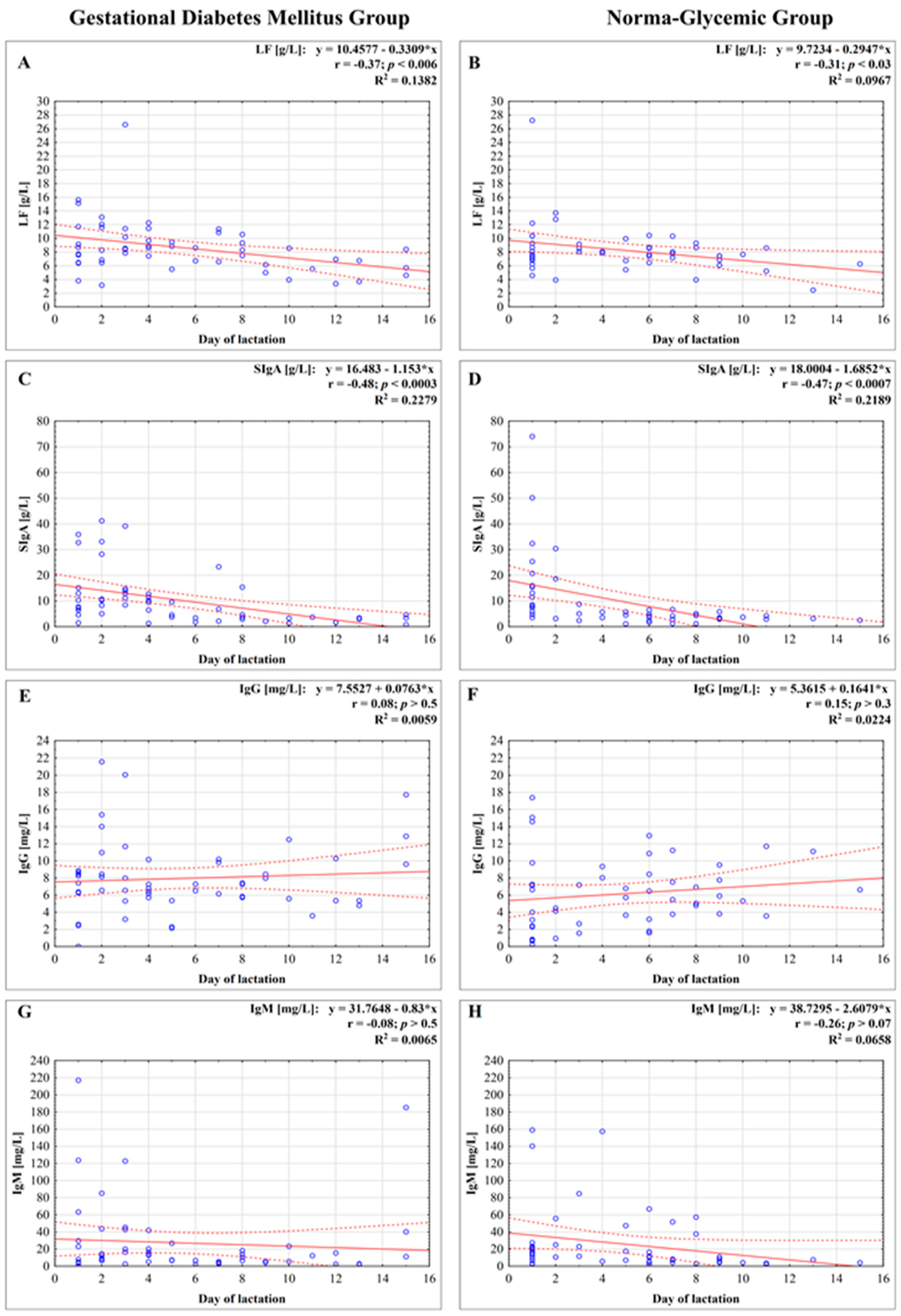
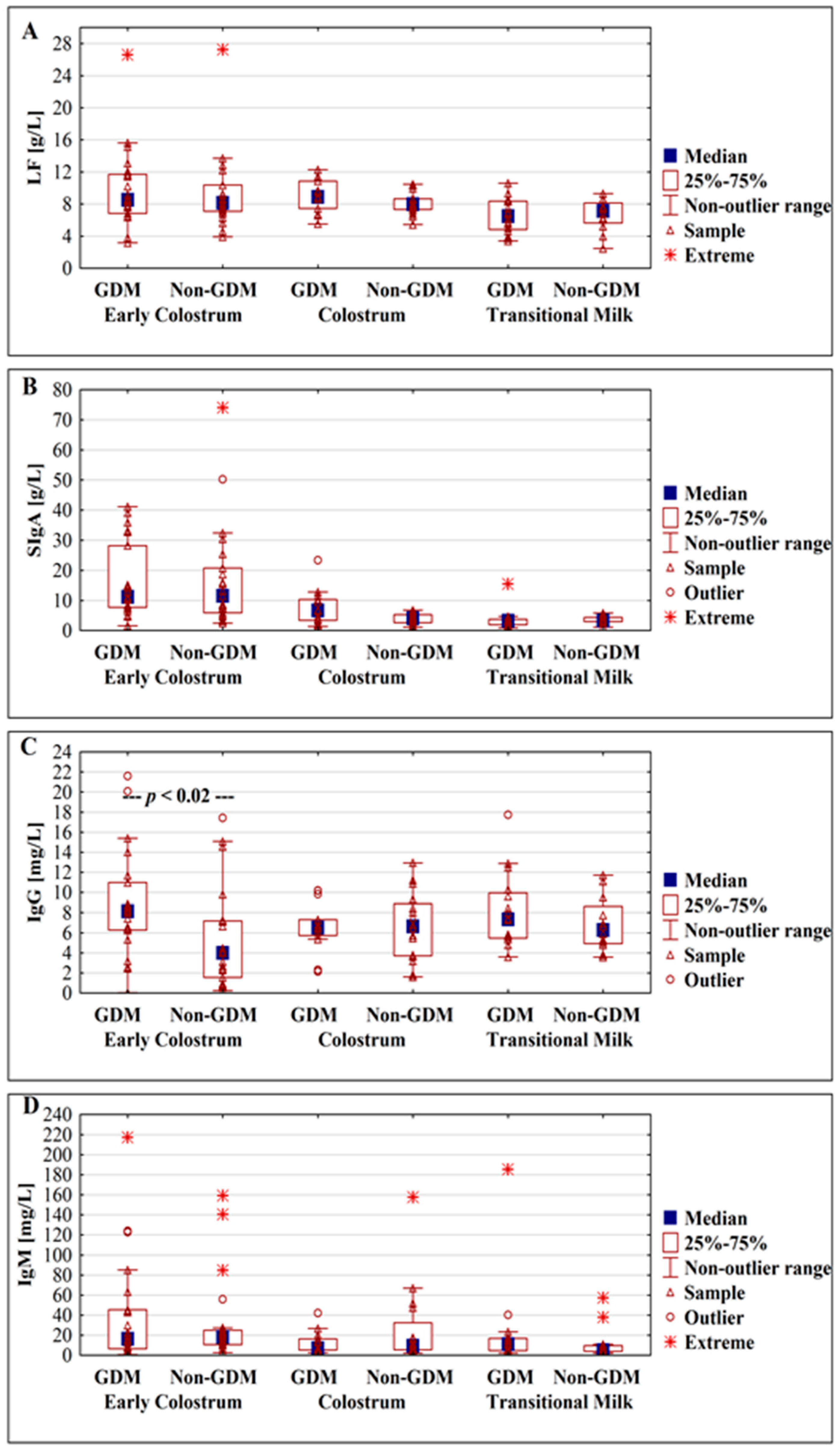
3.2. Concentration of Lactoferrin
3.3. Concentration of Immunoglobulins
3.3.1. Concentration of Secretory Immunoglobulin A
3.3.2. Concentration of IgG
3.3.3. Concentration of IgM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GDM | gestational diabetes mellitus |
| GDM G1 | diet-controlled GDM |
| GDM G2 | diet- and insulin-controlled GDM |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| LF | lactoferrin |
| SIgA | secretory immunoglobulin A |
References
- Eades, C.E.; Cameron, D.M.; Evans, J.M.M. Prevalence of gestational diabetes mellitus in Europe: A meta-analysis. Diabetes Res. Clin. Pract. 2017, 129, 173–181. [Google Scholar] [CrossRef]
- Buckley, B.S.; Harreiter, J.; Damm, P.; Corcoy, R.; Chico, A.; Simmons, D.; Vellinga, A.; Dunne, F.; DALI Core Investigator Group. Gestational diabetes mellitus in Europe: Prevalence, current screening practice and barriers to screening. A review. Diabet. Med. 2012, 29, 844–854. [Google Scholar] [CrossRef]
- Egan, A.M.; Vellinga, A.; Harreiter, J.; Simmons, D.; Desoye, G.; Corcoy, R.; Adelantado, J.M.; Devlieger, R.; Van Assche, A.; Galjaard, S.; et al. Epidemiology of gestational diabetes mellitus according to IADPSG/WHO criteria among obese pregnant women in Europe. Diabetologia 2017, 60, 1913–1921. [Google Scholar] [CrossRef]
- Benhalima, K.; Van Crombrugge, P.; Hanssens, M.; Devlieger, R.; Verhaeghe, J.; Mathieu, C. Gestational diabetes: Overview of the new consensus screening strategy and diagnostic criteria. Acta Clin. Belg. 2012, 67, 255–261. [Google Scholar]
- Buhary, B.M.; Almohareb, O.; Aljohani, N.; Alzahrani, S.H.; Elkaissi, S.; Sherbeeni, S.; Almaghamsi, A.; Almalki, M. Glycemic control and pregnancy outcomes in patients with diabetes in pregnancy: A retrospective study. Indian J. Endocrinol. Metab. 2016, 20, 481–490. [Google Scholar]
- Nilofer, A.R.; Raju, V.S.; Dakshayini, B.R.; Zaki, S.A. Screening in high-risk group of gestational diabetes mellitus with its maternal and fetal outcomes. Indian J. Endocrinol. Metab. 2012, 1, S74–S78. [Google Scholar]
- Kumari, R.; Dalal, V.; Kachhawa, G.; Sahoo, I.; Khadgawat, R.; Mahey, R.; Kulshrestha, V.; Vanamail, P.; Sharma, J.B.; Bhatla, N.; et al. Maternal and perinatal outcome in gestational diabetes mellitus in a tertiary care hospital in Delhi. Indian J. Endocrinol. Metab. 2018, 22, 116–120. [Google Scholar]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type diabetes and its impact on the immune system. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Zhong, J.; Gong, Q.; Mima, A. Inflammatory regulation in diabetes and metabolic dysfunction. J. Diabetes Res. 2017, 2017, 5165268. [Google Scholar] [CrossRef]
- Guo, X.; Meng, G.; Liu, F.; Zhang, Q.; Liu, L.; Wu, H.; Du, H.; Shi, H.; Xia, Y.; Liu, X.; et al. Serum levels of immunoglobulins in an adult population and their relationship with type diabetes. Diabetes Res. Clin. Pract. 2016, 115, 76–82. [Google Scholar] [CrossRef]
- Buchanan, T.A.; Xiang, A.H.; Page, K.A. Gestational diabetes mellitus: Risks and management during and after pregnancy. Nat. Rev. Endocrinol. 2012, 8, 639–649. [Google Scholar] [CrossRef]
- Sandsæter, H.L.; Horn, J.; Rich-Edwards, J.W.; Haugdahl, H.S. Preeclampsia, gestational diabetes and later risk of cardiovascular disease: Women’s experiences and motivation for lifestyle changes explored in focus group interviews. BMC Pregnancy Childbirth 2019, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Bryson, C.L.; Ioannou, G.N.; Rulyak, S.J.; Critchlow, C. Association between gestational diabetes and pregnancy-induced hypertension. Am. J. Epidemiol. 2003, 158, 1148–1153. [Google Scholar] [CrossRef]
- Metcalfe, A.; Sabr, Y.; Hutcheon, J.A.; Donovan, L.; Lyons, J.; Burrows, J.; Joseph, K.S. Trends in obstetric intervention and pregnancy outcomes of canadian women with diabetes in pregnancy from to 2015. J. Endocr. Soc. 2017, 1, 1540–1549. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Herath, H.; Herath, R.; Wickremasinghe, R. Gestational diabetes mellitus and risk of type diabetes years after the index pregnancy in Sri Lankan women-A community based retrospective cohort study. PLoS ONE 2017, 12, e0179647. [Google Scholar] [CrossRef]
- Hart, C.L.; Hole, D.J.; Lawlor, D.A.; Davey Smith, G. How many cases of Type diabetes mellitus are due to being overweight in middle age? Evidence from the Midspan prospective cohort studies using mention of diabetes mellitus on hospital discharge or death records. Diabet. Med. 2007, 241, 73–80. [Google Scholar] [CrossRef]
- Langer, O.; Mazze, R. The relationship between large-for-gestational-age infants and glycemic control in women with gestational diabetes. Am. J. Obstet. Gynecol. 1988, 159, 1478–1483. [Google Scholar] [CrossRef]
- Gandhi, P.; Bustani, R.; Madhuvrata, P.; Farrell, T. Introduction of metformin for gestational diabetes mellitus in clinical practice: Has it had an impact? Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 160, 147–150. [Google Scholar] [CrossRef]
- Suhonen, L.; Hiilesmaa, V.; Teramo, K. Glycaemic control during early pregnancy and fetal malformations in women with type I diabetes mellitus. Diabetologia 2000, 43, 79–82. [Google Scholar] [CrossRef]
- Guerin, A.; Nisenbaum, R.; Ray, J.G. Use of maternal GHb concentration to estimate the risk of congenital anomalies in the offspring of women with prepregnancy diabetes. Diabetes Care 2007, 30, 1920–1925. [Google Scholar] [CrossRef]
- Mitanchez, D.; Yzydorczyk, C.; Simeoni, U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J. Diabetes 2015, 6, 734–743. [Google Scholar] [CrossRef]
- Opara, P.I.; Jaja, T.; Onubogu, U.C. Morbidity and mortality amongst infants of diabetic mothers admitted into a special care baby unit in Port Harcourt, Nigeria. Ital. J. Pediatr. 2010, 36, 77. [Google Scholar] [CrossRef]
- Gillman, M.W.; Rifas-Shiman, S.; Berkey, C.S.; Field, A.E.; Colditz, G.A. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003, 111, e221–e226. [Google Scholar] [CrossRef]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sharma, A.J.; Callaghan, W.M. Gestational diabetes and childhood obesity: What is the link? Curr. Opin. Obstet. Gynecol. 2012, 24, 376–381. [Google Scholar] [CrossRef]
- Bider-Canfield, Z.; Martinez, M.P.; Wang, X.; Yu, W.; Bautista, M.P.; Brookey, J.; Page, K.A.; Buchanan, T.A.; Xiang, A.H. Maternal obesity, gestational diabetes, breastfeeding and childhood overweight at age years. Pediatr. Obes. 2017, 12, 171–178. [Google Scholar] [CrossRef]
- Kaul, P.; Bowker, S.L.; Savu, A.; Yeung, R.O.; Donovan, L.E.; Ryan, E.A. Association between maternal diabetes, being large for gestational age and breast-feeding on being overweight or obese in childhood. Diabetologia 2019, 62, 249–258. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
- Kollmann, T.R.; Kampmann, B.; Mazmanian, S.K.; Marchant, A.; Levy, O. Protecting the newborn and young infant from infectious diseases: Lessons from immune ontogeny. Immunity 2017, 46, 350–363. [Google Scholar] [CrossRef]
- Yu, J.C.; Khodadadi, H.; Malik, A.; Davidson, B.; Salles, É.D.S.L.; Bhatia, J.; Hale, V.L.; Baban, B. Innate immunity of neonates and infants. Front. Immunol. 2018, 9, 1759. [Google Scholar] [CrossRef]
- Rubarth, L.B. Infants of diabetic mothers. Neonatal Netw. 2013, 32, 416–418. [Google Scholar] [CrossRef]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast milk, a source of beneficial microbes and associated benefits for infant health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef]
- Martín-Álvarez, E.; Diaz-Castro, J.; Peña-Caballero, M.; Serrano-López, L.; Moreno-Fernández, J.; Sánchez-Martínez, B.; Martín-Peregrina, F.; Alonso-Moya, M.; Maldonado-Lozano, J.; Hurtado-Suazo, J.A.; et al. Oropharyngeal colostrum positively modulates the inflammatory response in preterm neonates. Nutrients 2020, 12, 413. [Google Scholar] [CrossRef]
- Liao, Y.; Alvarado, R.; Phinney, B.; Lönnerdal, B. Proteomic characterization of human milk whey proteins during a twelve-month lactation period. J. Proteome Res. 2011, 10, 1746–1754. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Erdmann, P.; Thakkar, S.K.; Sauser, J.; Destaillats, F. Longitudinal evolution of true protein, amino acids and bioactive proteins in breast milk: A developmental perspective. J. Nutr. Biochem. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Czosnykowska-Łukacka, M.; Orczyk-Pawiłowicz, M.; Broers, B.; Królak-Olejnik, B. Lactoferrin in human milk of prolonged lactation. Nutrients 2019, 11, 2350. [Google Scholar] [CrossRef]
- Czosnykowska-Łukacka, M.; Lis-Kuberka, J.; Królak-Olejnik, B.; Orczyk-Pawiłowicz, M. Changes in human milk immunoglobulin profile during prolonged lactation. Front. Pediatr. 2020, 8, 428. [Google Scholar] [CrossRef]
- Araújo, E.D.; Gonçalves, A.K.; Cornetta Mda, C.; Cunha, H.; Cardoso, M.L.; Morais, S.S.; Giraldo, P.C. Evaluation of the secretory immunoglobulin A levels in the colostrum and milk of mothers of term and pre-term newborns. Braz. J. Infect. Dis. 2005, 9, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Chirico, G.; Marzollo, R.; Cortinovis, S.; Fonte, C.; Gasparoni, A. Antiinfective properties of human milk. J. Nutr. 2008, 138, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr. 2016, 173, S43–S52. [Google Scholar] [CrossRef]
- Donovan, S.M. The role of lactoferrin in gastrointestinal and immune development and function: A preclinical perspective. J. Pediatr. 2016, 173, S16–S28. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Suresh, G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2017, 6. [Google Scholar] [CrossRef]
- Gopalakrishna, K.P.; Hand, T.W. Influence of maternal milk on the neonatal intestinal microbiome. Nutrients 2020, 12, 823. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Mathijssen, G.; Dapra, C.; Do, D.M.; Medo, E. Active free secretory component and secretory IgA in human milk: Do maternal vaccination, allergy, infection, mode of delivery, nutrition and active lifestyle change their concentrations? Pediatr. Res. 2020. [Google Scholar] [CrossRef]
- Ward, P.; Paz, E.; Conneely, O. Lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2540. [Google Scholar] [CrossRef]
- Lönnerdal, B. Bioactive proteins in human milk: Health, nutrition, and implications for infant formulas. J. Pediatr. 2016, 173, S4–S9. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin: Balancing ups and downs of inflammation due to microbial infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef]
- Redwan, E.M.; Uversky, V.N.; El-Fakharany, E.M.; Al-Mehdar, H. Potential lactoferrin activity against pathogenic viruses. C. R. Biol. 2014, 337, 581–595. [Google Scholar] [CrossRef]
- Lönnerdal, B. Nutritional roles of lactoferrin. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 293–297. [Google Scholar] [CrossRef]
- Vogel, H.J. Lactoferrin, a bird’s eye view. Biochem. Cell Biol. 2012, 90, 233–244. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Shin, K.; Lonnerdal, B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry 2001, 40, 15771–15779. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Jiang, R.; Lonnerdal, B. Biochemical and molecular impacts of lactoferrin on small intestinal growth and development during early life. Biochem Cell Biol. 2012, 90, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Rainard, P.; Lippolis, J.; Salmon, H.; Kacskovics, I. The mammary gland in mucosal and regional immunity. Mucosal Immunol. 2015, 2269–2306. [Google Scholar] [CrossRef]
- Goldman, A.S.; Chheda, S.; Garofalo, R.; Schmalstieg, F.C. Cytokines in human milk: Properties and potential effects upon the mammary gland and the neonate. J. Mammary Gland Biol. Neoplasia 1996, 1, 251–258. [Google Scholar] [CrossRef] [PubMed]
- McManaman, J.L.; Neville, M.C. Mammary physiology and milk secretion. Adv. Drug Deliv. Rev. 2003, 55, 629–641. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Liu, B.; Newburg, D.S. Human milk glycoproteins protect infants against human pathogens. Breastfeed Med. 2013, 8, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.; Cheah, W.Y.; Grinyer, J.; Packer, N. Glycoconjugates in human milk: Protecting infants from disease. Glycobiology 2013, 23, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Lis-Kuberka, J.; Orczyk-Pawiłowicz, M. The significance of fucosylated glycoconjugates of human milk in nutrition of newborns and infants. Pos. Hig. Med. Dosw. (Online) 2015, 69, 811–829. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, R.R.; Everett, M.L.; Palestrant, D. Human secretory immunoglobulin A may contribute to biofilm formation in the gut. Immunology 2003, 109, 580–587. [Google Scholar] [CrossRef]
- Lönnerdal, B. Bioactive proteins in breast milk. J. Paediatr. Child Health. 2013, 49, 1–7. [Google Scholar] [CrossRef]
- Rogier, E.; Frantz, A.; Bruno, M.; Wedlund, L.; Cohen, D.A.; Stromberg, A.J.; Kaetzel, C.S. Lessons from mother: Long-term impact of antibodies in breast milk on the gut microbiota and intestinal immune system of breastfed offspring. Gut Microbes. 2014, 5, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Hurley, W.L.; Theil, P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef]
- Broadhurst, M.; Beddis, K.; Black, J.; Henderson, H.; Nair, A.; Wheeler, T. Effect of gestation length on the levels of five innate defence proteins in human milk. Early Hum. Dev. 2015, 91, 7–11. [Google Scholar] [CrossRef]
- Shashiraj Faridi, M.M.A.; Singh, O.; Rusia, U. Mother’s iron status, breastmilk iron and lactoferrin—Are they related? Eur. J. Clin. Nutr. 2006, 60, 903–908. [Google Scholar] [CrossRef]
- Mastromarino, P.; Capobianco, D.; Campagna, G.; Laforgia, N.; Drimaco, P.; Dileone, A.; Baldassarre, M.E. Correlation between lactoferrin and beneficial microbiota in breast milk and infant’s feces. Biometals 2014, 27, 1077–1086. [Google Scholar] [CrossRef]
- Trend, S.; Strunk, T.; Lloyd, M.L.; Kok, C.H.; Metcalfe, J.; Geddes, D.T.; Lai, C.T.; Richmond, P.; Doherty, D.A.; Simmer, K.; et al. Levels of innate immune factors in preterm and term mothers’ breast milk during the 1st month postpartum. Br. J. Nutr. 2016, 115, 1178–1193. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, R.; Chen, Q.; Wang, J.; Duan, Y.; Pang, X.; Jiang, S.; Bi, Y.; Zhang, H.; Lönnerdal, B.; et al. Concentration of lactoferrin in human milk and its variation during lactation in different Chinese populations. Nutrients 2018, 10, 1235. [Google Scholar] [CrossRef]
- Goonatilleke, E.; Huang, J.; Xu, G.; Wu, L.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B. Human milk proteins and their glycosylation exhibit quantitative dynamic variations during lactation. J. Nutr. 2019, 149, 1317–1325. [Google Scholar] [CrossRef]
- Marquis, G.S.; Penny, M.E.; Zimmer, J.P.; Díaz, J.M.; Marín, R.M. An overlap of breastfeeding during late pregnancy is associated with subsequent changes in colostrum composition and morbidity rates among Peruvian Infants and their mothers. J. Nutr. 2003, 133, 2585–2591. [Google Scholar] [CrossRef]
- Houghton, M.R.; Gracey, M.; Burke, V.; Bottrell, C.; Spargo, R.M. Breast milk lactoferrin levels in relation to maternal nutritional status. J. Pediatr. Gastroenterol. Nutr. 1985, 4, 230–233. [Google Scholar] [CrossRef]
- Leelahakul, V.; Tanaka, F.; Sinsuksai, N.; Vichitsukon, K.; Pinyopasakul, W.; Kido, N.; Inukai, S. Comparison of the protein composition of breast milk and the nutrient intake between Thai and Japanese mothers. Nurs. Health Sci. 2009, 11, 180–184. [Google Scholar] [CrossRef]
- Lis-Kuberka, J.; Orczyk-Pawiłowicz, M.; Królak-Olejnik, B.; Berghausen-Mazur, M.; Barańska, K.; Kątnik-Prastowska, I. Lectin-based analysis of human milk immunoglobulin G fucosylated variants in relation to milk maturation and perinatal risk factors. J. Appl. Biomed. 2018, 16, 232–240. [Google Scholar] [CrossRef]
- Koenig, A.; de Albuquerque Diniz, E.M.; Barbosa, S.F.C.; Vaz, F.A.C. Immunologic factors in human milk: The effects of gestational age and pasteurization. J. Hum. Lact. 2005, 21, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Abuidhail, J.; Al-Shudiefat, A.A.; Darwish, M. Alterations of immunoglobulin G and immunoglobulin M levels in the breast milk of mothers with exclusive breastfeeding compared to mothers with non-exclusive breastfeeding during months postpartum: The Jordanian cohort study. Am. J. Hum. Biol. 2019, 31, 23197. [Google Scholar] [CrossRef] [PubMed]
- Berdi, M.; de Lauzon-Guillain, B.; Forhan, A.; Castelli, F.A.; Fenaille, F.; Charles, M.A.; Heude, B.; Junot, C.; Adel-Patient, K.; EDEN Mother-Child Cohort Study Group. Immune components of early breastmilk: Association with maternal factors and with reported food allergy in childhood. Pediatr. Allergy Immunol. 2019, 30, 107–116. [Google Scholar] [CrossRef]
- Peila, C.; Gazzolo, D.; Bertino, E.; Cresi, F.; Coscia, A. Influence of diabetes during pregnancy on human milk composition. Nutrients 2020, 12, 185. [Google Scholar] [CrossRef]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003, 1, S5–S20. [Google Scholar]
- Wender-Ożegowska, E.; Bomba-Opońl, D.; Brązert, J.; Celewicz, Z.; Czajkowski, K.; Gutaj, P.; Malinowska-Polubiec, A.; Zawiejska, A.; Wielgoś, M. Standards of Polish Society of Gynecologists and Obstetricians in management of women with diabetes. Ginekol. Pol. 2018, 89, 341–350. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Budzyński, A.; Cypryk, K.; Czech, A.; Czupryniak, L.; et al. Guidelines on the management of diabetic patients. A position of Diabetes Poland. Clin. Diabetol. 2020, 9, 1. [Google Scholar]
- Wesolowska, A.; Sinkiewicz-Darol, E.; Barbarska, O.; Strom, K.; Rutkowska, M.; Karzel, K.; Rosiak, E.; Oledzka, G.; Orczyk-Pawiłowicz, M.; Rzoska, S.; et al. New achievements in high-pressure processing to preserve human milk bioactivity. Front. Pediatr. 2018, 16, 323. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, L.; Roszer, T.; Ricote, M. Inflammatory mediators and insulin resistance in obesity: Role of nuclear receptor signaling in macrophages. Mediators Inflamm. 2010, 2010, 219583. [Google Scholar] [CrossRef] [PubMed]
- Sakowicz-Burkiewicz, M.; Pawelczyk, T. Recent advances in understanding the relationship between adenosine metabolism and the function of T and B lymphocytes in diabetes. J. Physiol. Pharmacol. 2011, 62, 505. [Google Scholar]
- Badillo-Suárez, P.A.; Rodríguez-Cruz, M.; Nieves-Morales, X. Impact of metabolic hormones secreted in human breast milk on nutritional programming in childhood obesity. J. Mammary Gland Biol. Neoplasia 2017, 22, 171–191. [Google Scholar] [CrossRef]
- Gallacher, S.J.; Thomson, G.; Fraser, W.D.; Fisher, B.M.; Gemmell, C.G.; MacCuish, A.C. Neutrophil bactericidal function in diabetes mellitus: Evidence for association with blood glucose control. Diabet. Med. 1995, 12, 916–920. [Google Scholar] [CrossRef]
- Rubinstein, R.; Genaro, A.; Motta, A.; Cremaschi, G.; Wald, M. Impaired immune responses in streptozotocin-induced type I diabetes in mice. Involvement of high glucose. Clin. Exp. Immunol. 2008, 154, 235–246. [Google Scholar] [CrossRef]
- Jafar, N.; Edriss, H.; Nugent, K. The effect of short-term hyperglycemia on the innate immune system. Am. J. Med. Sci. 2016, 351, 201–211. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, K.; Sun, Y.; Wang, J.; Xu, Y.; Yan, S.; Zhu, P.; Tao, F. Placenta response of inflammation and oxidative stress in low-risk term childbirth: The implication of delivery mode. B.M.C. Pregnancy Childbirth 2017, 17, 407. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.; Kent, J.C.; Lai, C.T.; Geddes, D.T. Comparison of maternal milk ejection characteristics during pumping using infant-derived and 2-phase vacuum patterns. Int. Breastfeed. J. 2019, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Trivedi, S.S.; Jain, A.; Bhattacharjee, J. Unusual changes in colostrum composition in lactating indian women having medical complications during pregnancy—A pilot study. Indian J. Clin. Biochem. 2002, 17, 68–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morceli, G.; Franca, E.; Magalha˜es, V.; Damasceno, D.; Calderon, I.; Honorio-Franca, A. Diabetes induced immunological and biochemical changes in human colostrum. Acta Paediatr. 2011, 100, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Franca, E.L.; Calderon, I.M.P.; Vieira, E.L.; Morceli, G.; Honorio-Franca, A.C. Transfer of maternal immunity to newborns of diabetic mothers. Clin. Dev. Immunol. 2012, 2012, 928187. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; Totten, S.M.; Huang, J.; Grapov, D.; Durham, H.A.; Lammi-Keefe, C.J.; Lebrilla, C.; German, J.B. human milk secretory immunoglobulin A and lactoferrin N-glycans are altered in women with gestational diabetes mellitus. J. Nutr. 2013, 143, 1906–1912. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Intermittent fasting, dietary modifications, and exercise for the control of gestational diabetes and maternal mood dysregulation: A review and a case report. Int. J. Environ. Res. Public Health 2020, 17, 9379. [Google Scholar] [CrossRef] [PubMed]
- Demers-Mathieu, V.; Huston, R.K.; Markell, A.M.; McCulley, E.A.; Martin, R.L.; Spooner, M.; Dallas, D.C. Differences in maternal immunoglobulins within mother’s own breast milk and donor breast milk and across digestion in preterm infants. Nutrients 2019, 11, 920. [Google Scholar] [CrossRef]
- Woof, J.M.; Russell, M.W. Structure and function relationships in IgA. Mucosal Immunol. 2011, 4, 590–597. [Google Scholar] [CrossRef]
- Koch, M.A.; Reiner, G.L.; Lugo, K.A.; Kreuk, L.S.; Stanbery, A.G.; Ansaldo, E.; Seher, T.D.; Ludington, W.B.; Barton, G.M. Maternal IgG and IgA antibodies dampen mucosal T helper cell responses in early life. Cell 2016, 165, 827–841. [Google Scholar] [CrossRef]
- Gomez de Aguero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef]
- Lawrence, R.M.; Pane, C.A. Human breast milk: Current concepts of immunology and infectious diseases. Curr. Probl. Pediatr. Adolesc. Health Care 2007, 37, 7–36. [Google Scholar] [CrossRef]
- Brandtzaeg, P. The mucosal immune system and its integration with the mammary glands. J. Pediatr. 2010, 156, 8–15. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Cisalpino, D.; Varadarajan, S.; Hellman, J.; Warren, H.S.; Cascalho, M.; Inohara, N.; Núñez, G. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity 2016, 44, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Flores, G.; Sakamoto, K.; Zeng, M.Y.; Wang, Y.; Hakim, J.; Matus-Acuña, V.; Inohara, N.; Núñez, G. Maternal immunization confers protection to the offspring against an attaching and effacing pathogen through delivery of IgG in breast milk. Cell Host Microbe 2019, 25, 313–323.e4. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, W.; Wu, M.; Song, X.; Caro, F.; Sun, X.; Gazzaniga, F.; Stefanetti, G.; Oh, S.; Mekalanos, J.J. Microbiota-targeted maternal antibodies protect neonates from enteric infection. Nature 2020, 577, 543–548. [Google Scholar]
- Royle, L.; Roos, A.; Harvey, D.J.; Wormald, M.R.; van Gijlswijk-Janssen, D.; Redwan, E.-R.M.; Wilson, I.A.; Daha, M.R.; Dwek, R.A.; Rudd, P.M. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J. Biol. Chem. 2003, 278, 20140–20153. [Google Scholar] [CrossRef]
- Lis-Kuberka, J.; Królak-Olejnik, B.; Berghausen-Mazur, M.; Orczyk-Pawiłowicz, M. Lectin-based method for deciphering human milk IgG sialylation. Molecules 2019, 24, 3797. [Google Scholar] [CrossRef]
- Pace, R.M.; Williams, J.E.; Järvinen, K.M.; Belfort, M.B.; Pace, C.D.; Lackey, K.A.; Gogel, A.C.; Nguyen-Contant, P.; Kanagaiah, P.; Fitzgerald, T.; et al. COVID-and human milk: SARS-CoV-2, antibodies, and neutralizing capacity. medRxiv 2020, 18. [Google Scholar] [CrossRef]
- Ruiz, L.; Espinosa-Martos, I.; García-Carral, C.; Manzano, S.; McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; et al. What’s normal? Immune profiling of human milk from healthy women living in different geographical and socioeconomic settings. Front Immunol. 2017, 8, 696. [Google Scholar] [CrossRef] [PubMed]
- Moirasgenti, M.; Doulougeri, K.; Panagopoulou, E.; Theodoridis, T. Psychological stress reduces the immunological benefits of breast milk. Stress Health. 2019, 35, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.D.; Phan, L.T.H.; Mathisen, R. The cost of not breastfeeding: Global results from a new tool. Health Policy Plan. 2019, 34, 407–417. [Google Scholar] [CrossRef] [PubMed]
| Gestational Diabetes Mellitus G1 N = 26 (% (n/N)) | Gestational Diabetes Mellitus G2 N = 23 (% (n/N)) | Normoglycemic Mothers N = 37 (% (n/N)) | Chi-Square Test χ2 | p-Value | |
|---|---|---|---|---|---|
Race/ethnicity
| 100% (26/26) | 100% (23/23) | 100% (37/37) | - | - |
Maternal age (mean ± SD)
| 33.8 ± 4.5 20.0% (5/25) 32.0% (8/25) 40.0% (10/25) 8.0% (2/25) | 33.3 ± 4.4 9.1% (2/22) 59.1% (13/22) 27.3% (6/22) 4.5% (1/22) | 32.5 ± 4.7 24.3% (9/37) 35.1% (13/37) 37.8% (14/37) 2.7% (1/37) | 5.78 | 0.45 |
| Maternal pre‑pregnancy BMI, kg/m2 (mean ± SD)
| 24.7 ± 5.3 15.5% (3/19) 36.8% (7/19) 31.6% (6/19) 15.5% (3/19) non | 27.5 ± 4.9 non 30.8% (4/13) 46.2% (6/13) 15.4% (2/13) 7.7% (1/13) | 22.7 ± 3.5 3.8% (1/26) 76.9% (20/26) 15.4% (4/26) 3.8% (1/26) non | 16.86 | 0.04 |
Parity
| 50.0% (12/24) 37.5% (9/24) 8.3% (2/24) 4.2% (1/24) | 50.0% (11/22) 36.4% (8/22) 9.1% (2/22) 4.5% (1/22) | 62.9% (22/35) 28.6% (10/35) 5.7% (2/35) 2.9% (1/35) | 1.39 | 0.97 |
Gestational age (mean ± SD)
| 37.4 ± 3.1 non 23.1% (6/26) 15.4% (4/26) 64.0% (16/26) | 36.8 ± 2.7 non 26.1% (6/23) 21.7% (5/23) 52.2% (12/23) | 37.2 ± 3.3 non 21.2% (7/33) 12.1% (4/33) 66.7% (22/33) | 1.41 | 0.85 |
Delivery mode
| 36% (9/25) 64% (16/25) 37.5% (6/16) 62.5% (10/16) | 26.1% (6/23) 73.9% (17/23) 58.8% (10/17) 41.2% (7/17) | 21.9% (7/32) 78.1% (25/32) 44% (11/25) 56% (14/25) | 1.44 | 0.49 |
Birth weight (g) (mean ± SD)
| 2883.0 ± 758.6 88.9% (24/27) 11.1% (3/27) | 2775.6 ± 758.1 95.7% (22/23) 4.3% (1/23) | 2956.4 ± 879.5 91.9% (34/37) 8.1% (3/37) | 0.77 | 0.69 |
Newborn’s gender
| 50.0% (13/26) 50.0% (13/26) | 41.7% (10/24) 58.3% (14/24) | 48.6% (17/35) 51.4% (18/35) | 3.59 | 0.17 |
Newborn’s overall condition (Apgar score)
| 66.7% (18/27) 33.3% (9/27) non | 72.7% (16/22) 27.3% (6/22) non | 72.2% (26/36) 27.8% (10/36) non | 0.29 | 0.87 |
Infections during pregnancy
| 4.0% (1/25) 24.0% (6/25) 16.0% (4/25) 28.0% (7/25) | 4.5% (1/22) 13.6% (3/22) 13.6% (3/22) 4.5% (1/22) | 18.9% (7/37) 13.5% (5/37) 13.5% (5/37) 13.5% (5/37) | 6.76 | 0.35 |
Mother’s diseases
| 12.0% (3/25) 28.0% (7/25) non 4.0% (1/25) | 17.4% (4/23) 26.1% (6/23) 8.7% (2/23) 4.3% (1/23) | 10.8% (4/37) 18.9% (7/37) 2.7% (1/37) 8.1% (3/37) | 3.36 | 0.77 |
Medicines during lactation (other than insulin)
| 72.0% (18/25) 28% (7/25) | 73.9% (17/23) 26.1% (6/23) | 81.1% (30/37) 18.9% (7/37) | 0.80 | 0.68 |
| LF and Immunoglobulins | Stage of Lactation | Group | p-Value GDM G1 vs. Normoglycemic | p-Value GDM G2 vs. Normoglycemic | p-Value GDM G1 vs. GDM G2 | ||
|---|---|---|---|---|---|---|---|
| GDM G1 n = 29 | GDM G2 n = 24 | Normoglycemic n = 49 | |||||
| Lactoferrin [g/L] | Early colostrum (Days 1–3) (n = 13/10/21) | 10.02 ± 3.23 8.68 (7.59–12.07) | 9.67 ± 6.56 8.14 (6.84–11.44) | 9.30 ± 4.81 8.12 (7.09–10.36) | p > 0.38 (NS) | p > 0.91 (NS) | p > 0.44 (NS) |
| Colostrum (Days 4–7) (n = 10/4/16) | 8.05 ± 1.38 8.64 (6.76–8.89) | 11.50 ± 0.58 11.42 (11.13–11.87) | 8.09 ± 1.37 7.97 (7.33–8.64) | p > 0.81 (NS) | p < 0.0004 | p < 0.001 | |
| Transitional milk (Days 8–15) (n = 6/10/12) | 6.71 ± 2.37 6.99 (4.63–8.58) | 6.45 ± 2. 12 6.47 (5.04–7.51) p 2 < 0.001 | 6.67 ± 2.01 7.13 (5.65–8.15) | p = 1 (NS) | p > 0.58 (NS) | p > 0.79 (NS) | |
| SIgA [g/L] | Early colostrum (Days 1–3) (n = 13/10/21) | 17.45 ± 10.86 12.97 (10.36–28.22) | 14.59 ± 14.06 9.39 (5.05–14.79) | 17.44 ± 17.63 11.52 (5.93–20.78) | p > 0.44 (NS) | p > 0.63 (NS) | p > 0.31 (NS) |
| Colostrum (Days 4–7) (n = 10/4/16) | 6.24 ± 3.98 5.58 (3.43–9.57) p 1 < 0.001 | 11.37 ± 8.79 9.92 (5.90–16.84) | 3.98 ± 1.75 4.05 (2.66–5.33) p 1 < 0.00006 | p > 0.20 (NS) | p > 0.08 (NS) | p > 0.37 (NS) | |
| Transitional milk (Days 8–15) (n = 6/10/12) | 3.23 ± 1.30 3.56 (2.93–3.76) | 3.92 ± 4.19 2.57 (1.75–3.47) | 3.56 ± 1.24 3.34 (2.94–4.33) | p > 0.89 (NS) | p > 0.28 (NS) | p > 0.63 (NS) | |
| IgG [mg/L] | Early colostrum (Days 1–3) (n = 13/10/21) | 7.65 ± 5.67 7.44 (3.20–8.85) | 10.01 ± 4.48 8.36 (6.58–11.70) | 5.40 ± 5.07 4.00 (1.56–7.20) | p > 0.15 (NS) | p < 0.008 | p > 0.23 (NS) |
| Colostrum (Days 4–7) (n = 10/4/16) | 6.31 ± 2.66 6.50 (5.36–7.30) | 7.33 ± 1.94 6.48 (6.15–8.50) | 6.69 ± 3.38 6.65 (3.72–8.89) | p > 0.77 (NS) | p > 0.75 (NS) | p > 0.63 (NS) | |
| Transitional milk (Days 8–15) (n = 6/10/12) | 7.96 ± 5.18 5.71 (5.37–9.62) | 8.27 ± 2.83 7.71 (5.74–10.28) | 6.85 ± 2.70 6.28 (4.93–8.65) | p > 0.89 (NS) | p > 0.20 (NS) | p > 0.56 (NS) | |
| IgM [mg/L] | Early colostrum (Days 1–3) (n = 13/10/21) | 49.75 ± 67.31 14.65 (6.61–85.22) | 26.26 ± 21.19 21.45 (8.37–43.81) | 32.86 ± 43.21 18.09 (10.67–24.92) | p > 0.91 (NS) | p > 0.98 (NS) | p > 0.97 (NS) |
| Colostrum (Days 4–7) (n = 10/4/16) | 13.44 ± 12.77 7.04 (5.21–20.25) | 9.71 ± 5.96 9.27 (4.71–14.71) | 26.54 ± 40.04 9.34 (5.52–32.54) | p > 0.51 (NS) | p > 0.61 (NS) | p > 0.83 (NS) | |
| Transitional milk (Days 8–15) (n = 6/10/12) | 13.08 ± 13.75 8.91 (5.40–12.29) | 28.19 ± 55.64 12.35 (4.25–18.22) | 12.44 ± 17.08 4.99 (3.91–9.66) | p > 0.43 (NS) | p > 0.38 (NS) | p > 0.87 (NS) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis-Kuberka, J.; Berghausen-Mazur, M.; Orczyk-Pawiłowicz, M. Lactoferrin and Immunoglobulin Concentrations in Milk of Gestational Diabetic Mothers. Nutrients 2021, 13, 818. https://doi.org/10.3390/nu13030818
Lis-Kuberka J, Berghausen-Mazur M, Orczyk-Pawiłowicz M. Lactoferrin and Immunoglobulin Concentrations in Milk of Gestational Diabetic Mothers. Nutrients. 2021; 13(3):818. https://doi.org/10.3390/nu13030818
Chicago/Turabian StyleLis-Kuberka, Jolanta, Marta Berghausen-Mazur, and Magdalena Orczyk-Pawiłowicz. 2021. "Lactoferrin and Immunoglobulin Concentrations in Milk of Gestational Diabetic Mothers" Nutrients 13, no. 3: 818. https://doi.org/10.3390/nu13030818
APA StyleLis-Kuberka, J., Berghausen-Mazur, M., & Orczyk-Pawiłowicz, M. (2021). Lactoferrin and Immunoglobulin Concentrations in Milk of Gestational Diabetic Mothers. Nutrients, 13(3), 818. https://doi.org/10.3390/nu13030818






