The Potential Role of Creatine in Vascular Health
Abstract
1. Introduction
2. Combatting the Development of Vascular Pathology
3. Existing Research on the Effects of Creatine Supplementation and Vascular Health
3.1. Creatine and the Macrovasculature
3.2. Creatine and the Microvasculature
4. The Presence and Function of Creatine within the Vascular Endothelium
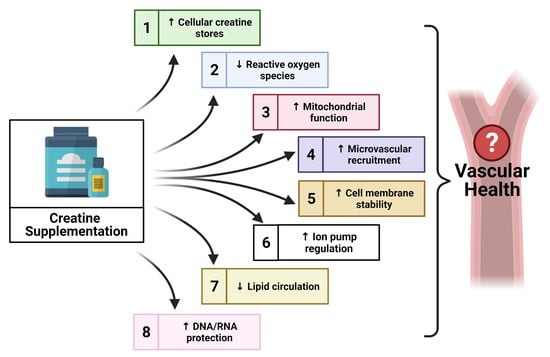
5. Possible Application of Creatine for the Promotion of Vascular Health
5.1. Creatine, Oxidative Stress and Nitric Oxide Bioavailability
5.2. Creatine and Endothelium-Derived Hyperpolarization Factors
5.3. Creatine, Endothelial Cell Integrity, and Inflammation
5.4. Creatine and Vascular DNA/RNA Protection
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update: A Report from the American Heart Association. Circulation 2020, E139–E596. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; De Ferranti, S.; Després, J.-P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics—2016 Update: A Report from the American Heart Association. American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the Future of Cardiovascular Disease in the United States. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Cuckler, G.A.; Sisko, A.M.; Poisal, J.A.; Keehan, S.P.; Smith, S.D.; Madison, A.J.; Wolfe, C.J.; Hardesty, J.C. National Health Expenditure Projections, 2017–2026: Despite Uncertainty, Fundamentals Primarily Drive Spending Growth. Health Aff. 2018, 37, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Celano, C.M.; Huffman, J.C. Depression and Cardiac Disease. Cardiol. Rev. 2011, 19, 130–142. [Google Scholar] [CrossRef]
- Stewart, J.C.; Rollman, B.L. Optimizing Approaches to Addressing Depression in Cardiac Patients: A Comment on O’Neil et al. Ann. Behav. Med. 2014, 48, 142–144. [Google Scholar] [CrossRef][Green Version]
- De Leon, C.F.M.; Krumholz, H.M.; Vaccarino, V.; Williams, C.S.; Glass, T.A.; Berkman, L.F.; Kas, S.V. A population-based perspective of changes in health-related quality of life after myocardial infarction in older men and women. J. Clin. Epidemiol. 1998, 51, 609–616. [Google Scholar] [CrossRef]
- Smith, R.N.; Agharkar, A.S.; Gonzales, E.B. A review of creatine supplementation in age-related diseases: More than a supplement for athletes. F1000Research 2014, 3, 222. [Google Scholar] [CrossRef]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.D. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lemus, L.A. The Dynamic Structure of Arterioles. Basic Clin. Pharmacol. Toxicol. 2011, 110, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Herring, N.; Paterson, D.J. Levick’s Introduction to Cardiovascular Physiology, 6th ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pract. 2014, 2014, 291–308. [Google Scholar] [CrossRef]
- Kohn, J.C.; Lampi, M.C.; Reinhart-King, C.A. Age-related vascular stiffening: Causes and consequences. Front. Genet. 2015, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Schwartz, B.G.; Economides, C.; Mayeda, G.S.; Burstein, S.; A Kloner, R. The endothelial cell in health and disease: Its function, dysfunction, measurement and therapy. Int. J. Impot. Res. 2009, 22, 77–90. [Google Scholar] [CrossRef]
- Barthelmes, J.; Nägele, M.P.; Ludovici, V.; Ruschitzka, F.; Sudano, I.; Flammer, A.J. Endothelial dysfunction in cardiovascular disease and Flammer syndrome—Similarities and differences. EPMA J. 2017, 8, 99–109. [Google Scholar] [CrossRef]
- Favero, G.; Paganelli, C.; Buffoli, B.; Rodella, L.F.; Rezzani, R. Endothelium and Its Alterations in Cardiovascular Diseases: Life Style Intervention. BioMed Res. Int. 2014, 2014, 801896 . [Google Scholar] [CrossRef]
- Henderson, K.K.; Byron, K.L. Vasopressin-induced vasoconstriction: Two concentration-dependent signaling pathways. J. Appl. Physiol. 2007, 102, 1402–1409. [Google Scholar] [CrossRef]
- Ye, G.J.; Nesmith, A.P.; Parker, K.K. The Role of Mechanotransduction on Vascular Smooth Muscle Myocytes Cytoskeleton and Contractile Function. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2014, 297, 1758–1769. [Google Scholar] [CrossRef]
- Thomas, G.D. Neural control of the circulation. Adv. Physiol. Educ. 2011, 35, 28–32. [Google Scholar] [CrossRef]
- Pugsley, M.K.; Tabrizchi, R. The vascular system. An overview of structure and function. J. Pharmacol. Toxicol. Methods 2000, 44, 333–340. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Castellon, X.; Bogdanova, V. Chronic Inflammatory Diseases and Endothelial Dysfunction. Aging Dis. 2016, 7, 81–89. [Google Scholar] [CrossRef]
- Kim, J.-A.; Montagnani, M.; Chandrasekran, S.; Quon, M.J. Role of Lipotoxicity in Endothelial Dysfunction. Heart Fail. Clin. 2012, 8, 589–607. [Google Scholar] [CrossRef]
- Popov, D. Endothelial cell dysfunction in hyperglycemia: Phenotypic change, intracellular signaling modification, ultrastructural alteration, and potential clinical outcomes. Int. J. Diabetes Mellit. 2010, 2, 189–195. [Google Scholar] [CrossRef]
- Pittilo, R.M. Cigarette smoking, endothelial injury and cardiovascular disease. Int. J. Exp. Pathol. 2001, 81, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Cui, R.; Kitamura, A.; Liu, K.; Imano, H.; Yamagishi, K.; Kiyama, M.; Okada, T.; Iso, H.; CIRCS Investigators. Heavy Alcohol Consumption is Associated with Impaired Endothelial Function: The Circulatory Risk in Communities Study (CIRCS). J. Atheroscler. Thromb. 2016, 23, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Lobato, N.; Filgueira, F.P.; Akamine, E.H.; Tostes, R.; De Carvalho, M.H.C.; Fortes, Z.B. Mechanisms of endothelial dysfunction in obesity-associated hypertension. Braz. J. Med. Biol. Res. 2012, 45, 392–400. [Google Scholar] [CrossRef]
- Fleg, J.L.; Strait, J. Age-associated changes in cardiovascular structure and function: A fertile milieu for future disease. Heart Fail. Rev. 2012, 17, 545–554. [Google Scholar] [CrossRef]
- Widlansky, M.E.; Gokce, N.; Keaney, J.F.; Vita, J.A. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003, 42, 1149–1160. [Google Scholar] [CrossRef]
- Kaiser Family Foundation. Data Note Prescription Drugs and Older Adults. Available online: https://www.kff.org/health-reform/issue-brief/data-note-prescription-drugs-and-older-adults/ (accessed on 26 December 2020).
- Council for Responsible Nutrition. 2018 Consumer Survey on Dietary Supplements. Available online: https://www.crnusa.org/sites/default/files/images/2018-survey/CRN-ConsumerSurvey-Infographic-2019f.pdf (accessed on 26 December 2020).
- Johnston, C. Functional Foods as Modifiers of Cardiovascular Disease. Am. J. Lifestyle Med. 2009, 3, 39S–43S. [Google Scholar] [CrossRef]
- Bode-Böger, S.M.; Muke, J.; Surdacki, A.; Brabant, G.; Böger, R.H.; Frölich, J.C. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc. Med. 2003, 8, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.-H.; Chen, J.-W.; Tsai, C.; Chiang, M.-C.; Young, M.S.; Lin, S.-J. L-arginine improves endothelial function and reduces LDL oxidation in patients with stable coronary artery disease. Clin. Nutr. 2005, 24, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-L.; Luk, T.-H.; Yiu, K.-H.; Wang, M.; Yip, P.M.; Lee, S.W.; Li, S.-W.; Tam, S.; Fong, B.; Lau, C.-P.; et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: A randomized controlled trial. Atherosclerosis 2011, 216, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in Cardiovascular and Metabolic Diseases: Current State of the Problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef]
- Walker, M.A.; Bailey, T.G.; McIlvenna, L.; Allen, J.D.; Green, D.J.; Askew, C.D. Acute Dietary Nitrate Supplementation Improves Flow Mediated Dilatation of the Superficial Femoral Artery in Healthy Older Males. Nutrients 2019, 11, 954. [Google Scholar] [CrossRef]
- Eskurza, I.; Myerburgh, L.A.; Kahn, Z.D.; Seals, U.R. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J. Physiol. 2005, 568, 1057–1065. [Google Scholar] [CrossRef]
- Romero, M.J.; Platt, D.H.; Caldwell, R.W. Therapeutic Use of Citrulline in Cardiovascular Disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef]
- Fusco, D.; Colloca, G.; Monaco, M.R.L.; Cesari, M. Effects of antioxidant supplementation on the aging process. Clin. Interv. Aging 2007, 2, 377–387. [Google Scholar] [PubMed]
- Durante, A.; Bronzato, S. Dietary supplements and cardiovascular diseases. Int. J. Prev. Med. 2018, 9, 80. [Google Scholar] [CrossRef]
- Sosnowska, B.; Penson, P.; Banach, M. The role of nutraceuticals in the prevention of cardiovascular disease. Cardiovasc. Diagn. Ther. 2017, 67, S21–S31. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.; Greenwood, M.; Kalman, D.; Antonio, J. Nutritional Supplements for Endurance Athletes. In Nutritional Supplements in Sports and Exercise; Humana Press: Totowa, NJ, USA, 2008; pp. 369–407. [Google Scholar]
- Hultman, E.; Soderlund, K.; Timmons, J.A.; Cederblad, G.; Greenhaff, P.L. Muscle creatine loading in men. J. Appl. Physiol. 1996, 81, 232–237. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 1–18. [Google Scholar] [CrossRef]
- Jäger, R.; Purpura, M.; Shao, A.; Inoue, T.; Kreider, R.B. Analysis of the efficacy, safety, and regulatory status of novel forms of creatine. Amino Acids 2011, 40, 1369–1383. [Google Scholar] [CrossRef]
- Cooper, R.; Naclerio, F.; Allgrove, J.; Jimenez, A. Creatine supplementation with specific view to exercise/sports performance: An update. J. Int. Soc. Sports Nutr. 2012, 9, 33. [Google Scholar] [CrossRef]
- Lawler, J.M.; Barnes, W.S.; Wu, G.; Song, W.; Demaree, S. Direct Antioxidant Properties of Creatine. Biochem. Biophys. Res. Commun. 2002, 290, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Sestili, P.; Martinelli, C.; Colombo, E.; Barbieri, E.; Potenza, L.; Sartini, S.; Fimognari, C. Creatine as an antioxidant. Amino Acids 2011, 40, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Nomura, A.; Zhang, M.; Sakamoto, T.; Ishii, Y.; Morishima, Y.; Mochizuki, M.; Kimura, T.; Uchida, Y.; Sekizawa, K. Anti-inflammatory activity of creatine supplementation in endothelial cells in vitro. Br. J. Pharmacol. 2003, 139, 715–720. [Google Scholar] [CrossRef]
- De Moraes, R.; Van Bavel, D.; De Moraes, B.S.; Tibiriçá, E. Effects of dietary creatine supplementation on systemic microvascular density and reactivity in healthy young adults. Nutr. J. 2014, 13, 115. [Google Scholar] [CrossRef]
- Van Bavel, D.; De Moraes, R.; Tibirica, E. Effects of dietary supplementation with creatine on homocysteinemia and systemic microvascular endothelial function in individuals adhering to vegan diets. Fundam. Clin. Pharmacol. 2019, 33, 428–440. [Google Scholar] [CrossRef]
- Pinto, C.L.; Botelho, P.B.; Pimentel, G.D.; Campos-Ferraz, P.L.; Mota, J.F. Creatine supplementation and glycemic control: A systematic review. Amino Acids 2016, 48, 2103–2129. [Google Scholar] [CrossRef]
- Meyer, L.E.; Machado, L.B.; Santiago, A.P.S.; Da-Silva, W.S.; De Felice, F.G.; Holub, O.; Oliveira, M.F.; Galina, A. Mitochondrial Creatine Kinase Activity Prevents Reactive Oxygen Species Generation. J. Biol. Chem. 2006, 281, 37361–37371. [Google Scholar] [CrossRef]
- Gualano, B.; Painelli, V.D.S.; Roschel, H.; Lugaresi, R.; Dorea, E.; Artioli, G.G.; Lima, F.R.; Da Silva, M.E.R.; Cunha, M.R.; Seguro, A.C.; et al. Creatine supplementation does not impair kidney function in type 2 diabetic patients: A randomized, double-blind, placebo-controlled, clinical trial. Graefe Arch. Clin. Exp. Ophthalmol. 2010, 111, 749–756. [Google Scholar] [CrossRef]
- Tarnopolsky, M.; Martin, J. Creatine monohydrate increases strength in patients with neuromuscular disease. Neurology 1999, 52, 854. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Mahoney, D.J.; Vajsar, J.; Rodriguez, C.; Doherty, T.J.; Roy, B.D.; Biggar, D. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology 2004, 62, 1771–1777. [Google Scholar] [CrossRef]
- Matthews, R.T.; Yang, L.; Jenkins, B.G.; Ferrante, R.J.; Rosen, B.R.; Kaddurah-Daouk, R.; Beal, M.F. Neuroprotective Effects of Creatine and Cyclocreatine in Animal Models of Huntington’s Disease. J. Neurosci. 1998, 18, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.T.; Ferrante, R.J.; Klivenyia, P.; Yanga, L.; Klein, A.M.; Muellera, G.; Daoukc, R.K.; Beala, M.F. Creatine and Cyclocreatine Attenuate MPTP Neurotoxicity. Exp. Neurol. 1999, 157, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, P.; Yu, Z.; Cong, Y.; Sun, H.; Zhang, J.; Zhang, J.; Sun, C.; Zhang, Y.; Ju, X. The effect of creatine and coenzyme q10 combination therapy on mild cognitive impairment in Parkinson’s disease. Eur. Neurol. 2015, 73, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Persky, A.M.; Rawson, E.S. Safety of Creatine Supplementation. Cholest. Bind. Cholest. Transp. Proteins 2007, 46, 275–289. [Google Scholar] [CrossRef]
- Korzun, W.J. Oral creatine supplements lower plasma homocysteine concentrations in humans. Am. Soc. Clin. Lab. Sci. 2004, 17, 102–106. [Google Scholar]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef]
- Willerson, J.T. Inflammation as a Cardiovascular Risk Factor. Circulation 2004, 109, II-2. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.S.; Law, M.; Morris, J.K. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ 2002, 325, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Hannibal, N.S.; Nindl, B.C.; Gentile, C.L.; Hamed, J.; Vukovich, M.D. Comparison of creatine ingestion and resistance training on energy expenditure and limb blood flow. Metabolism 2001, 50, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, M.A.; Wieder, R.; Kim, J.-S.; Vicil, F.; Figueroa, A. Creatine supplementation attenuates hemodynamic and arterial stiffness responses following an acute bout of isokinetic exercise. Graefe Arch. Clin. Exp. Ophthalmol. 2011, 111, 1965–1971. [Google Scholar] [CrossRef]
- Englund, D.A.; Kirn, D.R.; Koochek, A.; Zhu, H.; Travison, T.G.; Reid, K.F.; Von Berens, Å.; Melin, M.; Cederholm, T.; Gustafsson, T.; et al. Nutritional Supplementation With Physical Activity Improves Muscle Composition in Mobility-Limited Older Adults, The VIVE2 Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2018, 73, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Dawson, A.; Shaw, S.C.; Harvey, N.C.; Kanis, J.A.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.C.; Bruyère, O.; et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos. Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Cui, J.; Mascarenhas, V.; Moradkhan, R.; Blaha, C.; Sinoway, L.I. Effects of muscle metabolites on responses of muscle sympathetic nerve activity to mechanoreceptor(s) stimulation in healthy humans. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R458–R466. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-Rate Recovery Immediately after Exercise as a Predictor of Mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Söderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 1992, 83, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.H. Hyperlipidemia as a Risk Factor for Cardiovascular Disease. Prim. Care Clin. Off. Pract. 2013, 40, 195–211. [Google Scholar] [CrossRef]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Raddino, R.; Carretta, G.; Teli, M.; Bonadei, I.; Robba, D.; Zanini, G.; Madureri, A.; Nodari, S.; Cas, L.D. Nitric oxide and cardiovascular risk factors. Heart Int. 2007, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Sverdlov, A.L.; Ngo, D.T.; Chan, W.P.; Chirkov, Y.Y.; Horowitz, J.D. Aging of the Nitric Oxide System: Are We as Old as Our NO? J. Am. Heart Assoc. 2014, 3, e000973. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Kaviani, M.; Shaw, K.; Chilibeck, P.D. Benefits of Creatine Supplementation for Vegetarians Compared to Omnivorous Athletes: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 3041. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Vasan, R.S. Age as a Risk Factor. Med. Clin. N. Am. 2012, 96, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. The aging process: Major risk factor for disease and death. Proc. Natl. Acad. Sci. USA 1991, 88, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Snow, R.J.; Murphy, R.M. Creatine and the creatine transporter: A review. Mol. Cell. Biochem. 2001, 224, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Peral, M.J.; García-Delgado, M.; Calonge, M.L.; Durán, J.M.; De La Horra, M.C.; Wallimann, T.; Speer, O.; Ilundáin, A.A. Human, rat and chicken small intestinal Na+-Cl−-creatine transporter: Functional, molecular characterization and localization. J. Physiol. 2002, 545, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Thali, R.F.; Smolak, C.; Gong, F.; Alzamora, R.; Wallimann, T.; Scholz, R.; Pastor-Soler, N.M.; Neumann, D.; Hallows, K.R. Regulation of the creatine transporter by AMP-activated protein kinase in kidney epithelial cells. Am. J. Physiol. Physiol. 2010, 299, F167–F177. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O. Creatine and guanidinoacetate transport at blood-brain and blood-cerebrospinal fluid barriers. J. Inherit. Metab. Dis. 2012, 35, 655–664. [Google Scholar] [CrossRef]
- Decking, U.K.M.; Alves, C.; Wallimann, T.; Wyss, M.; Schrader, J. Functional aspects of creatine kinase isoenzymes in endothelial cells. Am. J. Physiol. Physiol. 2001, 281, C320–C328. [Google Scholar] [CrossRef]
- Wallimann, T.; Dolder, M.; Schlattner, U.; Eder, M.; Hornemann, T.; O’Gorman, E.; Rück, A.; Brdiczka, D. Some new aspects of creatine kinase (CK): Compartmentation, structure, function and regulation for cellular and mitochondrial bioenergetics and physiology. BioFactors 1998, 8, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Persky, A.M.; A Brazeau, G. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol. Rev. 2001, 53, 161–176. [Google Scholar] [PubMed]
- Wallimann, T.; Wyss, M.; Brdiczka, D.; Nicolay, K.; Eppenberger, H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 1992, 281, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Fitch, C.D.; Shields, R.P. Creatine metabolism in skeletal muscle. I. Creatine movement across muscle membranes. J. Biol. Chem. 1966, 241, 3611–3614. [Google Scholar] [CrossRef]
- Guerrero-Ontiveros, M.L.; Wallimann, T. Creatine supplementation in health and disease. Effects of chronic creatine ingestion in vivo: Down-regulation of the expression of creatine transporter isoforms in skeletal muscle. Mol. Cell. Biochem. 1998, 184, 427–437. [Google Scholar] [CrossRef]
- Loike, J.D.; Cao, L.; Brett, J.; Ogawa, S.; Silverstein, S.C.; Stern, D. Hypoxia induces glucose transporter expression in endothelial cells. Am. J. Physiol. Physiol. 1992, 263, C326–C333. [Google Scholar] [CrossRef]
- Sestili, P.; Martinelli, C.; Bravi, G.; Piccoli, G.; Curci, R.; Battistelli, M.; Falcieri, E.; Agostini, D.; Gioacchini, A.M.; Stocchi, V. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic. Biol. Med. 2006, 40, 837–849. [Google Scholar] [CrossRef]
- Gualano, B.; Artioli, G.G.; Poortmans, J.R.; Junior, A.H.L. Exploring the therapeutic role of creatine supplementation. Amino Acids 2009, 38, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Roschel, H.; Lancha, A.H., Jr.; Brightbill, C.E.; Rawson, E.S. In sickness and in health: The widespread application of creatine supplementation. Amino Acids 2011, 43, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Kandola, K.; Bowman, A.; Birch-Machin, M.A. Oxidative stress—A key emerging impact factor in health, ageing, lifestyle and aesthetics. Int. J. Cosmet. Sci. 2015, 37, 1–8. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J. Decreased blood antioxidant capacity and increased lipid peroxidation in young cigarette smokers compared to nonsmokers: Impact of dietary intake. Nutr. J. 2007, 6, 39. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, R.; Araya, H.; Navarro-Lisboa, R.; De Dicastillo, C.L. Evaluation of Polyphenol Content and Antioxidant Capacity of Fruits and Vegetables Using a Modified Enzymatic Extraction. Food Technol. Biotechnol. 2016, 54, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J.; Lowe, G.L. Carotenoids—Antioxidant Properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Djuric, Z.; Powell, L. Antioxidant capacity of lycopene-containing foods. Int. J. Food Sci. Nutr. 2001, 52, 143–149. [Google Scholar] [CrossRef]
- Lara-Padilla, E.; Kormanovski, A.; Grave, P.A.; Olivares-Corichi, I.M.; Santillan, R.M.; Hicks, J.J. Increased antioxidant capacity in healthy volunteers taking a mixture of oral antioxidants versus vitamin C or E supplementation. Adv. Ther. 2007, 24, 50–59. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. IJBS 2008, 4, 89–96. [Google Scholar]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Frei, B. Efficacy of Dietary Antioxidants to Prevent Oxidative Damage and Inhibit Chronic Disease. J. Nutr. 2004, 134, 3196S–3198S. [Google Scholar] [CrossRef]
- Temple, N.J. Antioxidants and disease: More questions than answers. Nutr. Res. 2000, 20, 449–459. [Google Scholar] [CrossRef]
- Fimognari, C.; Sestili, P.; Lenzi, M.; Cantelli-Forti, G.; Hrelia, P. Protective effect of creatine against RNA damage. Mutat. Res. Mol. Mech. Mutagen. 2009, 670, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Rambo, L.M.; Ribeiro, L.R.; Oliveira, M.S.; Furian, A.F.; Lima, F.D.; Souza, M.A.; Silva, L.F.A.; Retamoso, L.T.; Corte, C.L.D.; Puntel, G.O.; et al. Additive anticonvulsant effects of creatine supplementation and physical exercise against pentylenetetrazol-induced seizures. Neurochem. Int. 2009, 55, 333–340. [Google Scholar] [CrossRef]
- Sestili, P.; Barbieri, E.; Martinelli, C.; Battistelli, M.; Guescini, M.; Vallorani, L.; Casadei, L.; D’Emilio, A.; Falcieri, E.; Piccoli, G.; et al. Creatine supplementation prevents the inhibition of myogenic differentiation in oxidatively injured C2C12 murine myoblasts. Mol. Nutr. Food Res. 2009, 53, 1187–1204. [Google Scholar] [CrossRef]
- Hosamani, R.; Ramesh, S.R.; Muralidhara. Attenuation of Rotenone-Induced Mitochondrial Oxidative Damage and Neurotoxicty in Drosophila melanogaster Supplemented with Creatine. Neurochem. Res. 2010, 35, 1402–1412. [Google Scholar] [CrossRef]
- Wong, H.-S.; Dighe, P.A.; Mezera, V.; Monternier, P.-A.; Brand, M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017, 292, 16804–16809. [Google Scholar] [CrossRef]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef]
- Barbieri, E.; Guescini, M.; Calcabrini, C.; Vallorani, L.; Diaz, A.R.; Fimognari, C.; Canonico, B.; Luchetti, F.; Papa, S.; Battistelli, M.; et al. Creatine Prevents the Structural and Functional Damage to Mitochondria in Myogenic, Oxidatively Stressed C2C12 Cells and Restores Their Differentiation Capacity. Oxidative Med. Cell. Longev. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Rahimi, R. Creatine Supplementation Decreases Oxidative DNA Damage and Lipid Peroxidation Induced by a Single Bout of Resistance Exercise. J. Strength Cond. Res. 2011, 25, 3448–3455. [Google Scholar] [CrossRef] [PubMed]
- Gracia, K.C.; Llanas-Cornejo, D.; Husi, H. CVD and Oxidative Stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zuo, L. Redox Roles of Reactive Oxygen Species in Cardiovascular Diseases. Int. J. Mol. Sci. 2015, 16, 27770–27780. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative Stress and Vascular Disease. Arter. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nat. Cell Biol. 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.-M.; Papageorgiou, C.T.N.; Stefanadis, C. The Role of Nitric Oxide on Endothelial Function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Rees, D.D.; Palmer, R.M.; Moncada, S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc. Natl. Acad. Sci. USA 1989, 86, 3375–3378. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Ye, Z.-X.; Wang, X.-F.; Chang, J.; Yang, M.-W.; Zhong, H.-H.; Hong, F.-F.; Yang, S.-L. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed. Pharmacother. 2018, 97, 423–428. [Google Scholar] [CrossRef]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial Dysfunction: Cardiovascular Risk Factors, Therapy, and Outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Kingwell, B.A.; Formosa, M.; Muhlmann, M.; Bradley, S.J.; McConell, G.K. Nitric oxide synthase inhibition reduces glucose uptake during exercise in individuals with type 2 diabetes more than in control subjects. Diabetes 2002, 51, 2572–2580. [Google Scholar] [CrossRef]
- Wilkinson, I.B.; Maccallum, H.; Cockcroft, J.R.; Webb, D.J. Inhibition of basal nitric oxide synthesis increases aortic augmentation index and pulse wave velocity in vivo. Br. J. Clin. Pharmacol. 2002, 53, 189–192. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, G.; Chen, Z.-Q.; Mou, R.-T.; Feng, D.-X. The role of nitric oxide in stroke. Med. Gas Res. 2017, 7, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Giannitsi, S.; Bougiakli, M.; Bechlioulis, A.; Naka, K.; Mpougiaklh, M. Endothelial dysfunction and heart failure: A review of the existing bibliography with emphasis on flow mediated dilation. JRSM Cardiovasc. Dis. 2019, 8, 2048004019843047. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Huie, R.E.; Padmaja, S. The Reaction of no With Superoxide. Free Radic. Res. Commun. 1993, 18, 195–199. [Google Scholar] [CrossRef]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef]
- Wadley, A.J.; Van Zanten, J.J.C.S.V.; Aldred, S. The interactions of oxidative stress and inflammation with vascular dysfunction in ageing: The vascular health triad. AGE 2012, 35, 705–718. [Google Scholar] [CrossRef]
- Dupont, J.J.; Ramick, M.G.; Farquhar, W.B.; Townsend, R.R.; Edwards, D.G. NADPH oxidase-derived reactive oxygen species contribute to impaired cutaneous microvascular function in chronic kidney disease. Am. J. Physiol. Physiol. 2014, 306, F1499–F1506. [Google Scholar] [CrossRef]
- Baylis, C. Nitric oxide deficiency in chronic kidney disease. Am. J. Physiol. Physiol. 2008, 294, F1–F9. [Google Scholar] [CrossRef]
- Łuczak, A.; Madej, M.; Kasprzyk, A.; Doroszko, A. Role of the eNOS Uncoupling and the Nitric Oxide Metabolic Pathway in the Pathogenesis of Autoimmune Rheumatic Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 1417981. [Google Scholar] [CrossRef]
- Crabtree, M.J.; Channon, K.M. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide 2011, 25, 81–88. [Google Scholar] [CrossRef]
- Karbach, S.; Wenzel, P.; Waisman, A.; Munzel, T.; Daiber, A. eNOS Uncoupling in Cardiovascular Diseases—The Role of Oxidative Stress and Inflammation. Curr. Pharm. Des. 2014, 20, 3579–3594. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Ulrich, F.; Huige, L. Implication of eNOS Uncoupling in Cardiovascular Disease. React. Oxyg. Species 2017, 3, 38–46. [Google Scholar]
- Ahsan, A.; Han, G.; Pan, J.; Liu, S.; Padhiar, A.A.; Chu, P.; Sun, Z.; Zhang, Z.; Sun, B.; Wu, J.; et al. Phosphocreatine protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Apoptosis 2015, 20, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Varadharaj, S.; Kelly, O.J.; Khayat, R.N.; Kumar, P.S.; Ahmed, N.; Zweier, J.L. Role of Dietary Antioxidants in the Preservation of Vascular Function and the Modulation of Health and Disease. Front. Cardiovasc. Med. 2017, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Gokce, N.; Keaney, J.F.; Frei, B.; Holbrook, M.; Olesiak, M.; Zachariah, B.J.; Leeuwenburgh, C.; Heinecke, J.W.; Vita, J.A. Long-Term Ascorbic Acid Administration Reverses Endothelial Vasomotor Dysfunction in Patients With Coronary Artery Disease. Circulation 1999, 99, 3234–3240. [Google Scholar] [CrossRef]
- Wang-Polagruto, J.F.; Villablanca, A.C.; Polagruto, J.A.; Lee, L.; Holt, R.R.; Schrader, H.R.; Ensunsa, J.L.; Steinberg, F.M.; Schmitz, H.H.; Keen, C.L. Chronic Consumption of Flavanol-rich Cocoa Improves Endothelial Function and Decreases Vascular Cell Adhesion Molecule in Hypercholesterolemic Postmenopausal Women. J. Cardiovasc. Pharmacol. 2006, 47, S177–S186. [Google Scholar] [CrossRef]
- Tomasian, D.; Keaney, J.F.; Vita, J.A. Antioxidants and the bioactivity of endothelium-derived nitric oxide. Cardiovasc. Res. 2000, 47, 426–435. [Google Scholar] [CrossRef]
- Malinski, T.; Dawoud, H. Vitamin D3, L-Arginine, L-Citrulline, and antioxidant supplementation enhances nitric oxide bioavailability and reduces oxidative stress in the vascular endothelium—Clinical implications for cardiovascular system. Pharmacogn. Res. 2020, 12, 17. [Google Scholar] [CrossRef]
- Ting, H.H.; Timimi, F.K.; Boles, K.S.; Creager, S.J.; Ganz, P.; A Creager, M. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J. Clin. Investig. 1996, 97, 22–28. [Google Scholar] [CrossRef]
- Kirkby, N.S.; Lundberg, M.H.; Harrington, L.S.; Leadbeater, P.D.M.; Milne, G.L.; Potter, C.M.F.; Al-Yamani, M.; Adeyemi, O.; Warner, T.D.; Mitchell, J.A. Cyclooxygenase-1, not cyclooxygenase-2, is responsible for physiological production of prostacyclin in the cardiovascular system. Proc. Natl. Acad. Sci. USA 2012, 109, 17597–17602. [Google Scholar] [CrossRef]
- Ozkor, M.A.; Quyyumi, A.A. Endothelium-Derived Hyperpolarizing Factor and Vascular Function. Cardiol. Res. Pract. 2011, 2011, 156146. [Google Scholar] [CrossRef] [PubMed]
- Sandoo, A.; Van Zanten, J.J.V.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The Endothelium and Its Role in Regulating Vascular Tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef]
- Ozkor, M.A.; Murrow, J.R.; Rahman, A.M.; Kavtaradze, N.; Lin, J.; Manatunga, A.; Quyyumi, A.A. Endothelium-Derived Hyperpolarizing Factor Determines Resting and Stimulated Forearm Vasodilator Tone in Health and in Disease. Circulation 2011, 123, 2244–2253. [Google Scholar] [CrossRef]
- Scotland, R.S.; Madhani, M.; Chauhan, S.; Moncada, S.; Andresen, J.; Nilsson, H.; Hobbs, A.J.; Ahluwalia, A. Investigation of Vascular Responses in Endothelial Nitric Oxide Synthase/Cyclooxygenase-1 Double-Knockout Mice. Circulation 2005, 111, 796–803. [Google Scholar] [CrossRef]
- Jia, G.; Durante, W.; Sowers, J.R. Endothelium-Derived Hyperpolarizing Factors: A Potential Therapeutic Target for Vascular Dysfunction in Obesity and Insulin Resistance. Diabetes 2016, 65, 2118–2120. [Google Scholar] [CrossRef]
- Kang, K.-T. Endothelium-derived Relaxing Factors of Small Resistance Arteries in Hypertension. Toxicol. Res. 2014, 30, 141–148. [Google Scholar] [CrossRef]
- Godo, S.; Sawada, A.; Saito, H.; Ikeda, S.; Enkhjargal, B.; Suzuki, K.; Tanaka, S.; Shimokawa, H. Disruption of Physiological Balance Between Nitric Oxide and Endothelium-Dependent Hyperpolarization Impairs Cardiovascular Homeostasis in Mice. Arter. Thromb. Vasc. Biol. 2016, 36, 97–107. [Google Scholar] [CrossRef]
- Luksha, L.; Agewall, S.; Kublickiene, K. Endothelium-derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis 2009, 202, 330–344. [Google Scholar] [CrossRef]
- Spruce, A.E.; Standen, N.B.; Stanfield, P.R. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nat. Cell Biol. 1985, 316, 736–738. [Google Scholar] [CrossRef]
- Trube, G.; Rorsman, P.; Ohno-Shosaku, T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic β-cells. Pflügers Archiv. 1986, 407, 493–499. [Google Scholar] [CrossRef]
- Standen, N.B.; Quayle, J.M.; Davies, N.W.; Brayden, J.E.; Huang, Y.; Nelson, M.T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science 1989, 245, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Janigro, D.; West, G.A.; Gordon, E.L.; Winn, H.R. ATP-sensitive K+ channels in rat aorta and brain microvascular endothelial cells. Am. J. Physiol. Physiol. 1993, 265, C812–C821. [Google Scholar] [CrossRef]
- Schnitzler, M.M.Y.; Derst, C.; Daut, J.; Preisig-Müller, R. ATP-sensitive potassium channels in capillaries isolated from guinea-pig heart. J. Physiol. 2000, 525, 307–317. [Google Scholar] [CrossRef]
- Shi, W.-W.; Yang, Y.; Shi, Y.; Jiang, C.; Wei-Wei, S.; Yun, S.; Chun, J. K(ATP) channel action in vascular tone regulation: From genetics to diseases. Sheng Li Xue Bao Acta Physiol. Sin. 2012, 64, 1–13. [Google Scholar]
- Zhang, Y.-L.; Chen, Y.-P.; Wang, H. Targeting Small Arteries of Hypertensive Status with Novel ATP-Sensitive Potassium Channel Openers. Curr. Vasc. Pharmacol. 2005, 3, 119–124. [Google Scholar] [CrossRef]
- Wang, H. Cardiovascular ATP-sensitive K+ channel as a new molecular target for development of antihypertensive drugs. Zhongguo Yao Li Xue Bao Acta Pharmacol. Sin. 1998, 19, 397. [Google Scholar]
- Chutkow, W.A.; Pu, J.; Wheeler, M.T.; Wada, T.; Makielski, J.C.; Burant, C.F.; McNally, E.M. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 KATP channels. J. Clin. Investig. 2002, 110, 203–208. [Google Scholar] [CrossRef]
- Miki, T.; Suzuki, M.; Shibasaki, T.; Uemura, H.; Sato, T.; Yamaguchi, K.; Koseki, H.; Iwanaga, T.; Nakaya, H.; Seino, S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat. Med. 2002, 8, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Domingo, A.; Tan, B.-H.; Crotti, L.; Tester, D.J.; Eckhardt, L.; Cuoretti, A.; Kroboth, S.L.; Song, C.; Zhou, Q.; Kopp, D.; et al. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac KATP channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010, 7, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Tester, D.J.; Tan, B.-H.; Medeiros-Domingo, A.; Song, C.; Makielski, J.C.; Ackerman, M.J. Loss-of-Function Mutations in the KCNJ8 -Encoded Kir6.1 K ATP Channel and Sudden Infant Death Syndrome. Circ. Cardiovasc. Genet. 2011, 4, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Randak, C.O.; Welsh, M.J. ADP inhibits function of the ABC transporter cystic fibrosis transmembrane conductance regulator via its adenylate kinase activity. Proc. Natl. Acad. Sci. USA 2005, 102, 2216–2220. [Google Scholar] [CrossRef]
- Dzeja, P.P.; Terzic, A. Phosphotransfer reactions in the regulation of ATP-sensitive K + channels. FASEB J. 1998, 12, 523–529. [Google Scholar] [CrossRef]
- Selivanov, V.A.; Alekseev, A.E.; Hodgson, D.M.; Dzeja, P.P.; Terzic, A. Nucleotide-gated KATP channels integrated with creatine and adenylate kinases: Amplification, tuning and sensing of energetic signals in the compartmentalized cellular environment. Mol. Cell Biochem. 2004, 256–257, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.L.; Beron, J.; Spindler, B.; Groscurth, P.; Wallimann, T.; Verrey, F. Metabolic support of Na+ pump in apically permeabilized A6 kidney cell epithelia: Role of creatine kinase. Am. J. Physiol. Physiol. 1997, 272, C697–C706. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Endothelial Barrier and Its Abnormalities in Cardiovascular Disease. Front. Physiol. 2015, 6, 365. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F.; Webster, N.R. Physiology of the endothelium. Br. J. Anaesth. 2004, 93, 105–113. [Google Scholar] [CrossRef]
- Park-Windhol, C.; D’Amore, P.A. Disorders of Vascular Permeability. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 251–281. [Google Scholar] [CrossRef]
- Vestweber, D. Relevance of endothelial junctions in leukocyte extravasation and vascular permeability. Ann. N. Y. Acad. Sci. 2012, 1257, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, G.; Dejana, E. Endothelial Cell-to-Cell Junctions: Molecular Organization and Role in Vascular Homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef] [PubMed]
- Mundi, S.; Massaro, M.; Scoditti, E.; Carluccio, M.A.; Van Hinsbergh, V.W.M.; Iruela-Arispe, M.L.; De Caterina, R. Endothelial permeability, LDL deposition, and cardiovascular risk factors—A review. Cardiovasc. Res. 2018, 114, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Asadollahi, K.; Soleimannejad, K.; Khalighi, Z.; Mohsenzadeh, Y.; Hemati, R.; Moradkhani, A.; Abangah, G. The Effects of Creatine Monohydrate on Permeability of Coronary Artery Endothelium and Level of Blood Lipoprotein in Diabetic Rats. Ann. Clin. Lab. Sci. 2016, 46, 495–501. [Google Scholar] [PubMed]
- Wang, L.; Chen, Y.; Li, X.; Zhang, Y.; Gulbins, E.; Zhang, Y. Enhancement of endothelial permeability by free fatty acid through lysosomal cathepsin B-mediated Nlrp3 inflammasome activation. Oncotarget 2016, 7, 73229–73241. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Gottlieb, E.; Rounds, S. Effects of cigarette smoke on pulmonary endothelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L743–L756. [Google Scholar] [CrossRef]
- Luc, G.; Arveiler, D.; Evans, A.; Amouyel, P.; Ferrieres, J.; Bard, J.-M.; Elkhalil, L.; Fruchart, J.-C.; Ducimetiere, P. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and incident coronary heart disease: The PRIME Study. Atherosclerosis 2003, 170, 169–176. [Google Scholar] [CrossRef]
- Roldán, V.; Marín, F.; Lip, G.Y.H.; Blann, A.D. Soluble E-selectin in cardiovascular disease and its risk factors. Thromb. Haemost. 2003, 90, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Tokarska-Schlattner, M.; Epand, R.F.; Meiler, F.; Zandomeneghi, G.; Neumann, D.; Widmer, H.R.; Meier, B.H.; Epand, R.M.; Saks, V.; Wallimann, T.; et al. Phosphocreatine Interacts with Phospholipids, Affects Membrane Properties and Exerts Membrane-Protective Effects. PLoS ONE 2012, 7, e43178. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.H.; Lee, J.S.; Murphy, E.M.; Gerich, M.E.; Dran, R.; Glover, L.E.; Abdulla, Z.I.; Skelton, M.R.; Colgan, S.P. Creatine Transporter, Reduced in Colon Tissues from Patients with Inflammatory Bowel Diseases, Regulates Energy Balance in Intestinal Epithelial Cells, Epithelial Integrity, and Barrier Function. Gastroenterology 2020, 159, 984–998. [Google Scholar] [CrossRef] [PubMed]
- Glover, L.E.; Bowers, B.E.; Saeedi, B.; Ehrentraut, S.F.; Campbell, E.L.; Bayless, A.J.; Dobrinskikh, E.; Kendrick, A.A.; Kelly, C.J.; Burgess, A.; et al. Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc. Natl. Acad. Sci. USA 2013, 110, 19820–19825. [Google Scholar] [CrossRef] [PubMed]
- Lombard, D.B.; Chua, K.F.; Mostoslavsky, R.; Franco, S.; Gostissa, M.; Alt, F.W. DNA Repair, Genome Stability, and Aging. Cell 2005, 120, 497–512. [Google Scholar] [CrossRef]
- Negishi, H.; Ikeda, K.; Kuga, S.; Noguchi, T.; Kanda, T.; Njelekela, M.; Liu, L.; Miki, T.; Nara, Y.; Sato, T.; et al. The relation of oxidative DNA damage to hypertension and other cardiovascular risk factors in Tanzania. J. Hypertens. 2001, 19, 529–533. [Google Scholar] [CrossRef]
- Bhat, M.A.; Mahajan, N.; Gandhi, G. DNA and chromosomal damage in coronary artery disease patients. EXCLI J. 2013, 12, 872–884. [Google Scholar]
- Shah, N.R.; Mahmoudi, M. The role of DNA damage and repair in atherosclerosis: A review. J. Mol. Cell. Cardiol. 2015, 86, 147–157. [Google Scholar] [CrossRef]
- Brass, E.P.; Wang, H.; Hiatt, W.R. Multiple skeletal muscle mitochondrial DNA deletions in patients with unilateral peripheral arterial disease. Vasc. Med. 2000, 5, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.; Ivanova, E.; Sobenin, I.; Yet, S.-F.; Orekhov, A. The Role of Mitochondria in Cardiovascular Diseases. Biology 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, V.; Seluanov, A.; Mao, Z.; Hine, C. Changes in DNA repair during aging. Nucleic Acids Res. 2007, 35, 7466–7474. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 1–32. [Google Scholar] [CrossRef]
- Woods, D.; Turchi, J.J. Chemotherapy induced DNA damage response. Cancer Biol. Ther. 2013, 14, 379–389. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef]
- Vetrani, C.; Costabile, G.; Di Marino, L.; Rivellese, A.A. Nutrition and oxidative stress: A systematic review of human studies. Int. J. Food Sci. Nutr. 2012, 64, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Albano, E. Alcohol, oxidative stress and free radical damage. Proc. Nutr. Soc. 2006, 65, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.; Bonauer, A.; Dimmeler, S. RNA Therapeutics in Cardiovascular Disease. Circ. Res. 2018, 123, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Pradhan-Nabzdyk, L.; Huang, C.; LoGerfo, F.W.; Nabzdyk, C.S. Current siRNA targets in atherosclerosis and aortic aneurysm. Discov. Med. 2014, 17, 233–246. [Google Scholar]
- Nabzdyk, C.S.; Pradhan-Nabzdyk, L.; LoGerfo, F.W. RNAi therapy to the wall of arteries and veins: Anatomical, physiologic, and pharmacological considerations. J. Transl. Med. 2017, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Berneburg, M.; Gremmel, T.; Kürten, V.; Schroeder, P.; Hertel, I.; Von Mikecz, A.; Wild, S.; Chen, M.; Declercq, L.; Matsui, M.; et al. Creatine Supplementation Normalizes Mutagenesis of Mitochondrial DNA as Well as Functional Consequences. J. Investig. Dermatol. 2005, 125, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Fetterman, J.L.; Holbrook, M.; Westbrook, D.G.; Brown, J.A.; Feeley, K.P.; Bretón-Romero, R.; Linder, E.A.; Berk, B.D.; Weisbrod, R.M.; Widlansky, M.E.; et al. Mitochondrial DNA damage and vascular function in patients with diabetes mellitus and atherosclerotic cardiovascular disease. Cardiovasc. Diabetol. 2016, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.P.; Bennett, M.R. The role of mitochondrial DNA damage in the development of atherosclerosis. Free. Radic. Biol. Med. 2016, 100, 223–230. [Google Scholar] [CrossRef]
- Uryga, A.; Gray, K.; Bennett, M. DNA Damage and Repair in Vascular Disease. Annu. Rev. Physiol. 2016, 78, 45–66. [Google Scholar] [CrossRef]
- Mirzaei, B.; Rahmani-Nia, F.; Salehi, Z.; Rahimi, R. Effects of creatine monohydrate supplementation on oxidative DNA damage and lipid peroxidation induced by acute incremental exercise to exhaustion in wrestlers. Kinesiol. Int. J. Fundam. Appl. Kinesiol. 2013, 45, 30–40. [Google Scholar]
- Qasim, N.; Mahmood, R. Diminution of Oxidative Damage to Human Erythrocytes and Lymphocytes by Creatine: Possible Role of Creatine in Blood. PLoS ONE 2015, 10, e0141975. [Google Scholar] [CrossRef]
- Guidi, C.; Potenza, L.; Sestili, P.; Martinelli, C.; Guescini, M.; Stocchi, L.; Zeppa, S.; Polidori, E.; Annibalini, G.; Stocchi, V. Differential effect of creatine on oxidatively-injured mitochondrial and nuclear DNA. Biochim. Biophys. Acta (BBA) Gen. Subj. 2008, 1780, 16–26. [Google Scholar] [CrossRef]
- Anderson, O. Creatine propels British athletes to Olympic gold medals: Is creatine the one true ergogenic aid. Run. Res. News 1993, 9, 1–5. [Google Scholar]
- Bird, S.P. Creatine Supplementation and Exercise Performance: A Brief Review. J. Sports Sci. Med. 2003, 2, 123–132. [Google Scholar]
- Chilibeck, P.D.; Kaviani, M.; Candow, D.G.; A Zello, G. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: A meta-analysis. Open Access J. Sports Med. 2017, 8, 213–226. [Google Scholar] [CrossRef]
- Rawson, E.S.; Stec, M.J.; Frederickson, S.J.; Miles, M.P. Low-dose creatine supplementation enhances fatigue resistance in the absence of weight gain. Nutrition 2011, 27, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Ferreira, M.; Wilson, M.; Grindstaff, P.; Plisk, S.; Reinardy, J.; Cantler, E.; Almada, A.L. Effects of creatine supplementation on body composition, strength, and sprint performance. Med. Sci. Sports Exerc. 1998, 30, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Tsikas, D.; Bakker, S.J. Creatine is a Conditionally Essential Nutrient in Chronic Kidney Disease: A Hypothesis and Narrative Literature Review. Nutritions 2019, 11, 1044. [Google Scholar] [CrossRef]
- Kreider, R.B.; Melton, C.; Rasmussen, C.J.; Greenwood, M.; Lancaster, S.; Cantler, E.C.; Milnor, P.; Almada, A.L. Long-term creatine supplementation does not significantly affect clinical markers of health in athletes. Mol. Cell. Biochem. 2003, 244, 95–104. [Google Scholar] [CrossRef]
- Clarke, H.; Kim, D.-H.; Meza, C.A.; Ormsbee, M.J.; Hickner, R.C. The Evolving Applications of Creatine Supplementation: Could Creatine Improve Vascular Health? Nutrients 2020, 12, 2834. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarke, H.; Hickner, R.C.; Ormsbee, M.J. The Potential Role of Creatine in Vascular Health. Nutrients 2021, 13, 857. https://doi.org/10.3390/nu13030857
Clarke H, Hickner RC, Ormsbee MJ. The Potential Role of Creatine in Vascular Health. Nutrients. 2021; 13(3):857. https://doi.org/10.3390/nu13030857
Chicago/Turabian StyleClarke, Holly, Robert C. Hickner, and Michael J. Ormsbee. 2021. "The Potential Role of Creatine in Vascular Health" Nutrients 13, no. 3: 857. https://doi.org/10.3390/nu13030857
APA StyleClarke, H., Hickner, R. C., & Ormsbee, M. J. (2021). The Potential Role of Creatine in Vascular Health. Nutrients, 13(3), 857. https://doi.org/10.3390/nu13030857