Effectiveness of Oral Nutritional Supplements on Older People with Anorexia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Data Sources and Search Strategy
2.3. Intervention and Control Group Definitions
2.4. Study Selection
2.5. Outcome Measures
2.6. Data Extraction
2.7. Statistical Analyses
2.8. Assessment of Risk of Bias and Study Quality
3. Result
3.1. Systematic Review
3.2. Study Characteristics
3.3. ONS Characteristics
3.4. Methodological Quality of the Included Studies
3.5. Effect of ONS on Appetite
3.5.1. Effect of ONS on Overall Appetite
3.5.2. Effect of ONS on Hunger
3.5.3. Effect of ONS on Fullness
3.5.4. Effect of ONS on Desire to Eat
3.5.5. Effect of ONS on Consumption
3.5.6. Effect of ONS on Preoccupation
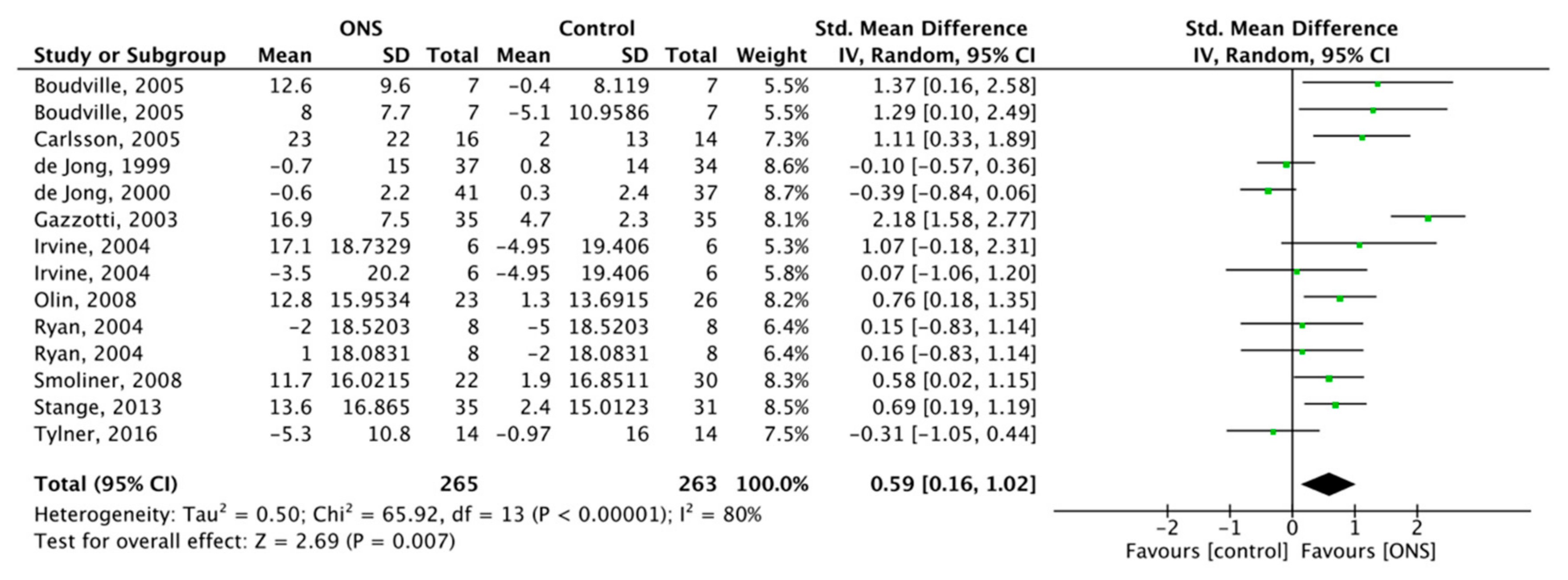
3.6. Effect of ONS on Intake
3.6.1. Effect of ONS on Overall Energy Intake (OEI)
3.6.2. Effect of ONS on Protein Intake
3.6.3. Effect of ONS on Fat Intake
3.6.4. Effect of ONS on Carbohydrate Intake
3.7. Effect of ONS on Body Weight
3.8. Effect of ONS on BMI
3.9. Effect of Other Parts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wojteczek, A.; Dardzinska, J.A.; Malgorzewicz, S.; Gruszecka, A.; Zdrojewski, Z. Prevalence of malnutrition in systemic sclerosis patients assessed by different diagnostic tools. Clin. Rheumatol. 2020, 39, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, R.; Chapman, I.M. Undernutrition and anorexia in the older person. Gastroenterol. Clin. N. Am. 2009, 38, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Kruizenga, H.M.; Van Tulder, M.W.; Seidell, J.C.; Thijs, A.; Ader, H.J.; Van Bokhorst-de van der Schueren, M.A. Effectiveness and cost-effectiveness of early screening and treatment of malnourished patients. Am. J. Clin. Nutr. 2005, 82, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Elia, M.; Normand, C.; Laviano, A.; Norman, K. A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in community and care home settings. Clin. Nutr. 2016, 35, 125–137. [Google Scholar] [CrossRef]
- Seretis, C.; Kaisari, P.; Wanigasooriya, K.; Shariff, U.; Youssef, H. Malnutrition is associated with adverse postoperative outcome in patients undergoing elective colorectal cancer resections. J. BUON 2018, 23, 36–41. [Google Scholar]
- Dent, E.; Hoogendijk, E.O.; Visvanathan, R.; Wright, O.R.L. Malnutrition Screening and Assessment in Hospitalised Older People: A Review. J. Nutr. Health Aging 2019, 23, 431–441. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Sisto, A.; Marzetti, E. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients 2016, 8, 69. [Google Scholar] [CrossRef]
- Cox, N.J.; Morrison, L.; Ibrahim, K.; Robinson, S.M.; Sayer, A.A.; Roberts, H.C. New horizons in appetite and the anorexia of ageing. Age Ageing 2020, 49, 526–534. [Google Scholar] [CrossRef]
- Jadczak, A.D.; Visvanathan, R. Anorexia of Aging—An Updated Short Review. J. Nutr. Health Aging 2019, 23, 306–309. [Google Scholar] [CrossRef]
- Morley, J.E. Pathophysiology of the anorexia of aging. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Wysokinski, A.; Sobow, T.; Kloszewska, I.; Kostka, T. Mechanisms of the anorexia of aging-a review. Age (Dordr) 2015, 37, 9821. [Google Scholar] [CrossRef]
- Dent, E. Anorexia of Aging and Avoidant/Restrictive Food Intake Disorder. J. Am. Med. Dir. Assoc. 2017, 18, 449–450. [Google Scholar] [CrossRef]
- Hara, L.M.; Freiria, C.N.; Silva, G.M.; Fattori, A.; Corona, L.P. Anorexia of Aging Associated with Nutrients Intake in Brazilian Elderly. J. Nutr. Health Aging 2019, 23, 606–613. [Google Scholar] [CrossRef]
- Malafarina, V.; Uriz-Otano, F.; Gil-Guerrero, L.; Iniesta, R. The anorexia of ageing: Physiopathology, prevalence, associated comorbidity and mortality. A systematic review. Maturitas 2013, 74, 293–302. [Google Scholar] [CrossRef]
- Chapman, I.M.; MacIntosh, C.G.; Morley, J.E.; Horowitz, M. The anorexia of ageing. Biogerontology 2002, 3, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.J.; Ibrahim, K.; Sayer, A.A.; Robinson, S.M.; Roberts, H.C. Assessment and Treatment of the Anorexia of Aging: A Systematic Review. Nutrients 2019, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Thomas, D.R. Anorexia and aging: Pathophysiology. Nutrition 1999, 15, 499–503. [Google Scholar] [CrossRef]
- Tsaousi, G.; Stavrou, G.; Ioannidis, A.; Salonikidis, S.; Kotzampassi, K. Pressure ulcers and malnutrition: Results from a snapshot sampling in a university hospital. Med. Princ. Pract. 2015, 24, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C. Importance of nutrition in preventing and treating pressure ulcers. Nurs. Older People 2017, 29, 33–39. [Google Scholar] [CrossRef]
- Ilhan, B.; Bahat, G.; Erdogan, T.; Kilic, C.; Karan, M.A. Anorexia Is Independently Associated with Decreased Muscle Mass and Strength in Community Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 202–206. [Google Scholar] [CrossRef]
- Morley, J.E. Anorexia of ageing: A key component in the pathogenesis of both sarcopenia and cachexia. J. Cachexia Sarcopenia Muscle 2017, 8, 523–526. [Google Scholar] [CrossRef]
- Morley, J.E. Anorexia, sarcopenia, and aging. Nutrition 2001, 17, 660–663. [Google Scholar] [CrossRef]
- Visvanathan, R. Anorexia of Aging. Clin. Geriatr. Med. 2015, 31, 417–427. [Google Scholar] [CrossRef]
- Tsutsumimoto, K.; Doi, T.; Makizako, H.; Hotta, R.; Nakakubo, S.; Makino, K.; Suzuki, T.; Shimada, H. Aging-related anorexia and its association with disability and frailty. J. Cachexia Sarcopenia Muscle 2018, 9, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Sanford, A.M. Anorexia of aging and its role for frailty. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Gaudreau, P.; Payette, H. A scoping review of anorexia of aging correlates and their relevance to population health interventions. Appetite 2016, 105, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Acar Tek, N.; Karaçil-Ermumcu, M. Determinants of Health Related Quality of Life in Home Dwelling Elderly Population: Appetite and Nutritional Status. J. Nutr. Health Aging 2018, 22, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Hoogendijk, E.O.; Wright, O.R.L. New insights into the anorexia of ageing: From prevention to treatment. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 44–51. [Google Scholar] [CrossRef]
- Giezenaar, C.; Chapman, I.; Luscombe-Marsh, N.; Feinle-Bisset, C.; Horowitz, M.; Soenen, S. Ageing Is Associated with Decreases in Appetite and Energy Intake—A Meta-Analysis in Healthy Adults. Nutrients 2016, 8, 28. [Google Scholar] [CrossRef]
- Johnson, K.O.; Shannon, O.M.; Matu, J.; Holliday, A.; Ispoglou, T.; Deighton, K. Differences in circulating appetite-related hormone concentrations between younger and older adults: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2019, 32, 1233–1244. [Google Scholar] [CrossRef]
- Soenen, S.; Chapman, I.M. Body weight, anorexia, and undernutrition in older people. J. Am. Med. Dir. Assoc. 2013, 14, 642–648. [Google Scholar] [CrossRef]
- Van Wymelbeke, V.; Brondel, L.; Bon, F.; Martin-Pfitzenmeyer, I.; Manckoundia, P. An innovative brioche enriched in protein and energy improves the nutritional status of malnourished nursing home residents compared to oral nutritional supplement and usual breakfast: FARINE+ project. Clin. Nutr. ESPEN 2016, 15, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Chapman, I.M. Weight loss in older persons. Med. Clin. N. Am. 2011, 95, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.R.; Akhondzadeh, S.; Keshavarz, S.A.; Mostafavi, S.A. The Characteristics, Reliability and Validity of the Persian Version of Simplified Nutritional Appetite Questionnaire (SNAQ). J. Nutr. Health Aging 2019, 23, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Perna, S.; Rondanelli, M.; Spadaccini, D.; Lenzi, A.; Donini, L.M.; Poggiogalle, E. Are the therapeutic strategies in anorexia of ageing effective on nutritional status? A systematic review with meta-analysis. J. Hum. Nutr. Diet. 2019, 32, 128–138. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed]
- Elia, M.; Normand, C.; Norman, K.; Laviano, A. A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in the hospital setting. Clin. Nutr. 2016, 35, 370–380. [Google Scholar] [CrossRef]
- Ryan, M.; Salle, A.; Favreau, A.M.; Simard, G.; Dumas, J.F.; Malthiery, Y.; Berrut, G.; Ritz, P. Oral supplements differing in fat and carbohydrate content: Effect on the appetite and food intake of undernourished elderly patients. Clin. Nutr. 2004, 23, 683–689. [Google Scholar] [CrossRef]
- Deutz, N.E.; Matheson, E.M.; Matarese, L.E.; Luo, M.; Baggs, G.E.; Nelson, J.L.; Hegazi, R.A.; Tappenden, K.A.; Ziegler, T.R. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin. Nutr. 2016, 35, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.T.; Cruz-Jentoft, A.J.; Landi, F.; Hickson, M.; Zamboni, M.; Pereira, S.L.; Hustead, D.S.; Mustad, V.A. Impacts of High-Protein Oral Nutritional Supplements Among Malnourished Men and Women with Sarcopenia: A Multicenter, Randomized, Double-Blinded, Controlled Trial. J. Am. Med. Dir. Assoc. 2016, 17, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; McLachlan, M.H.; Maayan, N.; Garner, P. Community-based supplementary feeding for food insecure, vulnerable and malnourished populations—An overview of systematic reviews. Cochrane Database Syst. Rev. 2018, 11, Cd010578. [Google Scholar] [CrossRef] [PubMed]
- de van der Schueren, M.A.E. Use and effects of oral nutritional supplements in patients with cancer. Nutrition 2019, 67–68, 110550. [Google Scholar] [CrossRef]
- Parsons, E.L.; Stratton, R.J.; Cawood, A.L.; Smith, T.R.; Elia, M. Oral nutritional supplements in a randomised trial are more effective than dietary advice at improving quality of life in malnourished care home residents. Clin. Nutr. 2017, 36, 134–142. [Google Scholar] [CrossRef]
- Cawood, A.L.; Elia, M.; Stratton, R.J. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res. Rev. 2012, 11, 278–296. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- National Institute for Hearth Research. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 7 October 2020).
- Brocker, P.; Vellas, B.; Albarede, J.L.; Poynard, T. A two-centre, randomized, double-blind trial of ornithine oxoglutarate in 194 elderly, ambulatory, convalescent subjects. Age Ageing 1994, 23, 303–306. [Google Scholar] [CrossRef]
- Pouyssegur, V.; Brocker, P.; Schneider, S.M.; Philip, J.L.; Barat, P.; Reichert, E.; Breugnon, F.; Brunet, D.; Civalleri, B.; Solere, J.P.; et al. An innovative solid oral nutritional supplement to fight weight loss and anorexia: Open, randomised controlled trial of efficacy in institutionalised, malnourished older adults. Age Ageing 2015, 44, 245–251. [Google Scholar] [CrossRef]
- Tylner, S.; Cederholm, T.; Faxen-Irving, G. Effects on Weight, Blood Lipids, Serum Fatty Acid Profile and Coagulation by an Energy-Dense Formula to Older Care Residents: A Randomized Controlled Crossover Trial. J. Am. Med. Dir. Assoc. 2016, 17, 275.e5–275.e11. [Google Scholar] [CrossRef] [PubMed]
- Irvine, P.; Mouzet, J.B.; Marteau, U.; Salle, A.; Genaitay, M.; Favreau, A.M.; Berrut, G.; Ritz, P. Short-term effect of a protein load on appetite and food intake in diseased mildly undernourished elderly people. Clin. Nutr. 2004, 23, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, T.C. The Nordic Cochrane Centre RevMan; Version 5.3; The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- Stange, I.; Bartram, M.; Liao, Y.; Poeschl, K.; Kolpatzik, S.; Uter, W.; Sieber, C.C.; Stehle, P.; Volkert, D. Effects of a Low-Volume, Nutrient- and Energy-Dense Oral Nutritional Supplement on Nutritional and Functional Status: A Randomized, Controlled Trial in Nursing Home Residents. J. Am. Med. Dir. Assoc. 2013, 14, 628.e1–628.e8. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Hubbard, G.P.; Bolch, R.; Holdoway, A.; Beams, A.; Kerr, A.; Robertson, D.; Stratton, R.J. A randomised, controlled trial of the effects of an energy-dense supplement on energy intake, appetite and blood lipids in malnourished community-based elderly patients. J. Hum. Nutr. Diet. 2008, 21, 390–391. [Google Scholar] [CrossRef]
- Odlund Olin, A.; Koochek, A.; Cederholm, T.; Ljungqvist, O. Minimal effect on energy intake by additional evening meal for frail elderly service flat residents--a pilot study. J. Nutr. Health Aging 2008, 12, 295–301. [Google Scholar] [CrossRef]
- Carlsson, P.; Tidermark, J.; Ponzer, S.; Soderqvist, A.; Cederholm, T. Food habits and appetite of elderly women at the time of a femoral neck fracture and after nutritional and anabolic support. J. Hum. Nutr. Diet. 2005, 18, 117–120. [Google Scholar] [CrossRef]
- Faxén-Irving, G.; Andrén-Olsson, B.; af Geijerstam, A.; Basun, H.; Cederholm, T. The effect of nutritional intervention in elderly subjects residing in group-living for the demented. Eur. J. Clin. Nutr. 2002, 56, 221–227. [Google Scholar] [CrossRef]
- Faxen-Irving, G.; Cederholm, T. Energy dense oleic acid rich formula to newly admitted geriatric patients—Feasibility and effects on energy intake. Clin. Nutr. 2011, 30, 202–208. [Google Scholar] [CrossRef]
- de Jong, N.; Chin, A.P.M.J.; de Groot, L.C.; de Graaf, C.; Kok, F.J.; van Staveren, W.A. Functional biochemical and nutrient indices in frail elderly people are partly affected by dietary supplements but not by exercise. J. Nutr. 1999, 129, 2028–2036. [Google Scholar] [CrossRef]
- de Jong, N.; Paw, M.; de Graaf, C.; van Staveren, W.A. Effect of dietary supplements and physical exercise on sensory perception, appetite, dietary intake and body weight in frail elderly subjects. Br. J. Nutr. 2000, 83, 605–613. [Google Scholar] [CrossRef]
- Wouters-Wesseling, W.; Wouters, A.E.; Kleijer, C.N.; Bindels, J.G.; de Groot, C.P.; van Staveren, W.A. Study of the effect of a liquid nutrition supplement on the nutritional status of psycho-geriatric nursing home patients. Eur. J. Clin. Nutr. 2002, 56, 245–251. [Google Scholar] [CrossRef]
- Smoliner, C.; Norman, K.; Scheufele, R.; Hartig, W.; Pirlich, M.; Lochs, H. Effects of food fortification on nutritional and functional status in frail elderly nursing home residents at risk of malnutrition. Nutrition 2008, 24, 1139–1144. [Google Scholar] [CrossRef]
- Boudville, A.; Bruce, D.G. Lack of meal intake compensation following nutritional supplements in hospitalised elderly women. Br. J. Nutr. 2005, 93, 879–884. [Google Scholar] [CrossRef]
- Gazzotti, C.; Arnaud-Battandier, F.; Parello, M.; Farine, S.; Seidel, L.; Albert, A.; Petermans, J. Prevention of malnutrition in older people during and after hospitalisation: Results from a randomised controlled clinical trial. Age Ageing 2003, 32, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, G.P.; Elia, M.; Holdoway, A.; Stratton, R.J.; Cawood, A.L.; Elia, M.; Stratton, R.J. A systematic review of compliance to oral nutritional supplements. Clin. Nutr. 2012, 31, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Ritch, C.R.; Cookson, M.S.; Clark, P.E.; Chang, S.S.; Fakhoury, K.; Ralls, V.; Thu, M.H.; Penson, D.F.; Smith, J.A., Jr.; Silver, H.J. Perioperative Oral Nutrition Supplementation Reduces Prevalence of Sarcopenia following Radical Cystectomy: Results of a Prospective Randomized Controlled Trial. J. Urol. 2019, 201, 470–477. [Google Scholar] [CrossRef]
- Ibarra, A.; Astbury, N.M.; Olli, K.; Alhoniemi, E.; Tiihonen, K. Effect of Polydextrose on Subjective Feelings of Appetite during the Satiation and Satiety Periods: A Systematic Review and Meta-Analysis. Nutrients 2016, 8, 45. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Hermans, R.C.; Hermsen, S.; Robinson, E.; Higgs, S.; Mars, M.; Frost, J.H. The effect of real-time vibrotactile feedback delivered through an augmented fork on eating rate, satiation, and food intake. Appetite 2017, 113, 7–13. [Google Scholar] [CrossRef]
- Oh, S.Y.; Koh, S.J.; Baek, J.Y.; Kwon, K.A.; Jeung, H.C.; Lee, K.H.; Won, Y.W.; Lee, H.J. Validity and Reliability of Korean Version of Simplified Nutritional Appetite Questionnaire in Patients with Advanced Cancer: A Multicenter, Longitudinal Study. Cancer Res. Treat. 2019, 51, 1612–1619. [Google Scholar] [CrossRef]
- Wang, T.; Shen, J. Usefulness of Simplified Nutritional Appetite Questionnaire (SNAQ) in Appetite Assessment in Elder Patients with Liver Cirrhosis. J. Nutr. Health Aging 2018, 22, 911–915. [Google Scholar] [CrossRef]
- Beaudart, C.; Rolland, Y.; Cruz-Jentoft, A.J.; Bauer, J.M.; Sieber, C.; Cooper, C.; Al-Daghri, N.; Araujo de Carvalho, I.; Bautmans, I.; Bernabei, R.; et al. Assessment of Muscle Function and Physical Performance in Daily Clinical Practice: A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif. Tissue Int. 2019, 105, 1–14. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhang, X.Z.; Ma, B.W.; Li, B.; Zhou, D.L.; Liu, Z.C.; Chen, X.L.; Shen, X.; Yu, Z.; Zhuang, C.L. A comparison of four common malnutrition risk screening tools for detecting cachexia in patients with curable gastric cancer. Nutrition 2020, 70, 110498. [Google Scholar] [CrossRef]
- Hung, Y.; Wijnhoven, H.A.H.; Visser, M.; Verbeke, W. Appetite and Protein Intake Strata of Older Adults in the European Union: Socio-Demographic and Health Characteristics, Diet-Related and Physical Activity Behaviours. Nutrients 2019, 11, 777. [Google Scholar] [CrossRef]
- Landi, F. Mediterranean diet and muscular function. Osteoporos. Int. 2018, 29, S117. [Google Scholar] [CrossRef]
- Loman, B.R.; Luo, M.; Baggs, G.E.; Mitchell, D.C.; Nelson, J.L.; Ziegler, T.R.; Deutz, N.E.; Matarese, L.E. Specialized High-Protein Oral Nutrition Supplement Improves Home Nutrient Intake of Malnourished Older Adults Without Decreasing Usual Food Intake. JPEN J. Parenter. Enter. Nutr. 2019, 43, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.C.; Biver, E.; Kaufman, J.M.; Bauer, J.; Branco, J.; Brandi, M.L.; Bruyère, O.; Coxam, V.; Cruz-Jentoft, A.; Czerwinski, E.; et al. The role of calcium supplementation in healthy musculoskeletal ageing: An expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos. Int. 2017, 28, 447–462. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Picca, A.; Tosato, M.; Martone, A.M.; Ortolani, E.; Sisto, A.; D’angelo, E.; Serafini, E.; Desideri, G.; et al. Body mass index is strongly associated with hypertension: Results from the longevity check-up 7+ study. Nutrients 2018, 10, 1976. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; D’Angelo, E.; Sisto, A.; Marzetti, E. Protein intake and muscle health in old age: From biological plausibility to clinical evidence. Nutrients 2016, 8, 295. [Google Scholar] [CrossRef]










| Population | Older people (mean age over 60) in any settings, with any health conditions |
| Interventions | Treatment that used ONS of any kind |
| Comparators | Standard diets with or without placebo |
| Outcomes | With at least one assessment among appetite, intake, and weight |
| Study (Author, Year, Country, Ref) | Study Design | Intervention Length | Setting | Participants | Participants Situation | Age # | Interventions | Control | Effect of the Interventions | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ONS State | Times/Day | ONS Characteristic | Energy Amount | |||||||||
| Boudville, 2005, Australia [65] | Within-subject design | 2–3 days (2 sessions) | Hospital | n = 14, F * = 100%, BMI = 22.6 (3.4) | Rehabilitation phase with an osteoporotic fracture | 79 (7.5) | Liquid | Once | For elderly women |
| Placebo |
|
| Brocker, 1994, France [49] | RCT | 4 months | Community (geriatric units) | n = 185, F * = U/K, BMI = 19.9 (2.6) | Recovering from acute illnesses | 74 (8) | Liquid | Once | Ornithine oxoglutarate | / | Standard diet |
|
| Carlsson, 2005, Sweden [58] | RCT | 6 months | Not institutionalized | n = 45, F * = 100%, BMI = 20.4 (2) | Nondemented with a recent hip fracture | 83 (5) | Liquid ONS/ in combination with medication | Once | / |
| Standard treatment |
|
| De jong, 1999, the Netherlands [61] | RCT | 17 weeks | Community | n = 145, F * = 70.3%, BMI = 24.3 (3.6) | Frail | 78 (5.7) | Solid and liquid | / | / |
| Regular products and social program |
|
| De jong, 2000, the Netherlands [62] | RCT | 17 weeks | Community | n = 159, F * = 71.1%, BMI = 24.5 (3.9) | Frail | 78.7 (5.6) | Solid and liquid ONS + exercise | / | High-density ONS (rich in vitamins and minerals) |
| Placebo |
|
| Faxen-Irving, 2001, Sweden [59] | NRSI | 6 months | Group-living, i.e., community assisted housing | n = 36, F * = 86%, BMI = 20.8 (3.2) | Demented | 84 (4) | Liquid ONS and nutritional education | Twice | / |
| Standard diet |
|
| Faxen-Irving, 2011, Sweden [60] | RCT | 8 days | Hospital | n = 51, F * = 53%, BMI = 21.3 (3.7) | Frail with several chronic disorders | 84 (7) | Liquid | Three times | Energy dense oleic acid rich formula |
| Usual care |
|
| Gazzotti, 2003, Belgium [66] | RCT | 2 months | Hospital | n = 80, F * = 76%, BMI = 25.9 (5.1) | At risk of undernutrition | 80.1 (6.9) | Liquid | Twice | / |
| Standard diet |
|
| Hubbard, 2008, United Kingdom [56] | RCT | 4 weeks | Community | n = 42, F * = U/K, BMI = 20.9 (3.5) | At medium or high risk of malnutrition | 84 (7) | Liquid ONS and dietary advice | Three times | / |
| Standardized diet |
|
| Irvine, 2004, France [52] | Within-Subject design | 3 days | Hospital | n = 12, F * = 33%, BMI = 21.3 (2.4) | Mildly undernourished people with disease | 84 (7.8) | Liquid | Once | Protein-rich |
| Usual care |
|
| Olin, 2008, Sweden [57] | NRSI | 6 months | Nursing homes (frail elderly service flat) | n = 49, F * = 71%, BMI = 23.1 (2.2) | Need regular assistance | 84 (6.2) | Liquid and solid | Once | Evening supplement |
| Regular meals |
|
| Pouyssegur, 2015, France [50] | RCT | 6 weeks | Nursing homes | n = 154, F * = 80%, BMI = 19.2 (2.9) | Malnourished | 86 (7.1) | Solid | / | Cookies |
| Standard institutional diet |
|
| Ryan, 2004, France [40] | Within-Subject design | 3 days | Hospital | n = 16, F * = 38%, BMI = 20 (3) | Malnourished | 77 (8) | Liquid | Once | 2 different sets of volume of fat and carbohydrate |
| Usual care |
|
| Smoliner, 2008, Germany [64] | RCT | 12 weeks | Nursing homes | n = 65, F * = 71%, BMI = 21.6 (3.6) | Malnourished or at risk of malnutrition | 85.2 (9.5) | Solid and liquid | / | / |
| Standard diet |
|
| Stange, 2013, Germany [54] | RCT | 12 weeks | Nursing homes | n = 77, F * = 91%, BMI = 21.5 (2.6) | Malnourished or at risk of malnutrition | 87 (6) | Liquid | Twice | Low-volume, nutrient- and energy-dense |
| Routine care |
|
| Tylner, 2016, Sweden [51] | RCT with crossover | 12 weeks | Care residential homes | n = 39, F * = 60%, BMI = 23 (3.7) | Frail, malnourished or at risk of malnutrition | 84 (7) | Liquid | Three times | ONS with oleic and linoleic acids, proteins, trace elements |
| Usual care |
|
| Wouters-Wesseling, 2002, the Netherlands [63] | RCT | 12 weeks | Nursing homes | n = 35, F * = 88%, BMI = 20.7 (2.9) | Demented, psycho-geriatric, at risk of undernutrition | 82 (8.6) | Liquid | Twice | Low-volume |
| Placebo |
|
| Primary Outcomes | Appetite | Overall Appetite | Increased |
| Hunger | No positive effect | ||
| Fullness | No positive effect | ||
| Desire to eat | No positive effect | ||
| How much do you think you can eat now? | Increased | ||
| How preoccupied are you with thoughts of food? | Increased | ||
| Secondary Outcomes | Intake | Overall energy intake | Increased |
| Protein intake | Increased | ||
| Fat intake | Increased | ||
| Carbohydrate intake | No positive effect | ||
| Body weight | Increased | ||
| Body mass index (BMI) | Increased | ||
| Diarrhea | Decreased | ||
| Pressure sores | Decreased | ||
| Quality of life (QoL) | Increased | ||
| Total health care cost indices | Decreased | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhao, S.; Wu, S.; Yang, X.; Feng, H. Effectiveness of Oral Nutritional Supplements on Older People with Anorexia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 835. https://doi.org/10.3390/nu13030835
Li M, Zhao S, Wu S, Yang X, Feng H. Effectiveness of Oral Nutritional Supplements on Older People with Anorexia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2021; 13(3):835. https://doi.org/10.3390/nu13030835
Chicago/Turabian StyleLi, Mengqi, Si Zhao, Shuang Wu, Xiufen Yang, and Hui Feng. 2021. "Effectiveness of Oral Nutritional Supplements on Older People with Anorexia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 13, no. 3: 835. https://doi.org/10.3390/nu13030835
APA StyleLi, M., Zhao, S., Wu, S., Yang, X., & Feng, H. (2021). Effectiveness of Oral Nutritional Supplements on Older People with Anorexia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 13(3), 835. https://doi.org/10.3390/nu13030835






