Effect of Cone of Pinus densiflora on DNCB-Induced Allergic Contact Dermatitis-Like Skin Lesion in Balb/c Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of the Cone of Pinus densiflora
2.3. Cell Culture and Cell Viability
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-PCR)
2.6. Western Blot
2.7. Animal Experiment
2.8. ACD Model and Drug Treatment
2.9. Histological and Immunohistochemical (IHC) Staining
2.10. Clinical Skin Severity Score
2.11. Statistical Analysis
3. Results
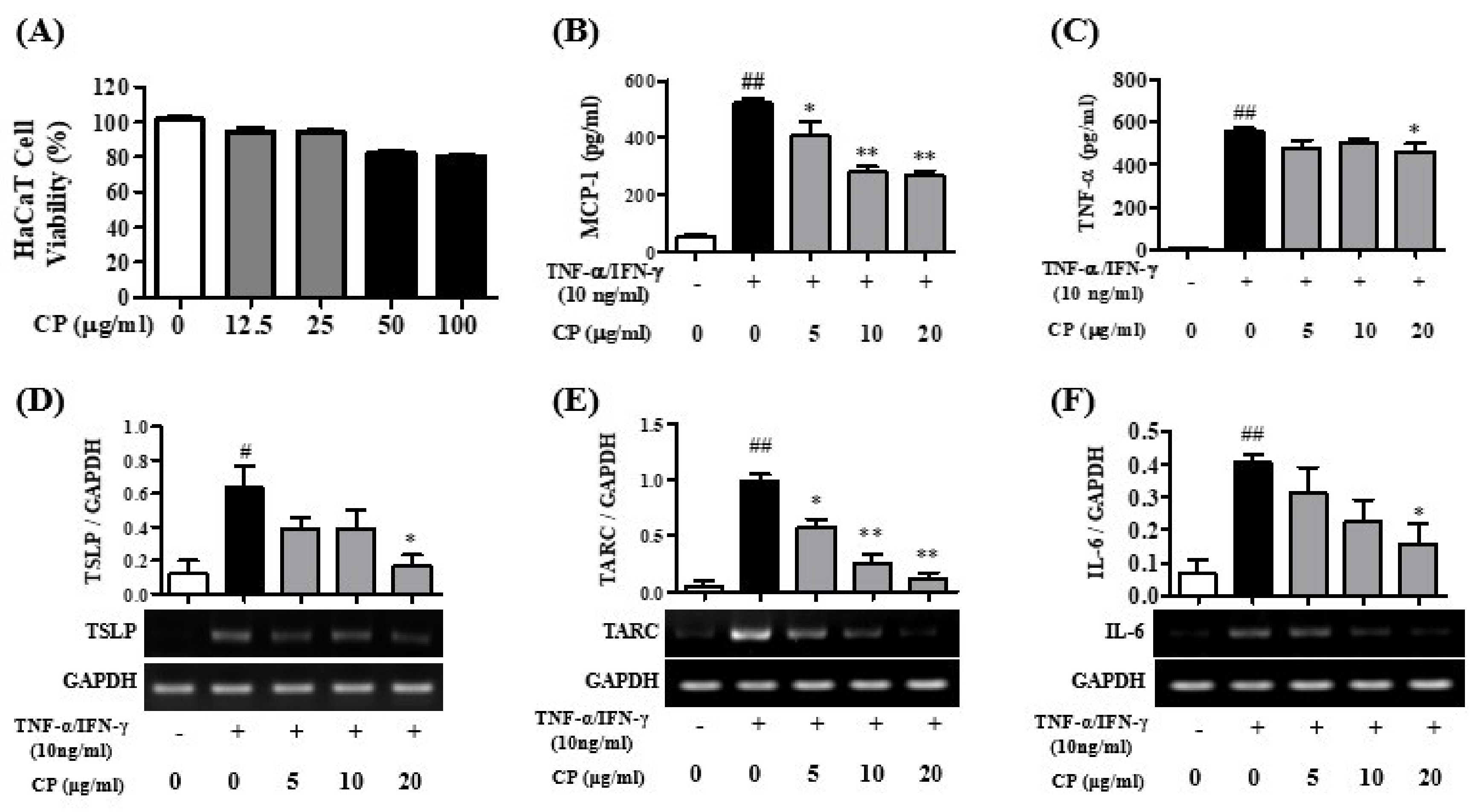
3.1. Cell Viability of CP and the Effects of CP for Pro-Inflammatory Cytokines in HaCaT Cells
3.2. Cell Viability of CP and the Effect of CP on Pro-Inflammatory Cytokines in HMC-1 Cells
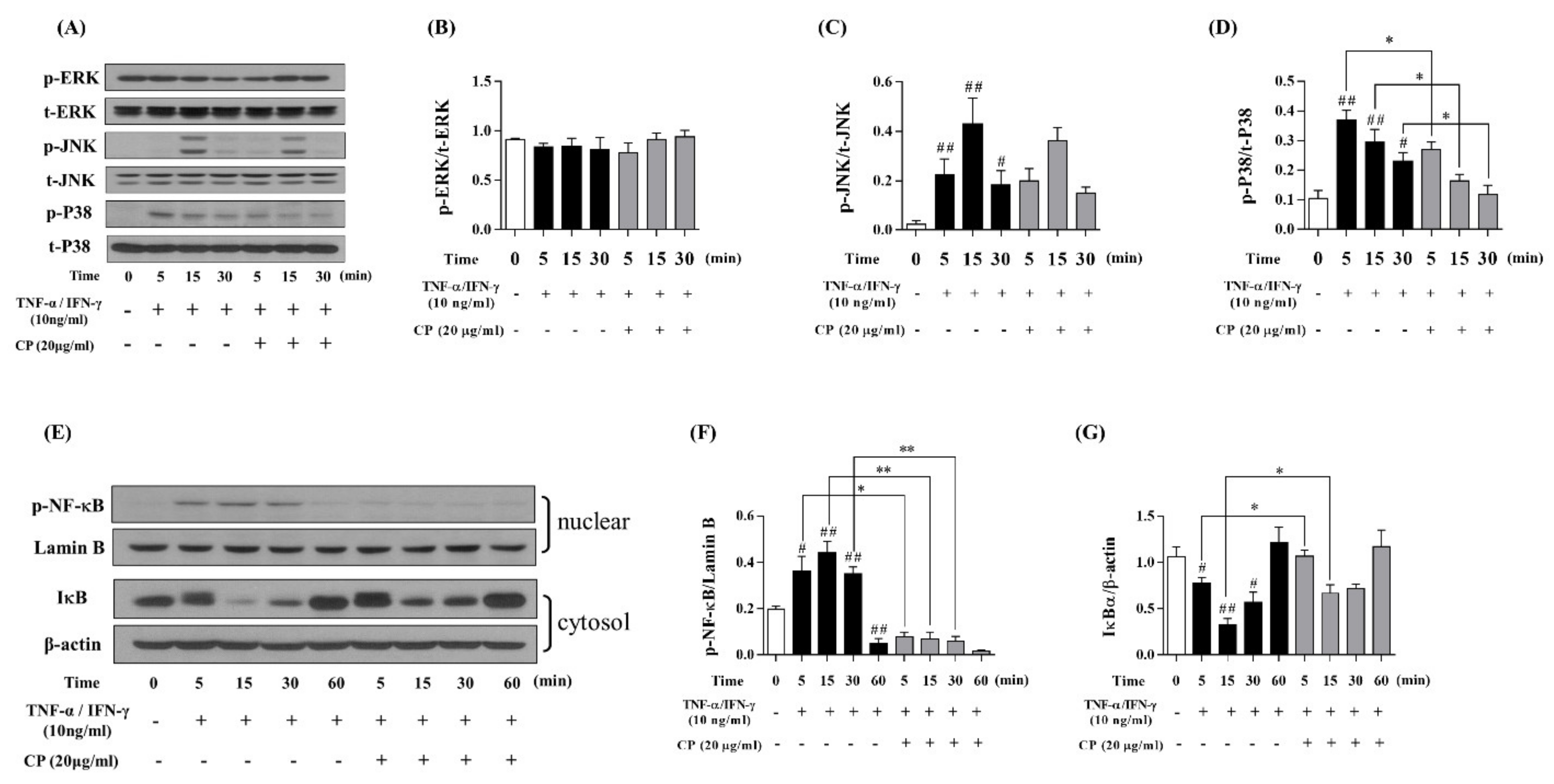
3.3. Effect of CP on the MAPKs and NF-κB/IκBα Signaling Pathway in TNF-α/IFN-γ-Stimulated HaCaT Cells
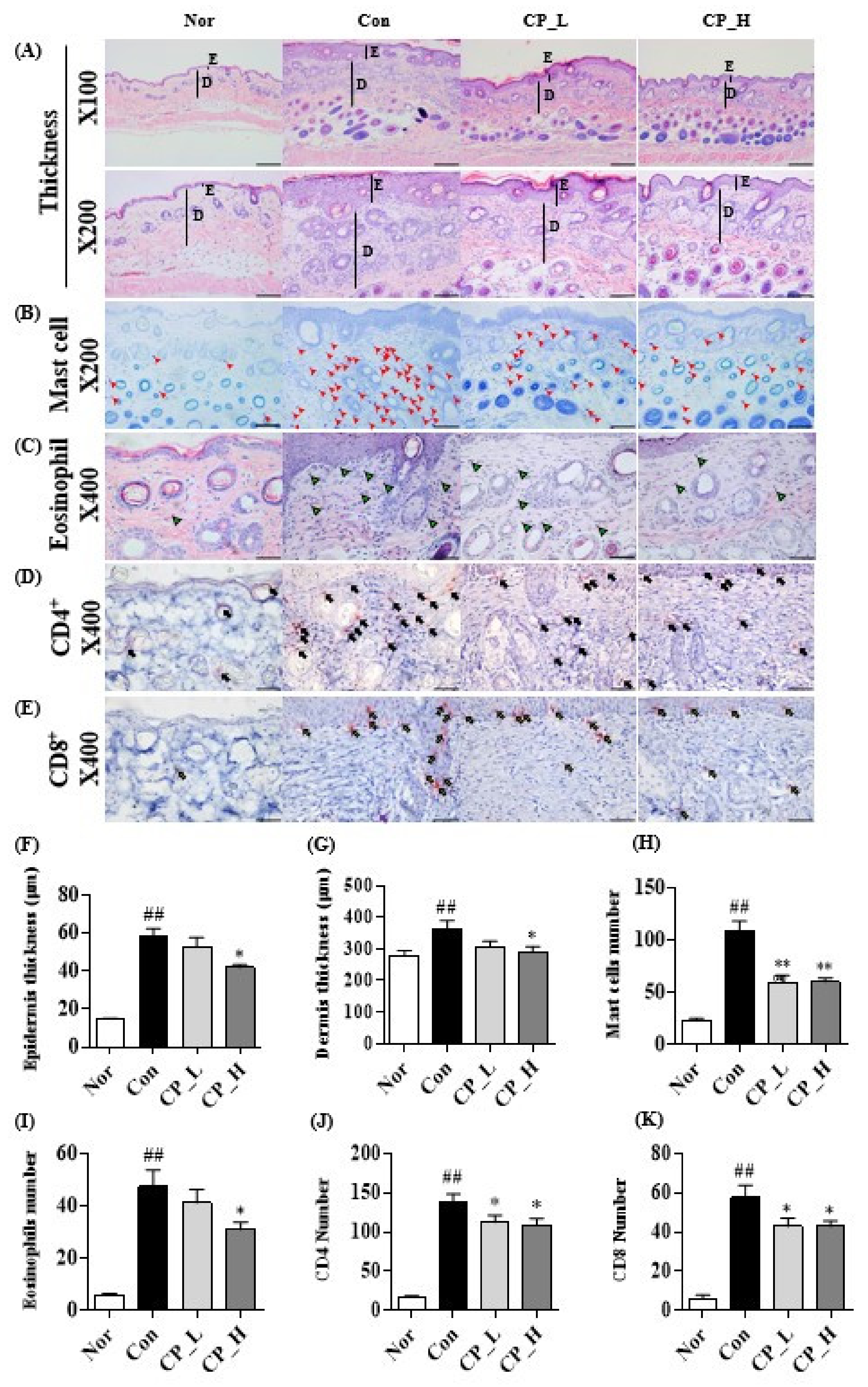
3.4. Effect of CP on DNCB-Induced ACD-Like Skin Lesion in the Balb/c Mouse Model
3.5. Effect of CP on the Epidermal or Dermal Thickness and Infiltration of Eosinophils, Mast Cells, CD4+ and CD8+ T Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avena-Woods, C. Overview of atopic dermatitis. Am. J. Manag. Care 2017, 23, S115–S123. [Google Scholar] [PubMed]
- Margolis, J.S.; Abuabara, K.; Bilker, W.; Hoffstad, O.; Margolis, D.J. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014, 150, 593–600. [Google Scholar] [CrossRef] [Green Version]
- Eckert, R.L. Structure, function, and differentiation of the keratinocyte. Physiol. Rev. 1989, 69, 1316–1346. [Google Scholar] [CrossRef]
- Esche, C.; de Benedetto, A.; Beck, L.A. Keratinocytes in atopic dermatitis: Inflammatory signals. Curr. Allergy Asthma Rep. 2004, 4, 276–284. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J. The skin: An indispensable barrier. Exp. Derm. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D. HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A review of the contribution of mast cells in wound healing: Involved molecular and cellular mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Yang-lin, H.; Gao, W.; Hong-ying, L.; Tang, J. The role of the mast cell in skin aging. J. Derm. Res. Ther. 2016, 2, 035. [Google Scholar] [CrossRef]
- Yang, H.; Liu, X.; Shen, Z.; Yao, W.; Gong, X.; Huang, H.; Ding, G. An investigation of the distribution and location of mast cells affected by the stiffness of substrates as a mechanical niche. Int. J. Biol. Sci. 2018, 14, 1142. [Google Scholar] [CrossRef]
- Marshall, J.S.; Portales-Cervantes, L.; Leong, E. Mast cell responses to viruses and pathogen products. Int. J. Mol. Sci. 2019, 20, 4241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metz, M.; Siebenhaar, F.; Maurer, M. Mast cell functions in the innate skin immune system. Immunobiology 2008, 213, 251–260. [Google Scholar] [CrossRef]
- Indra, A.K. Epidermal TSLP: A trigger factor for pathogenesis of atopic dermatitis. Expert Rev. Proteom. 2013, 10, 309–311. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Liu, Y.; Arima, K. Cellular and molecular mechanisms of TSLP function in human allergic disorders-TSLP programs the “Th2 code” in dendritic cells. Allergol. Int. 2012, 61, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A. Human epithelial cells trigger dendritic cell-mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, C.; Deleuran, M.; Gesser, B.; Larsen, C.G. Thymus-and activation-regulated chemokine (TARC/CCL17) induces a Th2-dominated inflammatory reaction on intradermal injection in mice. Exp. Derm. 2004, 13, 265–271. [Google Scholar] [CrossRef]
- Nakamura, K.; Williams, I.R.; Kupper, T.S. Keratinocyte-derived monocyte chemoattractant protein 1 (MCP-1): Analysis in a transgenic model demonstrates MCP-1 can recruit dendritic and Langerhans cells to skin. J. Investig. Derm. 1995, 105, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Theoharides, T.C.; Alysandratos, K.; Angelidou, A.; Delivanis, D.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A. Mast cells and inflammation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Kaburagi, Y.; Shimada, Y.; Nagaoka, T.; Hasegawa, M.; Takehara, K.; Sato, S. Enhanced production of CC-chemokines (RANTES, MCP-1, MIP-1α, MIP-1β, and eotaxin) in patients with atopic dermatitis. Arch. Derm. Res. 2001, 293, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Jessup, H.K.; Brewer, A.W.; Omori, M.; Rickel, E.A.; Budelsky, A.L.; Yoon, B.P.; Ziegler, S.F.; Comeau, M.R. Intradermal administration of thymic stromal lymphopoietin induces a T cell-and eosinophil-dependent systemic Th2 inflammatory response. J. Immunol. 2008, 181, 4311–4319. [Google Scholar] [CrossRef] [Green Version]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Finotto, S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011, 22, 83–89. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.H.; Roberts, A.I.; Das, J.; Xu, G.; Ren, G.; Zhang, Y.; Zhang, L.; Yuan, Z.R.; Tan, H.S.W. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: What we do and don’t know. Cell Res. 2006, 16, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Giustizieri, M.L.; Mascia, F.; Frezzolini, A.; De Pità, O.; Chinni, L.M.; Giannetti, A.; Girolomoni, G.; Pastore, S. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. J. Allergy Clin. Immunol. 2001, 107, 871–877. [Google Scholar] [CrossRef]
- Varricchi, G.; Rossi, F.W.; Galdiero, M.R.; Granata, F.; Criscuolo, G.; Spadaro, G.; de Paulis, A.; Marone, G. Physiological roles of mast cells: Collegium internationale allergologicum update. Int. Arch. Allergy Immunol. 2019, 179, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.Z.M.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, H.; Chan, L.S. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. Jak-Stat 2013, 2, e24137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Xue, F.; Qin, H.; Chen, X.; Liu, N.; Fleming, C.; Hu, X.; Zhang, H.; Chen, F.; Zheng, J. Differential roles of the mTOR-STAT3 signaling in dermal γδ T cell effector function in skin inflammation. Cell Rep. 2019, 27, 3034–3048.e5. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Ren, M.; Yang, C.; Hu, Y.; Ran, J.; Yan, L. Involvement of RAGE, MAPK and NF-κB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp. Derm. 2012, 21, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.T.; Stephens, L.R. PI3K signalling in inflammation. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 882–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef] [Green Version]
- Krens, S.G.; Spaink, H.P.; Snaar-Jagalska, B.E. Functions of the MAPK family in vertebrate-development. FEBS Lett. 2006, 580, 4984–4990. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.T.; Kudo, N.; Yoshida, M.; Miyamoto, S. A nuclear export signal in the N-terminal regulatory domain of IκBα controls cytoplasmic localization of inactive NF-κB/IκBα complexes. Proc. Natl. Acad. Sci. USA 2000, 97, 1014–1019. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Jang, T.W.; Nam, S.H.; Park, J.H. Antioxidant activity and inhibitory effect on oxidative DNA damage of ethyl acetate fractions extracted from cone of red pine (Pinus densiflora). Korean J. Plant. Resour. 2016, 29, 163–170. [Google Scholar] [CrossRef]
- Velmurugan, P.; Park, J.; Lee, S.; Jang, J.; Lee, K.; Han, S.; Lee, S.; Cho, M.; Oh, B. Synthesis and characterization of nanosilver with antibacterial properties using Pinus densiflora young cone extract. J. Photochem. Photobiol. B Biol. 2015, 147, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.H.; Hwang, I.S.; Kim, J.E.; Lee, Y.J.; Kwak, M.H.; Lee, Y.H.; Lee, J.H.; Hwang, D.Y.; Jung, Y.J. Anti-bacterial effects of aqueous extract purified from the immature cone of red pine (Pinus densiflora). Text. Coloration Finish. 2014, 26, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Jang, T.; Choi, J.; Mun, J.; Park, J. Inhibitory Effects of Pine Cone (Pinus densiflora) on Melanogenesis in B16F10 Melanoma Cells. Plant. Resour. Soc. Korea 2019, 32, 275–281. [Google Scholar]
- Lee, A.R.; Roh, S.; Lee, E.; Min, Y.; Lee, A.R.; Roh, S.; Lee, E.; Min, Y. Anti-oxidant and anti-melanogenic activity of the methanol extract of pine cone. Asian J. Beauty Cosmetol. 2016, 14, 301–308. [Google Scholar] [CrossRef]
- Ha, T.K.Q.; Lee, B.W.; Nguyen, N.H.; Cho, H.M.; Venkatesan, T.; Doan, T.P.; Kim, E.; Oh, W.K. Antiviral Activities of Compounds Isolated from Pinus densiflora (Pine Tree) against the Influenza A Virus. Biomolecules 2020, 10, 711. [Google Scholar] [CrossRef]
- Guo, D.; Chen, J.; Tan, L.; Jin, M.; Ju, F.; Cao, Z.; Deng, F.; Wang, L.; Gu, Y.; Deng, Y. Terpene glycosides from Sanguisorba officinalis and their anti-inflammatory effects. Molecules 2019, 24, 2906. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Burgos, E.; Gómez-Serranillos, M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef]
- Han, Y. Ginkgo terpene component has an anti-inflammatory effect on Candida albicans-caused arthritic inflammation. Int. Immunopharmacol. 2005, 5, 1049–1056. [Google Scholar] [CrossRef]
- Peng, J.; Li, K.; Zhu, W.; Nie, R.; Wang, R.; Li, C. Penta-O-galloyl-β-d-glucose, a hydrolysable tannin from Radix Paeoniae Alba, inhibits adipogenesis and TNF-α-mediated inflammation in 3T3-L1 cells. Chem. Biol. Interact. 2019, 302, 156–163. [Google Scholar] [CrossRef]
- Kim, H.J.; Baek, J.; Lee, J.R.; Roh, J.Y.; Jung, Y. Optimization of cytokine milieu to reproduce atopic dermatitis-related gene expression in HaCaT keratinocyte cell line. Immune Netw. 2018, 18. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal models of atopic dermatitis. J. Investig. Derm. 2009, 129, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.; Pfeiffer, S.; Witt, M.; Bräutigam, M.; Neumann, C.; Weichenthal, M.; Schwarz, T.; Fölster-Holst, R.; Proksch, E. Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2009, 124, R19–R28. [Google Scholar] [CrossRef] [PubMed]
- Correale, C.E.; Walker, C.; Murphy, L.; Craig, T.J. Atopic dermatitis: A review of diagnosis and treatment. Am. Fam. Physician 1999, 60, 1191. [Google Scholar] [PubMed]
- Simon, D.; Braathen, L.R.; Simon, H. Eosinophils and atopic dermatitis. Allergy 2004, 59, 561–570. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 402: Acute Dermal Toxicity, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2017. [Google Scholar] [CrossRef]
- Sinke, J.D.; Thepen, T.; Bihari, I.C.; Rutten, V.P.; Willemse, T. Immunophenotyping of skin-infiltrating T-cell subsets in dogs with atopic dermatitis. Vet. Immunol. Immunopathol. 1997, 57, 13–23. [Google Scholar] [CrossRef]
- Liu, X.; Bosselut, R. Duration of TCR signaling controls CD4-CD8 lineage differentiation in vivo. Nat. Immunol. 2004, 5, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Girolomoni, G.; Sebastiani, S.; Albanesi, C.; Cavani, A. T-cell subpopulations in the development of atopic and contact allergy. Curr. Opin. Immunol. 2001, 13, 733–737. [Google Scholar] [CrossRef]



| Gene Name | Orientation | Primer Sequence | Anneling Tm (°C) | Cycle | Reference |
|---|---|---|---|---|---|
| TSLP | Forward Reverse | 5′-TCC TCT GAA GAC CTG ACC-3′ 5′-TCT CCT TTC TCC CTA ATC CTC-3′ | 59.5 °C | 40 | kim et al. [45] |
| TARC | Forward Reverse | 5′-ACT GCT CCA GGG ATG CCA TCG TTT TT-3′ 5′-ACA AGG GGA TGG GAT CTC CCT CAC TG-3′ | 57.5 °C | 44 | NM_002987.3 |
| IL-6 | Forward Reverse | 5′-GAT GGC TGA AAA AGA TGG ATG C-3′ 5′-TGG TTG GGT CAG GGG TGG TT-3′ | 59 °C | 45 | NM_000600.4 |
| GAPDH | Forward Reverse | 5′-CGT CTA GAA AAA CCT GCC AA-3′ 5′-TGA AGT CAA AGG AGA CCA CC-3′ | 50 °C | 30 | NM_001256799.3 |
| Primary Antibody | Primary Antibody Dilution | System Used | Size |
|---|---|---|---|
| Phospho-ERK | 1:1000 | Western blot | 42, 44 kDa |
| Phospho-JNK | 1:1000 | Western blot | 46, 54 kDa |
| Phospho-P38 | 1:1000 | Western blot | 43 kDa |
| ERK | 1:1000 | Western blot | 42, 44 kDa |
| JNK | 1:1000 | Western blot | 46, 54 kDa |
| P38 | 1:1000 | Western blot | 43 kDa |
| Phospho-NF-κB | 1:1000 | Western blot | 65 kDa |
| IκBα | 1:1000 | Western blot | 39 kDa |
| Lamin B | 1:1000 | Western blot | 67 kDa |
| β-actin | 1:500 | Western blot | 43 kDa |
| CD4+ | 1:200 | Immunohistochemistry | - |
| CD8+ | 1:200 | Immunohistochemistry | - |
| Goat-anti-Rabbit IgE | 1:10,000 | Western blot | - |
| Goat-anti-mouse-IgE | 1:10,000 | Western blot | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, B.; Hong, S.Y.; Kim, E.-Y.; Kim, J.-H.; Kim, M.; Park, J.H.; Sohn, Y.; Jung, H.-S. Effect of Cone of Pinus densiflora on DNCB-Induced Allergic Contact Dermatitis-Like Skin Lesion in Balb/c Mice. Nutrients 2021, 13, 839. https://doi.org/10.3390/nu13030839
Kwon B, Hong SY, Kim E-Y, Kim J-H, Kim M, Park JH, Sohn Y, Jung H-S. Effect of Cone of Pinus densiflora on DNCB-Induced Allergic Contact Dermatitis-Like Skin Lesion in Balb/c Mice. Nutrients. 2021; 13(3):839. https://doi.org/10.3390/nu13030839
Chicago/Turabian StyleKwon, Boguen, Soo Yeon Hong, Eun-Young Kim, Jae-Hyun Kim, Minsun Kim, Jae Ho Park, Youngjoo Sohn, and Hyuk-Sang Jung. 2021. "Effect of Cone of Pinus densiflora on DNCB-Induced Allergic Contact Dermatitis-Like Skin Lesion in Balb/c Mice" Nutrients 13, no. 3: 839. https://doi.org/10.3390/nu13030839
APA StyleKwon, B., Hong, S. Y., Kim, E.-Y., Kim, J.-H., Kim, M., Park, J. H., Sohn, Y., & Jung, H.-S. (2021). Effect of Cone of Pinus densiflora on DNCB-Induced Allergic Contact Dermatitis-Like Skin Lesion in Balb/c Mice. Nutrients, 13(3), 839. https://doi.org/10.3390/nu13030839








